The Microbeam Insert at the White Beam Beamline P61A at the Synchrotron PETRA III/DESY: A New Tool for High Dose Rate Irradiation Research
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Beamline Design
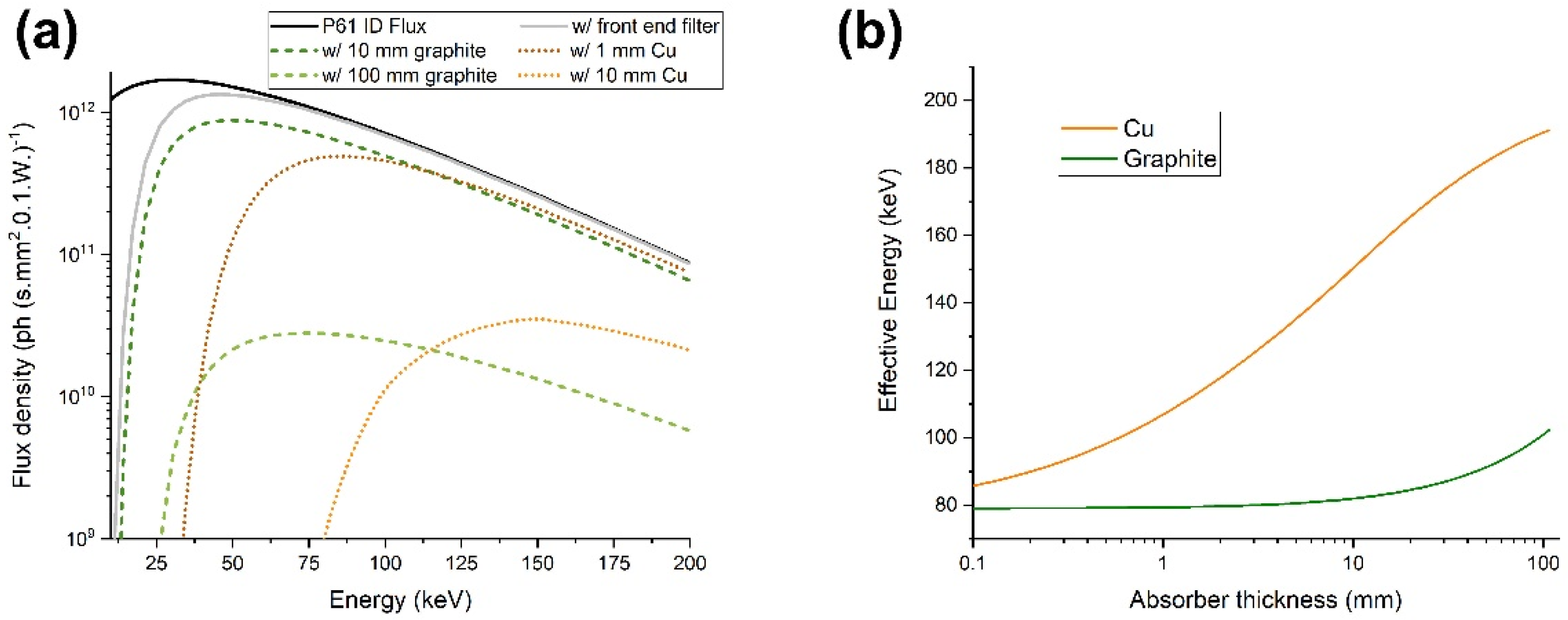
2.2. Beam Parameters and Spectrum
2.3. Experimental Setup for MRT
- (1)
- In detail, the pre-sample section consists of the following components, described in the beam direction:
- -
- Incident and secondary slits: The motorized slits (IB-C30-AIR, JJ X-ray A/S, Hørsholm, Denmark) are composed of four 10 mm thick, polished tungsten carbide blades that are slightly pitched for total reflection rejection. The slit system is flushed with gaseous nitrogen to slow beam-induced oxidation processes on the blades. The whole slit assembly can be adjusted in height via a vertically mounted linear stage. The first slits are used to further shape the beam coming from the P61A optics hutch and to remove possible uncollimated and scattered radiation. The purpose of the secondary slits is to remove scattered radiation generated by the multislit collimator. In addition, they can be used for beam characterisation by scanning the slits, when closed to a few micrometres.
- -
- Multi Slit Collimator (MSC): the fixed-spacing multislit collimator (UNT, Morbier, France) collimates the incident broad beam into an array of quasi-parallel, 50 µm wide microbeams spaced at a 400 µm center-to-center distance [35]. The slits are cut into an 8 mm thick tungsten block. The water cooled MSC is inserted into an inhouse built, nitrogen flushed chamber. It is mounted on a stage and thus can be rotated around its vertical axis as well as moved vertically and horizontally. The rotation stage is used to align the yaw direction of the collimator with the microslit lamellae precisely parallel to the beam direction and with the lateral translation symmetrically to the center of the beam so that uniform and well collimated microbeams can be obtained.
- -
- Ion chambers: The ion chambers were designed and manufactured by DESY. They are part of the P61A standard equipment used for intensity monitoring of the high energy beam and integrated into the beamline data acquisition system (40 mm electrode length, 6 mm electrode distance).
- -
- Fixed sample mask: Because of the considerable radiation background produced along the in-air beam path, a large anti-scatter shielding block, made of a massive lead-iron plate with an aperture, is installed in front of the sample stage.
- (2)
- Different sample setups can be placed on a small bread board table and positioned and translated vertically through the beam by a linear stage with a large stroke (Aerotech ECO115SL, Aerotech, Inc., Pittsburg, PA, USA). The stage has a load capacity of up to 5 kg and can be driven with up to 100 mm/s. The wide adjustability of the speed allows the variation of the dose by approximately two orders of magnitude when the sample is moved during an exposure vertically through the fixed synchrotron beam. The vertical stage itself is mounted onto a large stroke horizontal translation. In this manner the sample setup can be positioned within a rectangular range of 320 × 480 mm2 (horizontal × vertical).
- (3)
- The post-sample section contains another ion chamber for transmission measurements. Additionally, a scintillator-based X-ray camera for coarse sample imaging and beam alignment can be moved into the beam path. The CMOS camera (UI-5880CP-C-HQ Rev.2, IDS Imaging Development Systems GmbH, Obersulm, Germany) has a 5 times magnified optic and therefore an effective pixel size of 2.5 µm. A massive lead beam stop is installed at the end of the beam path.
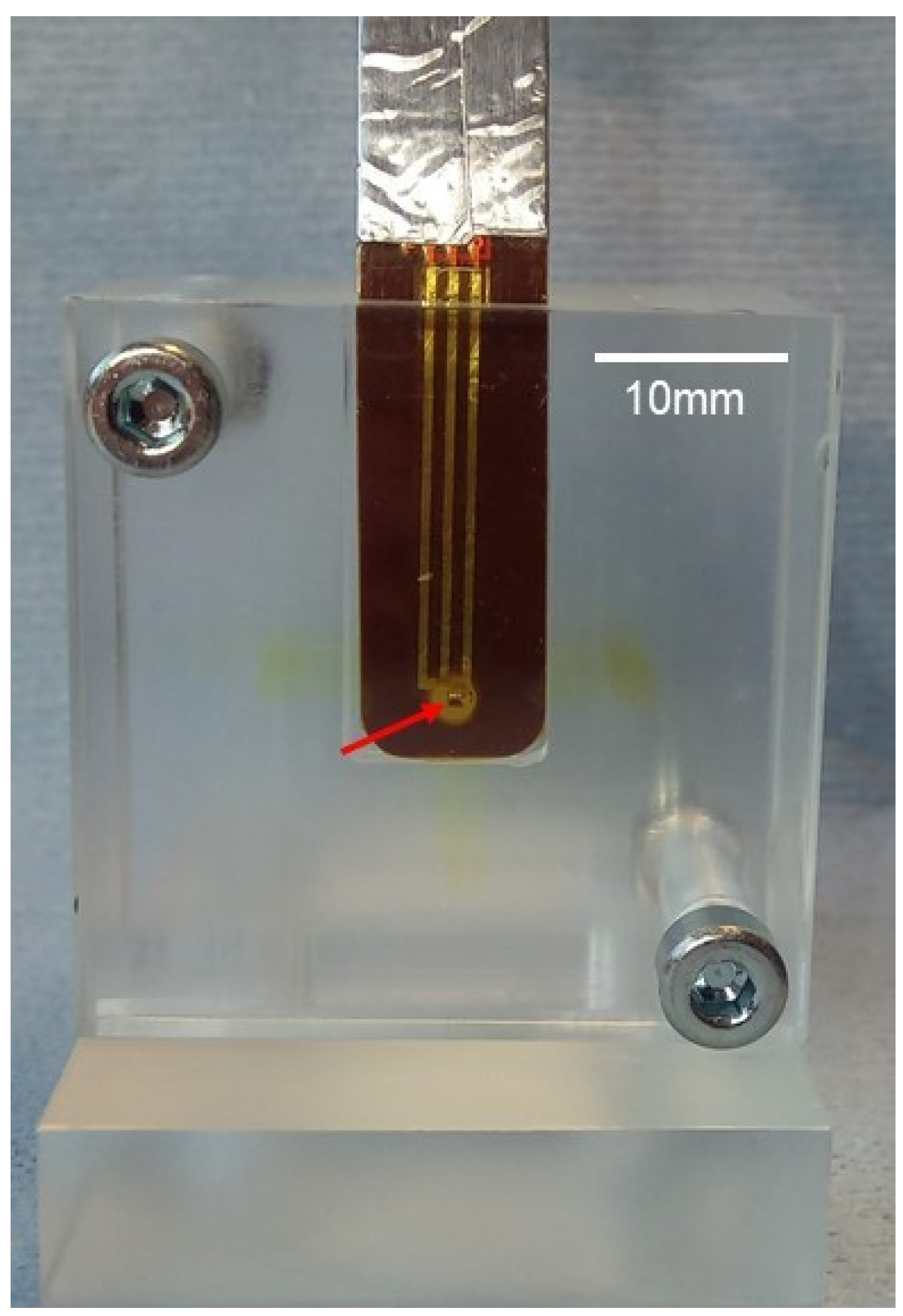
2.4. Dosimetry: Measurements, Recordings, and Monte Carlo Simulation
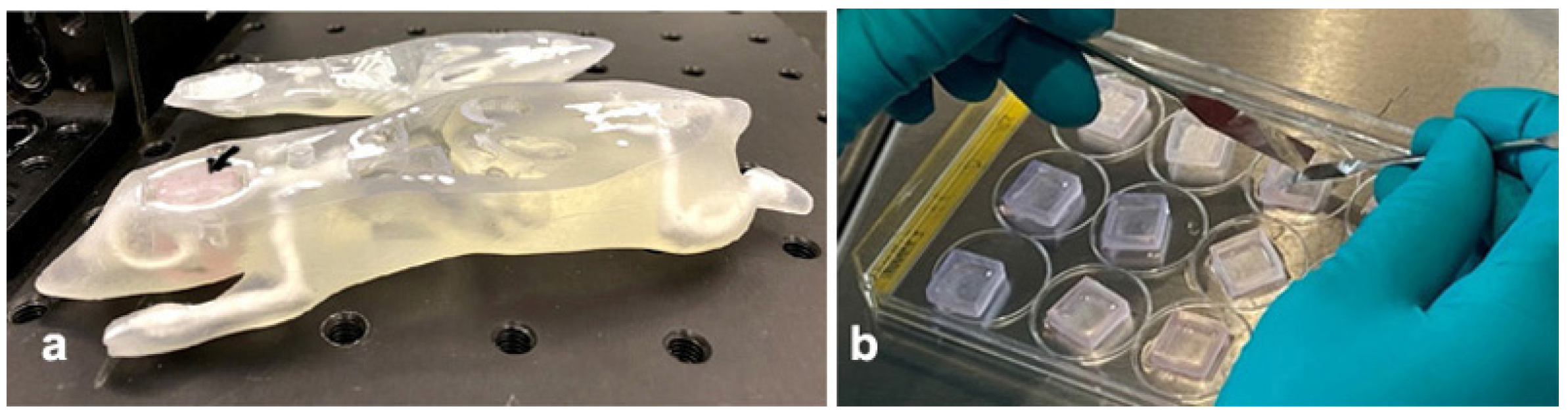
2.5. Cell Culture for Use in the Modified Alderson Phantom
3. Results
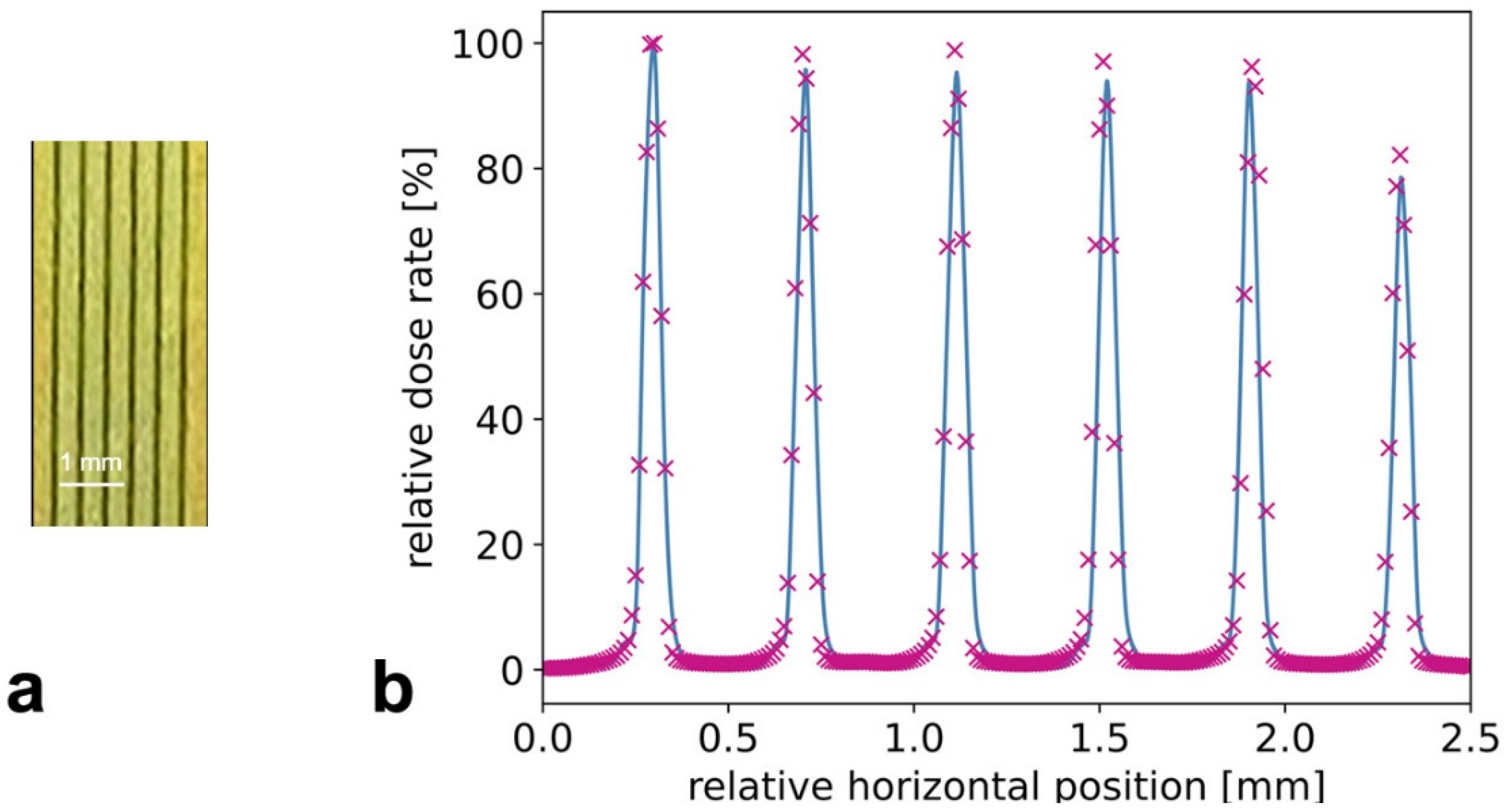
3.1. Characterization of Microbeams at Beamline P61A
3.2. Models Developed for Biomedical Work at Beamline P61A
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vora, S.A.; Wong, W.W.; Schild, S.E.; Ezzell, G.A.; Halyard, M.Y. Analysis of Biochemical Control and Prognostic Factors in Patients Treated With Either Low-Dose Three-Dimensional Conformal Radiation Therapy or High-Dose Intensity-Modulated Radiotherapy for Localized Prostate Cancer. Int. J. Radiat. Oncol. 2007, 68, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Yamada, Y.; Fuks, Z.; Zhang, Z.; Hunt, M.; Cahlon, O.; Park, J.; Shippy, A. Long-Term Results of Conformal Radiotherapy for Prostate Cancer: Impact of Dose Escalation on Biochemical Tumor Control and Distant Metastases-Free Survival Outcomes. Int. J. Radiat. Oncol. 2008, 71, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Viani, G.A.; Viana, B.S.; Martin, J.E.; Rossi, B.T.; Zuliani, G.; Stefano, E.J. Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. Cancer 2016, 122, 2004–2011. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.-M.; Shen, W.-B.; Xu, J.-R.; Li, Y.-M.; Li, S.-G.; Zhu, S.-C. Effect of SIB-IMRT-based selective dose escalation of local tumor on the prognosis of patients with esophageal cancer. Int. J. Clin. Oncol. 2021, 26, 1640–1649. [Google Scholar] [CrossRef]
- Li, J.-C.; Hu, C.-S.; Jiang, G.-L.; Mayr, N.; Wang, J.; He, X.-Y.; Wu, Y.-R. Dose Escalation of Three-dimensional Conformal Radiotherapy for Locally Recurrent Nasopharyngeal Carcinoma: A Prospective Randomised Study. Clin. Oncol. 2006, 18, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Balosso, J.; Febvey-Combes, O.; Iung, A.; Lozano, H.; Alloh, A.S.; Cornu, C.; Hervé, M.; Akkal, Z.; Lièvre, M.; Plattner, V.; et al. A randomized controlled phase III study comparing hadrontherapy with carbon ions versus conventional radiotherapy–including photon and proton therapy–for the treatment of radioresistant tumors: The ETOILE trial. BMC Cancer 2022, 22, 575. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef] [Green Version]
- Montay-Gruel, P.; Acharya, M.M.; Jorge, P.G.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron microbeam radiation therapy as a new approach for the treatment of radioresistant melanoma: Potential underlying bechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136. [Google Scholar] [CrossRef]
- Fouillade, C.; Curras-Alonso, S.; Giuranno, L.; Quelennec, E.; Heinrich, S.; Bonnet-Boissinot, S.; Beddok, A.; Leboucher, S.; Karakurt, H.U.; Bohec, M.; et al. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-induced Senescence. Clin. Cancer Res. 2020, 26, 1497–1506. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vozenin, M.-C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.-F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konradsson, E.; Arendt, M.L.; Jensen, K.B.; Børresen, B.; Hansen, A.E.; Bäck, S.; Kristensen, A.T.; Rosenschöld, P.M.A.; Ceberg, C.; Petersson, K. Establishment and Initial Experience of Clinical FLASH Radiotherapy in Canine Cancer Patients. Front. Oncol. 2021, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Schüler, E.; Acharya, M.; Montay-Gruel, P.; Loo, B.W., Jr.; Vozenin, M.C.; Maxim, P.G. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med. Phys. 2022, 49, 2082–2095. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Bouchet, A.; Bräuer-Krisch, E.; Prezado, Y.; El Atifi, M.; Rogalev, L.; Le Clec′H, C.; Laissue, J.A.; Pelletier, L.; Le Duc, G. Better Efficacy of Synchrotron Spatially Microfractionated Radiation Therapy Than Uniform Radiation Therapy on Glioma. Int. J. Radiat. Oncol. 2016, 95, 1485–1494. [Google Scholar] [CrossRef]
- Potez, M.; Bouchet, A.; Flaender, M.; Rome, C.; Collomb, N.; Grotzer, M.; Krisch, M.; Djonov, V.; Balosso, J.; Brun, E.; et al. Synchrotron X-Ray Boost Delivered by Microbeam Radiation Therapy After Conventional X-ray Therapy Fractionated in Time Improves F98 Glioma Control. Int. J. Radiat. Oncol. 2020, 107, 360–369. [Google Scholar] [CrossRef]
- Smyth, L.M.L.; Crosbie, J.C.; Sloggett, C.; Rogers, P.A.W.; Donoghue, J.F. Spatially Fractionated X-Ray Microbeams Elicit a More Sustained Immune and Inflammatory Response in the Brainstem than Homogenous Irradiation. Radiat. Res. 2021, 196, 355–365. [Google Scholar] [CrossRef]
- Adam, J.-F.; Balosso, J.; Bayat, S.; Berkvens, P.; Berruyer, G.; Bräuer-Krisch, E.; Brochard, T.; Chamel, G.; Desagneaux, A.; Drevon-Gaud, R.; et al. Toward Neuro-Oncologic Clinical Trials of High-Dose-Rate Synchrotron Microbeam Radiation Therapy: First Treatment of a Spontaneous Canine Brain Tumor. Int. J. Radiat. Oncol. 2022, 113, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Thomlinson, W.; Berkvens, P.; Berruyer, G.; Bertrand, B.; Blattmann, H.; Brauer-Krisch, E.; Brochard, T.; Charvet, A.M.; Corde, S.; Dimichiel, M.; et al. Research at the European Synchrotron Radiation Facility medical beamline. Cell. Mol. Biol. 2000, 46, 1053–1063. [Google Scholar] [PubMed]
- Livingstone, J.; Adam, J.-F.; Crosbie, J.C.; Hall, C.J.; Lye, J.E.; McKinlay, J.; Pelliccia, D.; Pouzoulet, F.; Prezado, Y.; Stevenson, A.W.; et al. Preclinical radiotherapy at the Australian Synchrotron’s Imaging and Medical Beamline: Instrumentation, dosimetry and a small-animal feasibility study. J. Synchrotron Radiat. 2017, 24, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, J.; Stevenson, A.W.; Häusermann, D.; Adam, J.-F. Experimental optimisation of the X-ray energy in microbeam radiation therapy. Phys. Med. 2018, 45, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Torikoshi, M.; Ohno, Y.; Yagi, N.; Umetani, K.; Furusawa, Y. Dosimetry for a microbeam array generated by synchrotron radiation at SPring-8. Eur. J. Radiol. 2008, 68, S114–S117. [Google Scholar] [CrossRef]
- Ohno, Y.; Torikoshi, M.; Suzuki, M.; Umetani, K.; Imai, Y.; Uesugi, K.; Yagi, N. Dose distribution of a 125 keV mean energy microplanar x-ray beam for basic studies on microbeam radiotherapy. Med. Phys. 2008, 35, 3252–3258. [Google Scholar] [CrossRef]
- Annabell, N.; Yagi, N.; Umetani, K.; Wong, C.; Geso, M. Evaluating the peak-to-valley dose ratio of synchrotron microbeams using PRESAGE fluorescence. J. Synchrotron Radiat. 2012, 19, 332–339. [Google Scholar] [CrossRef]
- Schültke, E.; Fiedler, S.; Menk, R.H.; Jaekel, F.; Dreossi, D.; Casarin, K.; Tromba, G.; Bartzsch, S.; Kriesen, S.; Hildebrandt, G.; et al. Perspectives for microbeam irradiation at the SYRMEP beamline. J. Synchrotron Radiat. 2021, 28, 410–418. [Google Scholar] [CrossRef]
- Al-Zeer, M.A.; Prehn, F.; Fiedler, S.; Lienert, U.; Krisch, M.; Berg, J.; Kurreck, J.; Hildebrandt, G.; Schültke, E. Evaluating the Suitability of 3D Bioprinted Samples for Experimental Radiotherapy: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 9951. [Google Scholar] [CrossRef]
- Farla, R.; Bhat, S.; Sonntag, S.; Chanyshev, A.; Ma, S.; Ishii, T.; Liu, Z.; Néri, A.; Nishiyama, N.; Faria, G.A.; et al. Extreme conditions research using the large-volume press at the P61B endstation, PETRA III. J. Synchrotron Radiat. 2022, 29, 409–423. [Google Scholar] [CrossRef]
- Bacher, R.; Balewski, K.; Brefeld, W.; Decking, W.; Ebert, M.; Eckoldt, H.J.; Grabe-Celik, H.; Jensen, J.P.; Klute, J.; Leuschner, A.; et al. PETRA III—Status of the Storage Ring. AIP Conf. Proc. 2007, 879, 175. [Google Scholar] [CrossRef]
- Tanaka, T.; Kitamura, H. SPECTRA: A synchrotron radiation calculatiion code. J. Synchrotron Radiat. 2001, 8, 1221–1228. [Google Scholar] [CrossRef]
- Seltzer, H. NIST Attenuation Data Base. 1989. Available online: https://www.nist.gov/pml/x-ray-mass-attenuation-coefficients (accessed on 1 July 2022).
- Wroblewski, T. RadSynch17. In Proceedings of the Ninth International Workshop on Radiation Safety at Synchrotron Radiation Sources (RadSynch17), NSRRC, Hsinchu, Taiwan, 19–21 April 2017; pp. 182–186. [Google Scholar]
- Bräuer-Krisch, E.; Requardt, H.; Brochard, T.; Berruyer, G.; Renier, M.; Laissue, J.A.; Bravin, A. New technology enables high precision multislit collimators for microbeam radiation therapy. Rev. Sci. Instrum. 2009, 80, 74301. [Google Scholar] [CrossRef] [PubMed]
- Petasecca, M.; Cullen, A.; Fuduli, I.; Espinoza, A.; Porumb, C.; Stanton, C.; Aldosari, A.H.; Brauer-Krisch, E.; Requardt, H.; Bravin, A.; et al. X-Tream: A novel dosimetry system for Synchrotron Microbeam Radiation Therapy. J. Instrum. 2012, 7, P07022. [Google Scholar] [CrossRef] [Green Version]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. Geant4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef] [Green Version]
- Wegner, M.; Gargioni, E.; Krause, D. Einsatzmöglichkeiten der additiven Fertigung in der Herstellung von Phantomen. In Konstruktion für die Additive Fertigung 2020; Springer Vieweg: Berlin/Heidelberg, Germany, 2021; pp. 267–282. [Google Scholar] [CrossRef]
- Schültke, E.; Juurlink, B.H.; Ataelmannan, K.; Laissue, J.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Minczewska, J.; Crosbie, J.; Taherian, H.; et al. Memory and survival after microbeam radiation therapy. Eur. J. Radiol. 2008, 68, S142–S146. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Bräuer-Krisch, E.; Blattmann, H.; Requardt, H.; Laissue, J.A.; Hildebrandt, G. Survival of rats bearing advanced intracerebral F 98 tumors after glutathione depletion and microbeam radiation therapy: Conclusions from a pilot project. Radiat. Oncol. 2018, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Engels, E.; Li, N.; Davis, J.; Paino, J.; Cameron, M.; Dipuglia, A.; Vogel, S.; Valceski, M.; Khochaiche, A.; O’Keefe, A.; et al. Toward personalized synchrotron microbeam radiation therapy. Sci. Rep. 2020, 10, 8833. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Markarian, M.; Allen, B.D.; Baddour, J.D.; Giedzinski, E.; Jorge, P.G.; Petit, B.; Bailat, C.; Vozenin, M.-C.; Limoli, C.; et al. Ultra-High-Dose-Rate FLASH Irradiation Limits Reactive Gliosis in the Brain. Radiat. Res. 2020, 194, 636–645. [Google Scholar] [CrossRef]
- Schültke, E.; Bayat, S.; Bartzsch, S.; Bräuer-Krisch, E.; Djonov, V.; Fiedler, S.; Fernandez-Palomo, C.; Jaekel, F.; Pellicioli, P.; Trappetti, V.; et al. A Mouse Model for Microbeam Radiation Therapy of the Lung. Int. J. Radiat. Oncol. 2020, 110, 521–525. [Google Scholar] [CrossRef]
- Trappetti, V.; Fernandez-Palomo, C.; Smyth, L.; Klein, M.; Haberthür, D.; Butler, D.; Barnes, M.; Shintani, N.; de Veer, M.; Laissue, J.A.; et al. Synchrotron Microbeam Radiation Therapy for the Treatment of Lung Carcinoma: A Preclinical Study. Int. J. Radiat. Oncol. 2021, 111, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Cotchin, E. Spontaneous tumours in young animals. Proc. R. Soc. Med. 1975, 68, 653–655. [Google Scholar] [PubMed]
- José-López, R.; Gutierrez-Quintana, R.; de la Fuente, C.; Manzanilla, E.G.; Suñol, A.; Castro, D.P.; Añor, S.; Sánchez-Masian, D.; Fernández-Flores, F.; Ricci, E.; et al. Clinical features, diagnosis, and survival analysis of dogs with glioma. J. Vet. Intern. Med. 2021, 35, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Palomo, C.; Mothersill, C.; Bräuer-Krisch, E.; Laissue, J.; Seymour, C.; Schültke, E. γ-H2AX as a Marker for Dose Deposition in the Brain of Wistar Rats after Synchrotron Microbeam Radiation. PLoS ONE 2015, 10, e0119924. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schültke, E.; Fiedler, S.; Mewes, C.; Gargioni, E.; Klingenberg, J.; Abreu Faria, G.; Lerch, M.; Petasecca, M.; Prehn, F.; Wegner, M.; et al. The Microbeam Insert at the White Beam Beamline P61A at the Synchrotron PETRA III/DESY: A New Tool for High Dose Rate Irradiation Research. Cancers 2022, 14, 5137. https://doi.org/10.3390/cancers14205137
Schültke E, Fiedler S, Mewes C, Gargioni E, Klingenberg J, Abreu Faria G, Lerch M, Petasecca M, Prehn F, Wegner M, et al. The Microbeam Insert at the White Beam Beamline P61A at the Synchrotron PETRA III/DESY: A New Tool for High Dose Rate Irradiation Research. Cancers. 2022; 14(20):5137. https://doi.org/10.3390/cancers14205137
Chicago/Turabian StyleSchültke, Elisabeth, Stefan Fiedler, Catharina Mewes, Elisabetta Gargioni, Johannes Klingenberg, Guilherme Abreu Faria, Michael Lerch, Marco Petasecca, Franziska Prehn, Marie Wegner, and et al. 2022. "The Microbeam Insert at the White Beam Beamline P61A at the Synchrotron PETRA III/DESY: A New Tool for High Dose Rate Irradiation Research" Cancers 14, no. 20: 5137. https://doi.org/10.3390/cancers14205137
APA StyleSchültke, E., Fiedler, S., Mewes, C., Gargioni, E., Klingenberg, J., Abreu Faria, G., Lerch, M., Petasecca, M., Prehn, F., Wegner, M., Scholz, M., Jaekel, F., & Hildebrandt, G. (2022). The Microbeam Insert at the White Beam Beamline P61A at the Synchrotron PETRA III/DESY: A New Tool for High Dose Rate Irradiation Research. Cancers, 14(20), 5137. https://doi.org/10.3390/cancers14205137




