Occupational Exposure to Pesticides Affects Pivotal Immunologic Anti-Tumor Responses in Breast Cancer Women from the Intermediate Risk of Recurrence and Death
Abstract
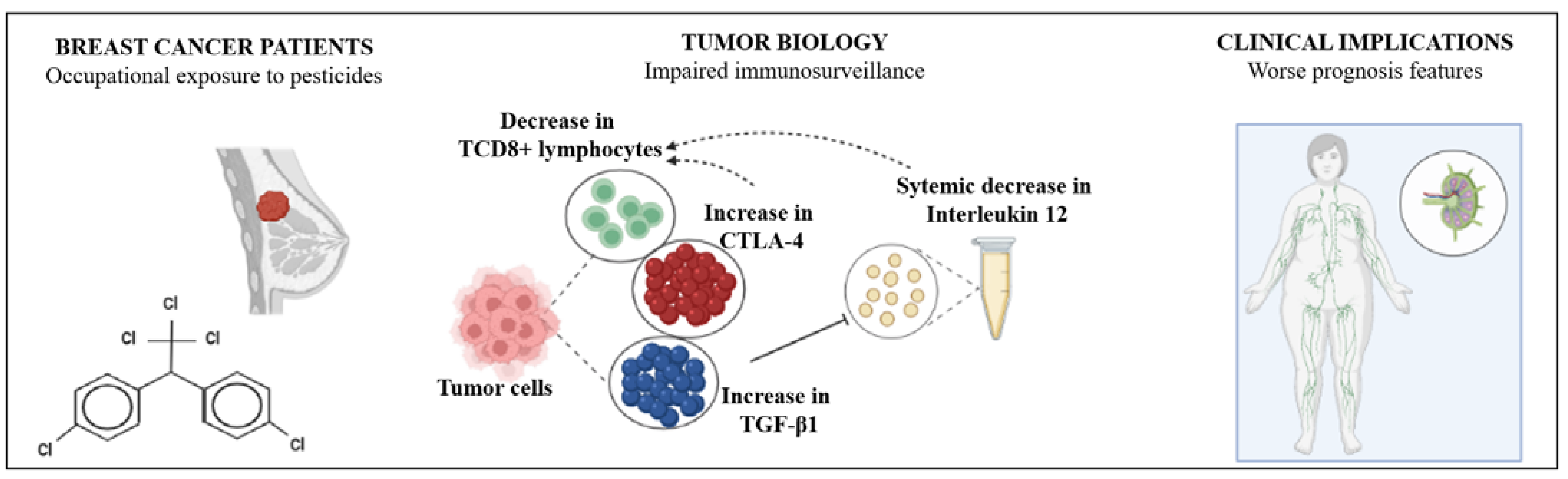
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Obtention and Determination of the Systemic Inflammatory Profile
2.3. Tumor Analysis: Immunofluorescence Labeling for Transforming Growth Factor-β1, Cytotoxic T-Lymphocyte-Associated Antigen 4, and CD4/CD8 Lymphocyte Labeling
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Gray, R.J.; Ravdin, P.M.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.C.; Goetz, M.P.; et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N. Engl. J. Med. 2019, 380, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, Y.; Wu, Q.; Chen, W.; Liu, Z.; Wang, T.; Ai, C.; Chen, F.; Wang, Z.; Ma, G.; et al. Effects of clinicopathological factors on prognosis of young patients with resected breast cancer. Medicine 2021, 100, e23693. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.-J.; Panel members. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- SBOC. Guideline 2011. Rev. Soc Bras. Oncol. Clín. 2011, 589. [Google Scholar]
- Pertile, E.; Matias, M.I.; Ribeiro, Z.D.S.; Poeta, J.; Roncada, C. Evidências experimentais e epidemiológicas entre exposição aos agrotóxicos e o desenvolvimento de câncer de mama. Rev. Bras. Pesqui Saúde/Brazilian J. Health Res. 2018, 20, 137–146. [Google Scholar] [CrossRef]
- Cepeda, S.; Forero-Castro, M.; Cárdenas-Nieto, D.; Martínez-Agüero, M.; Rondón-Lagos, M. Chromosomal instability in farmers exposed to pesticides: High prevalence of clonal and non-clonal chromosomal alterations. Risk Manag. Healthc. Policy 2020, 13, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Li, X. Alteration in the cytokine levels and histopathological damage in common carp induced by glyphosate. Chemosphere 2015, 128, 293–298. [Google Scholar] [CrossRef]
- Koureas, M.; Tsezou, A.; Tsakalof, A.; Orfanidou, T.; Hadjichristodoulou, C. Increased levels of oxidxative DNA damage in pesticide sprayers in Thessaly Region (Greece). Implications of pesticide exposure. Sci. Total Environ. 2014, 496, 358–364. [Google Scholar] [CrossRef]
- Weinberg, R.A. The Biology of Cancer; Artmed: Porto Alegre, Brasil, 2008. [Google Scholar]
- He, T.T.; Zuo, A.J.; Wang, J.G.; Zhao, P. Organochlorine pesticides accumulation and breast cancer: A hospital-based case-control study. Tumor. Biol. 2017, 39, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pizzatti, L.; Kawassaki, A.C.B.; Fadel, B.; Nogueira, F.C.S.; Evaristo, J.A.M.; Woldmar, N.; Teixeira, G.T.; Da Silva, J.C.; Scandolara, T.B.; Rech, D.; et al. Toxicoproteomics Disclose Pesticides as Downregulators of TNF-α, IL-1β and Estrogen Receptor Pathways in Breast Cancer Women Chronically Exposed. Front. Oncol. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- El-Zaemey, S.; Heyworth, J.; Fritschi, L. Noticing pesticide spray drift from agricultural pesticide application areas and breast cancer: A case-control study. Aust. N. Z. J. Public Health 2013, 37, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Mekonen, S.; Ibrahim, M.; Astatkie, H.; Abreha, A. Exposure to organochlorine pesticides as a predictor to breast cancer: A case-control study among Ethiopian women. PLoS ONE 2021, 16, e0257704. [Google Scholar] [CrossRef]
- Ferro, R.; Parvathaneni, A.; Patel, S.; Cheriyath, P. Pesticides and Breast Cancer. Adv. Breast Cancer Res. 2012, 1, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Engel, L.S.; Werder, E.; Satagopan, J.; Blair, A.; Hoppin, J.A.; Koutros, S.; Lerro, C.C.; Sandler, D.P.; Alavanja, M.C.; Freeman, L.E.B. Insecticide Use and Breast Cancer Risk among Farmers’ Wives in the Agricultural Health Study. Environ. Health Perspect. 2017, 125, 097002. [Google Scholar] [CrossRef] [PubMed]
- Panis, C.; Gaboardi, S.C.; Kawassaki, A.C.B.; Dias, E.C.M.; Teixeira, G.T.; da Silva, D.R.P.; Rech, D.; Candiotto, L.Z.P. Characterization of occupational exposure to pesticides and its impact on the health of rural women. Rev. Ciências Farm Básica E Apl-RCFBA 2022, 43, e748. [Google Scholar] [CrossRef]
- Panis, C.; Herrera, A.C.S.A.; Victorino, V.J.; Campos, F.C.; Freitas, L.F.; De Rossi, T.; Simão, A.N.C.; Cecchini, A.L.; Cecchini, R. Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res. Treat. 2012, 133, 89–97. [Google Scholar] [CrossRef]
- Victorino, V.J.; Campos, F.C.; Herrera, A.C.S.A.; Simão, A.N.C.; Cecchini, A.L.; Panis, C.; Cecchini, R. Overexpression of HER-2/neu protein attenuates the oxidative systemic profile in women diagnosed with breast cancer. Tumor Biol. 2014, 35, 3025–3034. [Google Scholar] [CrossRef]
- Panis, C.; Mazzuco, T.L.; Costa, C.Z.F.; Victorino, V.J.; Tatakihara, V.L.H.; Yamauchi, L.M.; Yamada-Ogatta, S.F.; Cecchini, R.; Rizzo, L.V.; Pinge-Filho, P. Trypanosoma cruzi: Effect of the absence of 5-lipoxygenase (5-LO)-derived leukotrienes on levels of cytokines, nitric oxide and iNOS expression in cardiac tissue in the acute phase of infection in mice. Exp. Parasitol. 2011, 127, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pacholak, L.M.; Kern, R.; de Oliveira, S.T.; Lúcio, L.C.; Amarante, M.K.; Guembarovski, R.L.; Watanabe, M.A.E.; Panis, C. Effects of GSTT1 and GSTM1 polymorphisms in glutathione levels and breast cancer development in Brazilian patients. Mol. Biol. Rep. 2021, 48, 33–40. [Google Scholar] [CrossRef]
- Scandolara, T.B.; da Silva, J.C.; Malanowski, J.; de Oliveira, J.A.; Rech, D.; Panis, C. Anti-neutrophil antibodies (anti-MPO-ANCAs) are associated with poor prognosis in breast cancer patients. Immunobiology 2020, 225, 152011. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, P.L.; Silva, A.P.D.B.C.D.; Rech, D.; Victorino, V.J.; Herrera, A.C.D.S.D.A.; Kern, R.; Pires, B.R.; Simão, A.N.C.; Bortoloti, D.S.; Panis, C.; et al. Low Plasmatic 25-hydroxyvitamin D at Diagnosis is Associated with Axillary Invasion, Chemoresistance and Metastasis in Women with Breast Cancer. Arch. Med. Res. 2020, 51, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.C.S.; Victorino, V.J.; Campos, F.C.; Verenitach, B.D.; Lemos, L.T.; Aranome, A.M.; Oliveira, S.R.; Cecchini, A.L.; Simão, A.N.C.; Abdelhay, E.; et al. Impact of tumor removal on the systemic oxidative profile of patients with breast cancer discloses lipid peroxidation at diagnosis as a putative marker of disease recurrence. Clin. Breast Cancer 2014, 14, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Sakurai, M.; Yamamoto, Y.; Suzuki, E.; Tsuda, M.; Kataoka, T.R.; Hirata, M.; Nishie, M.; Nojiri, T.; Kumazoe, M.; et al. Alteration of specific cytokine expression patterns in patients with breast cancer. Sci. Rep. 2019, 9, 2924. [Google Scholar] [CrossRef] [Green Version]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Zarzynska, J.M. Two Faces of TGF-Beta1 in Breast Cancer. Mediators Inflamm. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Panis, C.; Herrera, A.C.; Victorino, V.J.; Aranome, A.M.F.; Cecchini, R. Screening of circulating TGF-β levels and its clinicopathological significance in human breast cancer. Anticancer Res. 2013, 33, 737–742. [Google Scholar] [PubMed]
- Miret, N.; Pontillo, C.; Ventura, C.; Carozzo, A.; Chiappini, F.; de Pisarev, D.K.; Fernández, N.; Cocca, C.; Randi, A. Hexachlorobenzene modulates the crosstalk between the aryl hydrocarbon receptor and transforming growth factor-β1 signaling, enhancing human breast cancer cell migration and invasion. Toxicology 2016, 366, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Kern, R.; Panis, C. CTLA-4 Expression and Its Clinical Significance in Breast Cancer. Arch. Immunol. Ther. Exp. 2021, 69, 16. [Google Scholar] [CrossRef] [PubMed]
- Kassardjian, A.; Shintaku, P.I.; Moatamed, N.A. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), in female breast carcinomas. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Hui, J.F.; Sun, M.Y.; Liu, X.S.; Lu, W.H.; Ma, C.H.; Zhang, Q.B. Hematological effects of glyphosate in mice revealed by traditional toxicology and transcriptome sequencing. Environ. Toxicol. Pharmacol. 2022, 92, 103866. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hui, J.F.; Sun, M.Y.; Liu, X.S.; Lu, W.H.; Ma, C.H.; Zhang, Q.B. Direct Effects of Glyphosate on In Vitro T Helper Cell Differentiation and Cytokine Production. Front. Immunol. 2022, 13, 854837. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hui, J.F.; Sun, M.Y.; Liu, X.S.; Lu, W.H.; Ma, C.H.; Zhang, Q.B. Effect of organophosphorus pesticide exposure on the immune cell phenotypes among farm women and their children. Arch. Environ. Occup. Health 2021, 24, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An Epigenetic Role of Mitochondria in Cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Goodwin, P.J. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J. Clin. Oncol. 2016, 34, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Malone, K.E.; Tang, M.T.C.; Li, C.I. Height, body mass index (BMI), BMI change, and the risk of estrogen receptor-positive, HER2-positive, and triple-negative breast cancer among women ages 20 to 44 years. Cancer 2014, 120, 1548–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Wang, P.; Li, X.; Zhao, B.; Yang, H.; Yu, H.; Li, C. Lymph node status have a prognostic impact in breast cancer patients with distant metastasis. PLoS ONE 2017, 12, e0182953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonellotto, F.; Bergmann, A.; Abrahao, K.D.S.; De Aguiar, S.S.; Bello, M.A.; Thuler, L.C.S. Impact of Number of Positive Lymph Nodes and Lymph Node Ratio on Survival of Women with Node-Positive Breast Cancer. Eur. J. Breast. Health 2019, 15, 76–84. [Google Scholar] [CrossRef] [PubMed]





| Low risk | Negative lymph nodes and all the following criteria: |
| pT under 2 cm; | |
| Histological grade 1; | |
| ER or PR positive; | |
| HER-2 negative; | |
| Molecular subtype luminal A; and | |
| Age equal to or above 35 years old. | |
| Intermediate risk | Negative lymph nodes and at least one of the following criteria: |
| pT higher than 2 cm; or | |
| Histological grade 2–3; or | |
| ER or PR negative; or | |
| Molecular subtype luminal B (HER-2 negative); or | |
| Age under 35 years old; or yet | |
| 1 to 3 affected lymph nodes if ER and PR positive. | |
| High risk | 4 or more positive lymph nodes; or |
| Lymph nodes negative with ER. PR and HER-2 negative. pT higher than 2 cm; or | |
| Lymph node negative. pT higher than 1 cm and HER-2 positive. |
| Antibody | Clone | Reactivity | Titration | Incubation |
|---|---|---|---|---|
| Anti-TGF-β | Monoclonal antibody | Mouse anti-human | 1:300 | 2 h in dark and humid chamber at room temperature |
| Alexa Fluor 488 a | Superclonal recombinant secondary antibody | Goat anti-mouse IgG | 1:500 | 1 h in dark and humid chamber at room temperature |
| Anti-CTLA-4 | BNI3 monoclonal antibody | Mouse anti-human CD152 | 1:1000 | overnight in dark and humid chamber at 4 °C |
| Alexa Fluor 488 b | Superclonal recombinant secondary antibody | Goat anti-mouse IgG | 1:1000 | 1 h in dark and humid chamber at room temperature |
| Anti-CD4 | Monoclonal antibody | Mouse anti-human | 1:1000 | overnight in dark and humid chamber at 4 °C |
| Anti-CD8 | Monoclonal antibody | Mouse anti-human | 1:1000 | overnight. in dark and humid chamber at 4 °C |
| Texas Red c | Superclonal recombinant secondary antibody | Goat anti-mouse IgG | 1:1000 | 2 h in dark and humid chamber at room temperature |
| Variable | Group | Category | % | p Value |
|---|---|---|---|---|
| Estrogen receptor | Exposed | Negative | 7.79 | >0.05 |
| Positive | 92.21 | |||
| Unexposed | Negative | 5.88 | ||
| Positive | 94.11 | |||
| Progesterone receptor | Exposed | Negative | 44.16 | >0.05 |
| Positive | 55.84 | |||
| Unexposed | Negative | 45.09 | ||
| Positive | 54.9 | |||
| KI67 (%) | Exposed | <14 | 41.09 | >0.05 |
| >14 | 58.9 | |||
| Unexposed | <14 | 45.83 | ||
| >14 | 54.16 | |||
| Molecular subtype | Exposed | Luminal A | 38.96 | >0.05 |
| Luminal B | 57.14 | |||
| Triple negative | 3.9 | |||
| Unexposed | Luminal A | 43.13 | ||
| Luminal B | 47.05 | |||
| Triple negative | 9.8 | |||
| Tumor size (cm) | Exposed | <2 | 35.61 | >0.05 |
| >2 | 64.38 | |||
| Unexposed | <2 | 40 | ||
| >2 | 60 | |||
| Histological grade | Exposed | I and II | 81.33 | >0.05 |
| III | 18.66 | |||
| Unexposed | I and II | 80.39 | ||
| III | 19.6 | |||
| Lymphnodal metastases | Exposed | None affected | 60 | 0.0353 * |
| At least one affected | 40 | |||
| Unexposed | None affected | 74 | ||
| At least one affected | 26 | |||
| Age at diagnosis | Exposed | <50 | 32.47 | >0.05 |
| >50 | 67.53 | |||
| Unexposed | <50 | 39.21 | ||
| >50 | 60.78 | |||
| Menopause status at diagnosis | Exposed | No | 27.27 | >0.05 |
| Yes | 72.73 | |||
| Unexposed | No | 35.29 | ||
| Yes | 64.7 | |||
| BMI | Exposed | Eutrophic | 25 | 0.0478 * |
| Overweight/obese | 75 | |||
| Unexposed | Eutrophic | 38 | ||
| Overweight/obese | 62 |
| Marker | INE | IE | p Value Δ |
|---|---|---|---|
| TGF-β1 tumor a | 70% | 80% | NS |
| TGF-β1 infiltrate a | 40% | 90% | <0.001 * |
| CTLA-4 tumor a | 40% | 10% | <0.001 * |
| CTLA-4 infiltrate a | 70% | 100% | <0.002 * |
| CD4 infiltrate b | 50% | 60% | NS |
| CD8 infiltrate b | 50% | 30% | 0.0059 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, J.C.; Scandolara, T.B.; Kern, R.; Jaques, H.d.S.; Malanowski, J.; Alves, F.M.; Rech, D.; Silveira, G.F.; Panis, C. Occupational Exposure to Pesticides Affects Pivotal Immunologic Anti-Tumor Responses in Breast Cancer Women from the Intermediate Risk of Recurrence and Death. Cancers 2022, 14, 5199. https://doi.org/10.3390/cancers14215199
da Silva JC, Scandolara TB, Kern R, Jaques HdS, Malanowski J, Alves FM, Rech D, Silveira GF, Panis C. Occupational Exposure to Pesticides Affects Pivotal Immunologic Anti-Tumor Responses in Breast Cancer Women from the Intermediate Risk of Recurrence and Death. Cancers. 2022; 14(21):5199. https://doi.org/10.3390/cancers14215199
Chicago/Turabian Styleda Silva, Janaína Carla, Thalita Basso Scandolara, Rodrigo Kern, Hellen dos Santos Jaques, Jessica Malanowski, Fernanda Mara Alves, Daniel Rech, Guilherme Ferreira Silveira, and Carolina Panis. 2022. "Occupational Exposure to Pesticides Affects Pivotal Immunologic Anti-Tumor Responses in Breast Cancer Women from the Intermediate Risk of Recurrence and Death" Cancers 14, no. 21: 5199. https://doi.org/10.3390/cancers14215199
APA Styleda Silva, J. C., Scandolara, T. B., Kern, R., Jaques, H. d. S., Malanowski, J., Alves, F. M., Rech, D., Silveira, G. F., & Panis, C. (2022). Occupational Exposure to Pesticides Affects Pivotal Immunologic Anti-Tumor Responses in Breast Cancer Women from the Intermediate Risk of Recurrence and Death. Cancers, 14(21), 5199. https://doi.org/10.3390/cancers14215199






