The Prognostic and Therapeutic Potential of DNA Damage Repair Pathway Alterations and Homologous Recombination Deficiency in Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
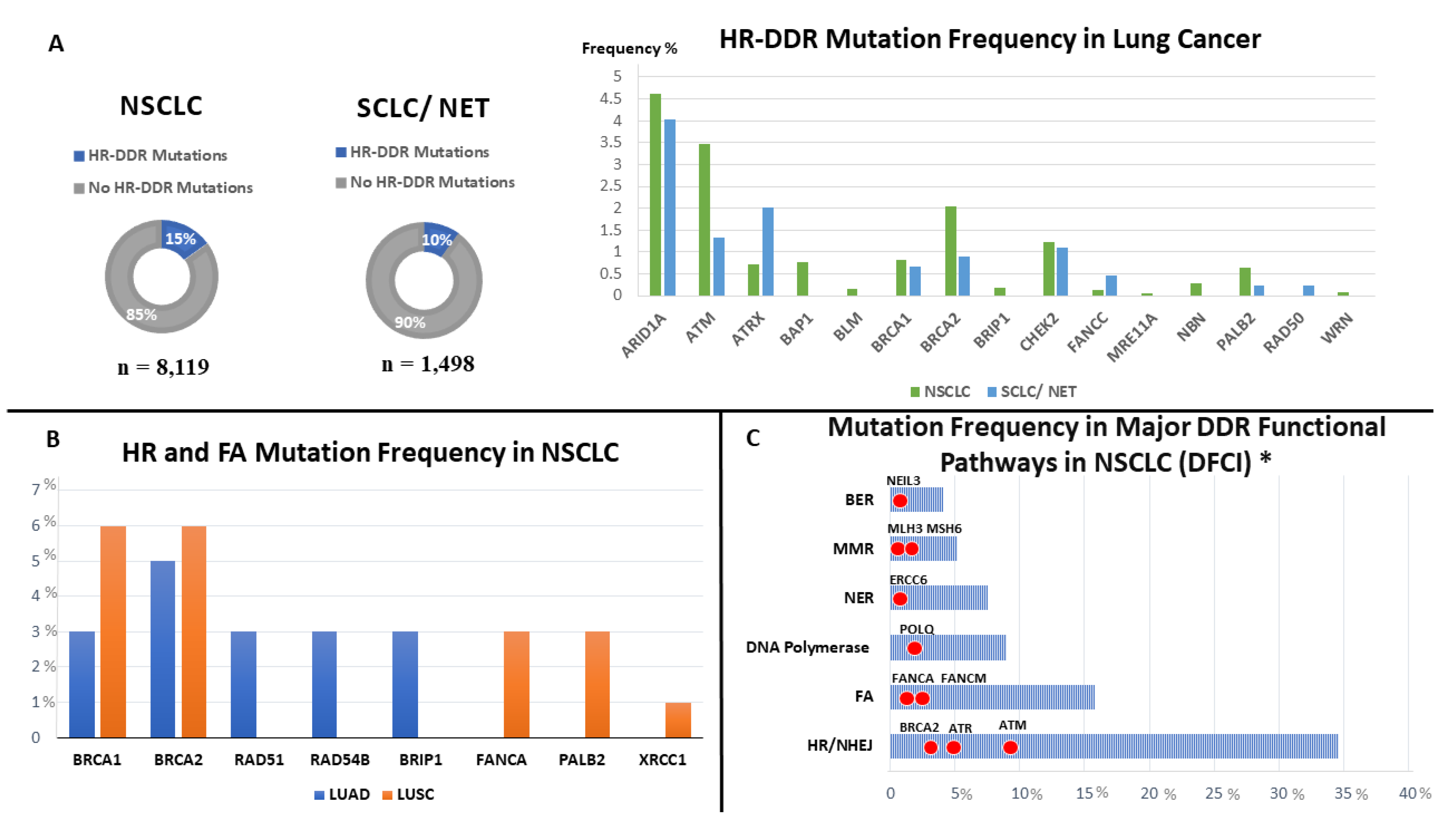
2. Frequency of DDR Alterations in Lung Cancer
3. Prognostic and Predictive Impact of Alterations in DNA Damage Repair Genes in NSCLC
4. The Clinical Relevance of Targeting HR Pathway in Patients with HRD in NSCLC
5. Prognostic and Predictive Biomarkers in DNA Damage Pathway Alterations in SCLC
6. Clinical Relevance of Targeting HR Pathway in Patients with HRD in SCLC
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef]
- Imielinski, M.; Berger, A.H.; Hammerman, P.S.; Hernandez, B.; Pugh, T.J.; Hodis, E.; Cho, J.; Suh, J.; Capelletti, M.; Sivachenko, A.; et al. Mapping the Hallmarks of Lung Adenocarcinoma with Massively Parallel Sequencing. Cell 2012, 150, 1107–1120. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. IMpower010 Investigators. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Akinboro, O.; Vallejo, J.J.; Mishra-Kalyani, P.S.; Larkins, E.A.; Drezner, N.L.; Tang, S.; Pazdur, R.; Beaver, J.A.; Harpreet, S. Outcomes of anti-PD-(L1) therapy in combination with chemotherapy versus immunotherapy (IO) alone for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC) with PD-L1 score 1–49%: FDA pooled analysis. J. Clin. Oncol. 2021, 39, 9001. [Google Scholar] [CrossRef]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S.; et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018, 23, 239–254.e6. [Google Scholar] [CrossRef]
- Burgess, J.T.; Rose, M.; Boucher, D.; Plowman, J.; Molloy, C.; Fisher, M.; O’Leary, C.; Richard, D.J.; O’Byrne, K.J.; Bolderson, E. The Therapeutic Potential of DNA Damage Repair Pathways and Genomic Stability in Lung Cancer. Front. Oncol. 2020, 10, 1256. [Google Scholar] [CrossRef]
- Melis, J.P.; van Steeg, H.; Luijten, M. Oxidative DNA Damage and Nucleotide Excision Repair. Antioxid. Redox Signal. 2013, 18, 2409–2419. [Google Scholar] [CrossRef]
- Beard, W.A.; Horton, J.K.; Prasad, R.; Wilson, S.H. Eukaryotic Base Excision Repair: New Approaches Shine Light on Mechanism. Annu. Rev. Biochem. 2019, 88, 137–162. [Google Scholar] [CrossRef]
- Takaoka, M.; Miki, Y. BRCA1 gene: Function and deficiency. Int. J. Clin. Oncol. 2017, 23, 36–44. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 81–104. [Google Scholar] [CrossRef]
- Nguyen, L.W.M.; Martens, J.; Van Hoeck, A.; Cuppen, E. Pan-cancer landscape of homologous recombination deficiency. Nat. Commun. 2020, 11, 5584. [Google Scholar] [CrossRef]
- Abkevich, V.; Timms, K.M.; Hennessy, B.T.; Potter, J.; Carey, M.S.; Meyer, L.A.; Smith-McCune, K.; Broaddus, R.; Lu, K.H.; Chen, J.; et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer 2012, 107, 1776–1782. [Google Scholar] [CrossRef]
- Popova, T.; Manié, E.; Rieunier, G.; Caux-Moncoutier, V.; Tirapo, C.; Dubois, T.; Delattre, O.; Sigal-Zafrani, B.; Bollet, M.; Longy, M.; et al. Ploidy and Large-Scale Genomic Instability Consistently Identify Basal-like Breast Carcinomas with BRCA1/2 Inactivation. Cancer Res. 2012, 72, 5454–5462. [Google Scholar] [CrossRef]
- Birkbak, N.J.; Wang, Z.C.; Kim, J.-Y.; Eklund, A.C.; Li, Q.; Tian, R.; Bowman-Colin, C.; Li, Y.; Greene-Colozzi, A.; Iglehart, J.D.; et al. Telomeric Allelic Imbalance Indicates Defective DNA Repair and Sensitivity to DNA-Damaging Agents. Cancer Discov. 2012, 2, 366–375. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.; Hurvitz, S.; Mina, L.; Rugo, H.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Gowers, K.H.C.; Lee-Six, H.; Chandrasekharan, D.P.; Coorens, T.; Maughan, E.F.; Beal, K.; Menzies, A.; Millar, F.R.; Anderson, E.; et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 2020, 578, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Hopkin, J.M.; Evans, H.J. Cigarette smoke-induced DNA damage and lung cancer risks. Nature 1980, 283, 388–390. [Google Scholar] [CrossRef]
- Zhao, H.; Albino, A.P.; Jorgensen, E.; Traganos, F.; Darzynkiewicz, Z. DNA damage response induced by tobacco smoke in normal human bronchial epithelial and A549 pulmonary adenocarcinoma cells assessed by laser scanning cytometry. Cytom. Part A 2009, 75, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.-J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of Homologous Recombination–Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Ding, L.; Getz, G.; Wheeler, D.A.; Mardis, E.R.; McLellan, M.D.; Cibulskis, K.; Sougnez, C.; Greulich, H.; Muzny, D.M.; Morgan, M.B.; et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008, 455, 1069–1075. [Google Scholar] [CrossRef]
- Parry, E.M.; Gable, D.L.; Stanley, S.E.; Khalil, S.E.; Antonescu, V.; Florea, L.; Armanios, M. Germline Mutations in DNA Repair Genes in Lung Adenocarcinoma. J. Thorac. Oncol. 2017, 12, 1673–1678. [Google Scholar] [CrossRef]
- Campbell, J.D.; Alexandrov, A.; Kim, J.; Wala, J.; Berger, A.H.; Pedamallu, C.S.; Shukla, S.A.; Guo, G.; Brooks, A.N.; Murray, B.A.; et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 2016, 48, 607–616. [Google Scholar] [CrossRef]
- Yoshino, T.; Pentheroudakis, G.; Mishima, S.; Overman, M.; Yeh, K.-H.; Baba, E.; Naito, Y.; Calvo, F.; Saxena, A.; Chen, L.-T.; et al. JSCO—ESMO—ASCO—JSMO—TOS: International expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann. Oncol. 2020, 31, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.N.; Devarakonda, S.H.K.; Michel, L.S.; Maggi, L.B.; Watson, M.; Guebert, K.; Carpenter, D.; Sleckman, B.P.; Govindan, R.; Morgensztern, D. BRCAness in non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2014, 32, 11033. [Google Scholar] [CrossRef]
- Ricciuti, B.; Recondo, G.; Spurr, L.F.; Li, Y.Y.; Lamberti, G.; Venkatraman, D.; Umeton, R.; Cherniack, A.D.; Nishino, M.; Sholl, L.M.; et al. Impact of DNA Damage Response and Repair (DDR) Gene Mutations on Efficacy of PD-(L)1 Immune Checkpoint Inhibition in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 4135–4142. [Google Scholar] [CrossRef]
- Helin, K.; Holm, K.; Niebuhr, A.; Eiberg, H.; Tommerup, N.; Hougaard, S.; Poulsen, H.S.; Spang-Thomsen, M.; Nørgaard, P. Loss of the retinoblastoma protein-related p130 protein in small cell lung carcinoma. Proc. Natl. Acad. Sci. USA 1997, 94, 6933–6938. [Google Scholar] [CrossRef]
- Miller, C.W.; Simon, K.; Aslo, A.; Kok, K.; Yokota, J.; Buys, C.H.; Terada, M.; Koeffler, H.P. p53 mutations in human lung tumors. Cancer Res. 1992, 52, 1695–1698. [Google Scholar] [PubMed]
- Park, S.; Lee, H.; Lee, B.; Lee, S.-H.; Sun, J.-M.; Park, W.-Y.; Ahn, J.S.; Ahn, M.-J.; Park, K. DNA Damage Response and Repair Pathway Alteration and Its Association With Tumor Mutation Burden and Platinum-Based Chemotherapy in SCLC. J. Thorac. Oncol. 2019, 14, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Byers, L.A.; Wang, J.; Nilsson, M.B.; Fujimoto, J.; Saintigny, P.; Yordy, J.; Giri, U.; Peyton, M.; Fan, Y.H.; Diao, L.; et al. Proteomic Profiling Identifies Dysregulated Pathways in Small Cell Lung Cancer and Novel Therapeutic Targets Including PARP1. Cancer Discov. 2012, 2, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Cosaert, J.; Quoix, E. Platinum drugs in the treatment of non-small-cell lung cancer. Br. J. Cancer 2002, 87, 825–833. [Google Scholar] [CrossRef]
- Olaussen, K.A.; Dunant, A.; Fouret, P.; Brambilla, E.; André, F.; Haddad, V.; Taranchon, E.; Filipits, M.; Pirker, R.; Popper, H.H.; et al. DNA Repair by ERCC1 in Non–Small-Cell Lung Cancer and Cisplatin-Based Adjuvant Chemotherapy. N. Engl. J. Med. 2006, 355, 983–991. [Google Scholar] [CrossRef]
- Vilmar, A.; Santoni-Rugiu, E.; Sørensen, J. ERCC1 and histopathology in advanced NSCLC patients randomized in a large multicenter phase III trial. Ann. Oncol. 2010, 21, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.R.; Schell, M.J.; Begum, M.; Kim, J.; Chiappori, A.; Haura, E.; Antonia, S.; Bepler, G. Preliminary indication of survival benefit fromERCC1andRRM1-tailored chemotherapy in patients with advanced nonsmall cell lung cancer: Evidence from an individual patient analysis. Cancer 2012, 118, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, T.; Tsubata, Y.; Tanino, R.; Nakao, M.; Hotta, T.; Hamaguchi, M.; Hamaguchi, S.; Araki, A.; Isobe, T. ERCC1 Is a Predictive Biomarker for Non-small Cell Lung Cancer But Is Antibody-dependent. Anticancer Res. 2021, 41, 2653–2660. [Google Scholar] [CrossRef]
- Malottki, K.; Popat, S.; Deeks, J.J.; Riley, R.D.; Nicholson, A.G.; Billingham, L. Problems of variable biomarker evaluation in stratified medicine research—A case study of ERCC1 in non-small-cell lung cancer. Lung Cancer 2016, 92, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Bandlamudi, C.; Cheng, M.L.; Srinivasan, P.; Chavan, S.S.; Friedman, N.D.; Rosen, E.Y.; Richards, A.L.; Bouvier, N.; Selcuklu, S.D.; et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature 2019, 571, 576–579. [Google Scholar] [CrossRef]
- Diossy, M.; Sztupinszki, Z.; Borcsok, J.; Krzystanek, M.; Tisza, V.; Spisak, S.; Rusz, O.; Timar, J.; Csabai, I.; Fillinger, J.; et al. A subset of lung cancer cases shows robust signs of homologous recombination deficiency associated genomic mutational signatures. NPJ Precis. Oncol. 2021, 5, 55. [Google Scholar] [CrossRef]
- Huang, Z.; Xiong, G. BRCA1 expression associated with the prognostic value of platinum-based chemotherapy for stage II–IV non-small cell lung cancer: A meta-analysis. Int. J. Biol. Markers 2022, 37, 170–177. [Google Scholar] [CrossRef]
- Papadaki, C.; Sfakianaki, M.; Ioannidis, G.; Lagoudaki, E.; Trypaki, M.; Tryfonidis, K.; Mavroudis, D.; Stathopoulos, E.; Georgoulias, V.; Souglakos, J. ERCC1 and BRAC1 mRNA Expression Levels in the Primary Tumor Could Predict the Effectiveness of the Second-Line Cisplatin-Based Chemotherapy in Pretreated Patients with Metastatic Non-small Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 663–671. [Google Scholar] [CrossRef]
- Wang, L.; Meng, L.; Wang, X.-W.; Ma, G.-Y.; Chen, J.-H. Expression of RRM1 and RRM2 as a novel prognostic marker in advanced non-small cell lung cancer receiving chemotherapy. Tumor Biol. 2014, 35, 1899–1906. [Google Scholar] [CrossRef]
- Caiola, E.; Salles, D.; Frapolli, R.; Lupi, M.; Rotella, G.; Ronchi, A.; Garassino, M.C.; Mattschas, N.; Colavecchio, S.; Broggini, M.; et al. Base excision repair-mediated resistance to cisplatin in KRAS(G12C) mutant NSCLC cells. Oncotarget 2015, 6, 30072–30087. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, L.; Xu, J.; Shen, H.; Hu, Z.; Ma, H.; Shu, Y.; Shao, Y.; Yin, Y. Polymorphisms in the base excision repair pathway modulate prognosis of platinum-based chemotherapy in advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 2013, 71, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Jiang, M.; He, K.; Wang, H.; Chen, P.; Guo, H.; Zhao, W.; Lu, H.; He, Y.; Zhou, C. DNA Damage Response and Repair Gene Alterations Increase Tumor Mutational Burden and Promote Poor Prognosis of Advanced Lung Cancer. Front. Oncol. 2021, 11, 708294. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Williams, T.M.; Bertino, E.M.; Mo, X.; Webb, A.; Schweitzer, C.; Liu, T.; Roychowdhury, S.; Timmers, C.D.; Otterson, G.A. Homologous recombination and DNA repair mutations in patients treated with carboplatin and nab-paclitaxel for metastatic non-small cell lung cancer. Lung Cancer 2019, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gudikote, J.; Giri, U.; Yan, J.; Deng, W.; Ye, R.; Jiang, W.; Li, N.; Hobbs, B.P.; Wang, J.; et al. RAD50 Expression Is Associated with Poor Clinical Outcomes after Radiotherapy for Resected Non–small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jin, G.; Cai, F.; Chen, X.; Cao, N.; Zhang, X.; Liu, J.; Chen, F.; Wang, F.; Dong, W.; et al. Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response. Exp. Mol. Med. 2019, 51, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zou, N.; Xie, G.; Cui, T.; Srivastava, A.K.; Qu, M.; Yang, L.; Wei, S.; Zheng, Y.; Wang, Q.-E. DDB2 increases radioresistance of NSCLC cells by enhancing DNA damage responses. Tumor Biol. 2016, 37, 14183–14191. [Google Scholar] [CrossRef]
- Postel-Vinay, S.; Vanhecke, E.; Olaussen, K.A.; Lord, C.; Ashworth, A.; Soria, J.-C. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat. Rev. Clin. Oncol. 2012, 9, 144–155. [Google Scholar] [CrossRef]
- Chae, Y.K.; Davis, A.A.; Raparia, K.; Agte, S.; Pan, A.; Mohindra, N.; Villaflor, V.; Giles, F. Association of Tumor Mutational Burden With DNA Repair Mutations and Response to Anti–PD-1/PD-L1 Therapy in Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, 88–96.e6. [Google Scholar] [CrossRef]
- Chae, Y.K.; Anker, J.F.; Oh, M.S.; Bais, P.; Namburi, S.; Agte, S.; Giles, F.J.; Chuang, J.H. Mutations in DNA repair genes are associated with increased neoantigen burden and a distinct immunophenotype in lung squamous cell carcinoma. Sci. Rep. 2019, 9, 3235. [Google Scholar] [CrossRef]
- Xiong, A.; Nie, W.; Zhou, Y.; Li, C.; Gu, K.; Zhang, D.; Chen, S.; Wen, F.; Zhong, H.; Han, B.; et al. Comutations in DDR Pathways Predict Atezolizumab Response in Non-Small Cell Lung Cancer Patients. Front. Immunol. 2021, 12, 708558. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; Van Der Burg, E.; Nygren, A.O.H.; Zander, S.A.L.; Derksen, P.W.B.; De Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Feng, Y.; Yu, G.K.; Ru, Y.; Tang, S.-W.; Shen, Y.; Pommier, Y. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget 2016, 7, 76534–76550. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Swisher, E.M.; Kwan, T.T.; Oza, A.M.; Tinker, A.V.; Ray-Coquard, I.; Oaknin, A.; Coleman, R.L.; Aghajanian, C.; Konecny, G.E.; O’Malley, D.M.; et al. Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2). Nat. Commun. 2021, 12, 2487. [Google Scholar] [CrossRef]
- Morgan, R.D.; Clamp, A.R.; Evans, D.G.R.; Edmondson, R.J.; Jayson, G.C. PARP inhibitors in platinum-sensitive high-grade serous ovarian cancer. Cancer Chemother. Pharmacol. 2018, 81, 647–658. [Google Scholar] [CrossRef]
- Fennell, D.A.; Lester, J.F.; Danson, S.; Blackhall, F.H.; Nicolson, M.; Nixon, L.S.; Porter, C.; Gardner, G.M.; White, A.; Griffiths, G.O.; et al. A randomized phase II trial of olaparib maintenance versus placebo monotherapy in patients with chemosensitive advanced non-small cell lung cancer. J. Clin. Oncol. 2020, 38, e21649. [Google Scholar] [CrossRef]
- Riess, J.W.; Redman, M.W.; Wheatley-Price, P.; Faller, B.A.; Villaruz, L.C.; Corum, L.R.; Gowda, A.C.; Srkalovic, G.; Osarogiagbon, R.U.; Baumgart, M.A.; et al. A phase II study of rucaparib in patients with high genomic LOH and/or BRCA 1/2 mutated stage IV non-small cell lung cancer (Lung-MAP Sub-Study, S1900A). J. Clin. Oncol. 2021, 39, 9024. [Google Scholar] [CrossRef]
- Waddington, T.; Mambetsariev, I.; Pharaon, R.; Fricke, J.; Baroz, A.R.; Romo, H.; Ghanem, B.; Gray, S.; Salgia, R. Therapeutic Potential of Olaparib in Combination with Pembrolizumab in a Young Patient with a Maternally Inherited BRCA2 Germline Variant: A Research Report. Clin. Lung Cancer 2021, 22, e703–e707. [Google Scholar] [CrossRef]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Banna, G.; Malapelle, U.; Pisapia, P.; Addeo, A. Next Generation Sequencing and Genetic Alterations in Squamous Cell Lung Carcinoma: Where Are We Today? Front. Oncol. 2019, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Redman, M.W.; Byers, L.A.; Hirsch, F.R.; Mack, P.C.; Schwartz, L.H.; Bradley, J.D.; Stinchcombe, T.E.; Leighl, N.B.; Al Baghdadi, T.; et al. Phase 2 Study of Talazoparib in Patients with Homologous Recombination Repair–Deficient Squamous Cell Lung Cancer: Lung-MAP Substudy S1400G. Clin. Lung Cancer 2021, 22, 187–194.e1. [Google Scholar] [CrossRef] [PubMed]
- Paz-Elizur, T.; Krupsky, M.; Blumenstein, S.; Elinger, D.; Schechtman, E.; Livneh, Z. DNA Repair Activity for Oxidative Damage and Risk of Lung Cancer. JNCI J. Natl. Cancer Inst. 2003, 95, 1312–1319. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, H.; Li, Y.; Yin, J.; Guo, S.; Lin, S.-Y.; McGrail, D.J. PARP inhibitors synergize with gemcitabine by potentiating DNA damage in non-small-cell lung cancer. Int. J. Cancer 2019, 144, 1092–1103. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Blais, N.; Mazieres, J.; Reck, M.; Jones, C.M.; Juhasz, E.; Urban, L.; Orlov, S.; Barlesi, F.; Kio, E.; et al. Randomized, Placebo-Controlled, Phase II Study of Veliparib in Combination with Carboplatin and Paclitaxel for Advanced/Metastatic Non–Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 1937–1944. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Novello, S.; Guclu, S.Z.; Bentsion, D.; Zvirbule, Z.; Szilasi, M.; Bernabe, R.; Syrigos, K.; Byers, L.A.; Clingan, P.; et al. Veliparib in Combination With Platinum-Based Chemotherapy for First-Line Treatment of Advanced Squamous Cell Lung Cancer: A Randomized, Multicenter Phase III Study. J. Clin. Oncol. 2021, 39, 3633–3644. [Google Scholar] [CrossRef]
- Wilkerson, M.D.; Schallheim, J.M.; Hayes, D.N.; Roberts, P.J.; Bastien, R.R.; Mullins, M.; Yin, X.; Miller, C.R.; Thorne, L.B.; Geiersbach, K.B.; et al. Prediction of Lung Cancer Histological Types by RT-qPCR Gene Expression in FFPE Specimens. J. Mol. Diagn. 2013, 15, 485–497. [Google Scholar] [CrossRef]
- Govindan, R.; Lind, M.; Insa, A.; Khan, S.A.; Uskov, D.; Tafreshi, A.; Guclu, S.; Bar, J.; Kato, T.; Lee, K.H.; et al. Veliparib Plus Carboplatin and Paclitaxel Versus Investigator’s Choice of Standard Chemotherapy in Patients With Advanced Non–Squamous Non–Small Cell Lung Cancer. Clin. Lung Cancer 2022, 23, 214–225. [Google Scholar] [CrossRef]
- Li, A.; Yi, M.; Qin, S.; Chu, Q.; Luo, S.; Wu, K. Prospects for combining immune checkpoint blockade with PARP inhibition. J. Hematol. Oncol. 2019, 12, 98. [Google Scholar] [CrossRef]
- Clarke, J.M.; Patel, J.D.; Robert, F.; Kio, E.A.; Thara, E.; Camidge, D.R.; Dunbar, M.; Nuthalapati, S.; Dinh, M.H.; Bach, B.A. Veliparib and nivolumab in combination with platinum doublet chemotherapy in patients with metastatic or advanced non-small cell lung cancer: A phase 1 dose escalation study. Lung Cancer 2021, 161, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fumet, J.-D.; Limagne, E.; Thibaudin, M.; Truntzer, C.; Bertaut, A.; Rederstorff, E.; Ghiringhelli, F. Precision medicine phase II study evaluating the efficacy of a double immunotherapy by durvalumab and tremelimumab combined with olaparib in patients with solid cancers and carriers of homologous recombination repair genes mutation in response or stable after olaparib treatment. BMC Cancer 2020, 20, 748. [Google Scholar] [CrossRef]
- Passiglia, F.; Bironzo, P.; Righi, L.; Listì, A.; Arizio, F.; Novello, S.; Volante, M.; Scagliotti, G.V. A Prospective Phase II Single-arm Study of Niraparib Plus Dostarlimab in Patients With Advanced Non–small-cell Lung Cancer and/or Malignant Pleural Mesothelioma, Positive for PD-L1 Expression and Germline or Somatic Mutations in the DNA Repair Genes: Rationale and Study Design. Clin. Lung Cancer 2021, 22, e63–e66. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Campelo, R.; Arrieta, O.; Massuti, B.; Rodriguez-Abreu, D.; Granados, A.L.O.; Majem, M.; Vicente, D.; Lianes, P.; Bosch-Barrera, J.; Insa, A.; et al. Combination of gefitinib and olaparib versus gefitinib alone in EGFR mutant non-small-cell lung cancer (NSCLC): A multicenter, randomized phase II study (GOAL). Lung Cancer 2020, 150, 62–69. [Google Scholar] [CrossRef]
- de Haan, R.; van der Heuvel, M.M.; van Diessen, J.; Peulen, H.M.; van Werkhoven, E.; de Langen, A.J.; Lalezari, F.; Pluim, D.; Verwijs-Janssen, M.; Vens, C.; et al. Phase I and Pharmacologic Study of Olaparib in Combination with High-dose Radiotherapy with and without Concurrent Cisplatin for Non–Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 1256–1266. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Thara, E.; Awad, M.M.; Dowlati, A.; Haque, B.; Stinchcombe, T.E.; Dy, G.K.; Spigel, D.R.; Lu, S.; Singh, N.I.; et al. JASPER: Phase 2 trial of first-line niraparib plus pembrolizumab in patients with advanced non–small cell lung cancer. Cancer 2022, 128, 65–74. [Google Scholar] [CrossRef]
- Chabot, P.; Hsia, T.-C.; Ryu, J.-S.; Gorbunova, V.; Belda-Iniesta, C.; Ball, D.; Kio, E.; Mehta, M.; Papp, K.; Qin, Q.; et al. Veliparib in combination with whole-brain radiation therapy for patients with brain metastases from non-small cell lung cancer: Results of a randomized, global, placebo-controlled study. J. Neuro-Oncol. 2017, 131, 105–115. [Google Scholar] [CrossRef]
- Marzio, A.; Kurz, E.; Sahni, J.M.; Di Feo, G.; Puccini, J.; Jiang, S.; Hirsch, C.A.; Arbini, A.A.; Wu, W.L.; Pass, H.I.; et al. EMSY inhibits homologous recombination repair and the interferon response, promoting lung cancer immune evasion. Cell 2022, 185, 169–183.e19. [Google Scholar] [CrossRef]
- Vendetti, F.P.; Lau, A.; Schamus, S.; Conrads, T.P.; O’Connor, M.J.; Bakkenist, C.J. The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget 2015, 6, 44289–44305. [Google Scholar] [CrossRef]
- Dunne, V.; Ghita, M.; Small, D.M.; Coffey, C.B.; Weldon, S.; Taggart, C.C.; Osman, S.O.; McGarry, C.K.; Prise, K.M.; Hanna, G.G.; et al. Inhibition of ataxia telangiectasia related-3 (ATR) improves therapeutic index in preclinical models of non-small cell lung cancer (NSCLC) radiotherapy. Radiother. Oncol. 2017, 124, 475–481. [Google Scholar] [CrossRef]
- Yap, T.A.; Krebs, M.G.; Postel-Vinay, S.; Bang, Y.J.; El-Khoueiry, A.; Abida, W.; Harrington, K.; Sundar, R.; Carter, L.; Castanon-Alvarez, E.; et al. Phase I modular study of AZD6738, a novel oral, potent and selective ataxia telangiectasia Rad3-related (ATR) inhibitor in combination (combo) with carboplatin, olaparib or durvalumab in patients (pts) with advanced cancers. Eur. J. Cancer 2016, 69, S2. [Google Scholar] [CrossRef]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef]
- Lok, B.H.; Gardner, E.E.; Schneeberger, V.E.; Ni, A.; Desmeules, P.; Rekhtman, N.; De Stanchina, E.; Teicher, B.A.; Riaz, N.; Powell, S.N.; et al. PARP Inhibitor Activity Correlates with SLFN11 Expression and Demonstrates Synergy with Temozolomide in Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 523–535. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Waqar, S.N.; Krug, L.M.; Dowlati, A.; Hann, C.L.; Chiappori, A.; Owonikoko, T.K.; Woo, K.M.; Cardnell, R.J.; Fujimoto, J.; et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021, 39, 346–360.e7. [Google Scholar] [CrossRef] [PubMed]
- Cardnell, R.J.; Feng, Y.; Diao, L.; Fan, Y.-H.; Masrorpour, F.; Wang, J.; Shen, Y.; Mills, G.B.; Minna, J.D.; Heymach, J.V.; et al. Proteomic Markers of DNA Repair and PI3K Pathway Activation Predict Response to the PARP Inhibitor BMN 673 in Small Cell Lung Cancer. Clin. Cancer Res. 2013, 19, 6322–6328. [Google Scholar] [CrossRef]
- Sen, T.; Tong, P.; Stewart, C.A.; Cristea, S.; Valliani, A.; Shames, D.S.; Redwood, A.B.; Fan, Y.H.; Li, L.; Glisson, B.S.; et al. CHK1 Inhibition in Small-Cell Lung Cancer Produces Single-Agent Activity in Biomarker-Defined Disease Subsets and Combination Activity with Cisplatin or Olaparib. Cancer Res. 2017, 77, 3870–3884. [Google Scholar] [CrossRef]
- de Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017, 7, 620–629. [Google Scholar] [CrossRef]
- Woll, P.; Gaunt, P.; Danson, S.; Steele, N.; Ahmed, S.; Mulatero, C.; Shah, R.; Bhosle, J.; Hodgkinson, E.; Watkins, B.; et al. Olaparib as maintenance treatment in patients with chemosensitive small cell lung cancer (STOMP): A randomised, double-blind, placebo-controlled phase II trial. Lung Cancer 2022, 171, 26–33. [Google Scholar] [CrossRef]
- Atrafi, F.; Groen, H.J.; Byers, L.A.; Garralda, E.; Lolkema, M.P.; Sangha, R.S.; Viteri, S.; Chae, Y.K.; Camidge, D.R.; Gabrail, N.Y.; et al. A Phase I Dose-Escalation Study of Veliparib Combined with Carboplatin and Etoposide in Patients with Extensive-Stage Small Cell Lung Cancer and Other Solid Tumors. Clin. Cancer Res. 2019, 25, 496–505. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Dahlberg, S.E.; Sica, G.L.; Wagner, L.I.; Wade, J.L.; Srkalovic, G.; Lash, B.W.; Leach, J.W.; Leal, T.B.; Aggarwal, C.; et al. Randomized Phase II Trial of Cisplatin and Etoposide in Combination With Veliparib or Placebo for Extensive-Stage Small-Cell Lung Cancer: ECOG-ACRIN 2511 Study. J. Clin. Oncol. 2019, 37, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Farago, A.F.; Yeap, B.Y.; Stanzione, M.; Hung, Y.P.; Heist, R.S.; Marcoux, J.P.; Zhong, J.; Rangachari, D.; Barbie, D.A.; Phat, S.; et al. Combination Olaparib and Temozolomide in Relapsed Small-Cell Lung Cancer. Cancer Discov. 2019, 9, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.H.; Lok, B.H.; Ma, J.; Bell, A.; de Stanchina, E.; Poirier, J.T.; Rudin, C.M. Talazoparib Is a Potent Radiosensitizer in Small Cell Lung Cancer Cell Lines and Xenografts. Clin. Cancer Res. 2018, 24, 5143–5152. [Google Scholar] [CrossRef] [PubMed]
- Foy, V.; Schenk, M.W.; Baker, K.; Gomes, F.; Lallo, A.; Frese, K.K.; Forster, M.; Dive, C.; Blackhall, F. Targeting DNA damage in SCLC. Lung Cancer 2017, 114, 12–22. [Google Scholar] [CrossRef]
- Lallo, A.; Frese, K.K.; Morrow, C.J.; Sloane, R.; Gulati, S.; Schenk, M.W.; Trapani, F.; Simms, N.; Galvin, M.; Brown, S.; et al. The Combination of the PARP Inhibitor Olaparib and the WEE1 Inhibitor AZD1775 as a New Therapeutic Option for Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 5153–5164. [Google Scholar] [CrossRef]
- Gardner, E.E.; Lok, B.H.; Schneeberger, V.E.; Desmeules, P.; Miles, L.A.; Arnold, P.K.; Ni, A.; Khodos, I.; de Stanchina, E.; Nguyen, T.; et al. Chemosensitive Relapse in Small Cell Lung Cancer Proceeds through an EZH2-SLFN11 Axis. Cancer Cell 2017, 31, 286–299. [Google Scholar] [CrossRef]
- Barayan, R.; Ran, X.; Lok, B.H. PARP inhibitors for small cell lung cancer and their potential for integration into current treatment approaches. J. Thorac. Dis. 2020, 12, 6240–6252. [Google Scholar] [CrossRef]
- Liu, J.; Barry, W.; Birrer, M.; Lee, J.-M.; Buckanovich, R.; Fleming, G.; Rimel, B.; Buss, M.; Nattam, S.; Hurteau, J.; et al. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann. Oncol. 2019, 30, 551–557. [Google Scholar] [CrossRef]
- Kaplan, A.R.; Gueble, S.E.; Liu, Y.; Oeck, S.; Kim, H.; Yun, Z.; Glazer, P.M. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD. Sci. Transl. Med. 2019, 11, eaav4508. [Google Scholar] [CrossRef]
- Cardnell, R.J.; Feng, Y.; Mukherjee, S.; Diao, L.; Tong, P.; Stewart, C.A.; Masrorpour, F.; Fan, Y.; Nilsson, M.; Shen, Y.; et al. Activation of the PI3K/mTOR Pathway following PARP Inhibition in Small Cell Lung Cancer. PLoS ONE 2016, 11, e0152584. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, W.; Ju, Z.; Wang, L.; Peng, Y.; Labrie, M.; Yap, T.A.; Mills, G.B.; Peng, G. PARPi Triggers the STING-Dependent Immune Response and Enhances the Therapeutic Efficacy of Immune Checkpoint Blockade Independent of BRCAness. Cancer Res. 2019, 79, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Vilimas, R.; Trindade, C.; Erwin-Cohen, R.; Roper, N.; Xi, L.; Krishnasamy, V.; Levy, E.; Mammen, A.; Nichols, S.; et al. Durvalumab in Combination with Olaparib in Patients with Relapsed SCLC: Results from a Phase II Study. J. Thorac. Oncol. 2019, 14, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Peyraud, F.; Italiano, A. Combined PARP Inhibition and Immune Checkpoint Therapy in Solid Tumors. Cancers 2020, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tao, H.; Zhang, S.; Wang, X.; Ma, J.; Li, R.; Liu, Z.; Wang, J.; Cui, P.; Chen, S.; et al. Efficacy and safety of anti-PD-1-based therapy in combination with PARP inhibitors for patients with advanced solid tumors in a real-world setting. Cancer Immunol. Immunother. 2021, 70, 2971–2980. [Google Scholar] [CrossRef]
| Clinical Trial | Medication | Tumor Type | Inclusion Criteria | Exclusion Criteria | Selective Biomarkers | End Point | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| NCT01513174 | Olaparib + Gefitinb vs. Gefitinib alone Phase 1 and Phase 2 | NSCLC | Stage 4 disease ECOG score ≤ 2. Life expectancy of ≥ 3 months. | Prior treatment with PARPi History of ILD 2nd neoplasm | EGFR+ | Primary: Maximum tolerated dose Secondary: PFS, ORR, OS | mPFS 12.8 vs. 10.9 (p = 0.124) mOS 23.3 vs. 23.1 (p = 0.345) | [84] |
| NCT01562210 | Olaparib dose escalating + RT with or without cisplatin Phase 1 | NSCLC | Stage 2/3 non-operable disease Life expectancy of ≥ 3 months | Prior radiation/chemotherapy Significant pulmonary, cardiovascular, hepatic disease | Targetable mutations | Primary: dose-limiting toxicity Secondary: PFS, ORR | Dose limiting toxicity due to esophageal, hematologic and pulmonary toxicity | [85] |
| NCT03775486 | Durvalumab + Olaparib vs Durvalumab Phase 2 | NSCLC | Stage 4 NSCLC, not amenable to curative surgery or radiation | History of autoimmune disease Prior chemo/immune therapy | EGFR mutation negative ALK fusions negative | Primary: PFS Secondary: OS, ORR | N/A | Ongoing trial |
| NCT03976362 | Pembrolizumab +/- maintenance Olaparib Phase 3 | squamous NSCLC | Stage 4, life expectancy ≥ 3 months | History of autoimmune disease History of ILD | N/A | Primary: PFS, OS Secondary: AE, QLQ-C30 | N/A | Ongoing trial |
| NCT04380636 | Pembrolizumab with chemoradiation, followed by pembrolizumab +/− Olaparib Phase 3 | NSCLC | Stage 3, unresectable, life expectancy ≥ 6 months | Prior treatment History of autoimmune disease | N/A | Primary: PFS, OS Secondary: AE, ORR, DOR, QLQ-C30 | N/A | Ongoing trial |
| NCT03976323 | Pembrolizumab followed by maintenance Olaparib vs. pemetrexed Phase 3 | Non-squamous NSCLC | Stage 4, life expectancy ≥ 3 months | History of autoimmune disease Prior therapy with PARPi CNS disease | EGFR, ROS1 mutations negative, ALK fusions negative | Primary: PFS, OS Secondary: AE, ORR, QLQ-C30 | N/A | Ongoing trial |
| NCT03334617 | Umbrella study of novel agents (Olaparib) +Durvalumab Phase 2 | NSCLC | Stage 4, progressed on anti PD1/PD-L1, life expectancy ≥ 3 months | History of primary immunodeficiency or autoimmune disorders, CNS disease | EGFR mutation negative ALK fusions negative ROS1, BRAF, MET, RET negative | Primary: ORR Secondary: DCR, DOR, PFS, OS | N/A | Ongoing trial |
| NCT04550104 | Platform study of novel agents (olaparib) with radiotherapy Phase 1B | NSCLC | Stage 2B/3A/3B, not eligible for concurrent chemoradiotherapy/surgery | Prior autoimmune or inflammatory disorders, history of interstitial pneumonitis | N/A | Primary: Dose-limiting toxicities Secondary: ORR, OS, PFS, QLQ-C30 | N/A | Ongoing trial |
| NCT03559049 | Rucaparib and Pembrolizumab for maintenance therapy Phase 1/2 | Non-squamous NSCLC | Stage 4, life expectancy ≥ 3 months | Prior systemic therapy, prior treatment with PARPi, history of autoimmune disease | EGFR mutation negative ALK fusions negative ROS1 negative | Primary: PFS Secondary: OS, ORR | N/A | Ongoing trial |
| NCT03845296 | Rucaparib (LUNG-MAP Sub-Study) Phase 2 | NSCLC | Stage 4 | Prior treatment with PARPi, Grade 3/4 CV disease, | Genomic LOH or BRCA 1, 2 mutations ALK fusions negative EGFR, ROS1, BRAF mutations negative | Primary: ORR Secondary: IA-PFS, OS, DOS | N/A | N/A |
| NCT03891615 | Phase 1—Niraparib with combination osimertinib in EGFR mutated lung cancer | NSCLC | Stage 4, EGFR mutant NSCLC, life expectancy > 6 months | No prior PARP inhibitor, prior immunotherapy, ILD, | EGFR | Primary: Max tolerated dose Secondary: Rate of toxicity in combined, ORR niraparib, median PFS niraparid | N/A | Ongoing trial |
| NCT03497429 | Phase 1 niraparib in Japanese pts w/advanced solid tumors | Adv Solid tumor | Met or locally advanced solid tumor malignancy, Japanese, >20 years old | Prior PARPi, CNS mets, | N/A | Primary: Dose-limiting toxicity Secondary: Plasma concentration, AUC | N/A | N/A |
| NCT05169437 | Phase 2 niraparib in locally advanced on met PALB2 mutations | Adv Solid Tumor | Met or locally advanced, Pathogenic PALB2 | Ovarian or prostate ca, prior ParpI therapy, BRCA1 or 2, | Pathogenic PALB2 +, BRCA 1 and BRCA 2 negative | Primary: ORR Secondary: PFS, ORR, AE events, OS | N/A | Ongoing trial |
| NCT04475939 | ZEAL-1L—Phase 3 RCT, Niraparib + pembro vs. Placebo + pembro in NSCLC | NSCLC | Met or locally advanced, life expectancy > 12 wks | Prior ParpI, brain mets, autoimmune disorder, | N/A | Primary: PFS, OS Secondary: TTP, PFS by PDL1 status, IRAEs | Results from phase 2 trial: ORR 56.3% with TPS ≥ 50 (Cohort 1) and 20% in TPS 1–49% (Cohort 2). mPFS: 8.4 in cohort 1 and 4.2 in cohort 2. mOS NR in cohort 1 and 7.7 in cohort 2 | [86] |
| NCT03308942 | Phase 2 niraparib + anti -PD-1 (Pembro vs. Dostarlimab) | NSCLC (cohort 3—sqaumous) | Met or locally advanced, Cohort 1—PDL-1 > 50% cohort 2- PDL-1 1–49%; Cohort 3—SqCC, | Cohort 1/2; Prior Anti-PD1, PDL-1 or PDl-2 therapy All cohorts- hx of immunodeficiency, hx of autoimmune disease, ILD or pneumonitis | ROS-, ALK-, EGFR- | Primary: ORR Secondary: Non-SAEs, SAEs, duration of response, PFS | N/A | N/A |
| NCT03209401 | Phase 1—Niraparib + carboplatin in HRR deficient adv solid tumors | Adv solid tumor | Life expectancy > 3 mo, | Prior platinum chemotherapy w/in last 6 mo. Prior PARPi, CNS disease, | HR pathway mutations | Primary: Grade 3–4 toxicities, ORR Secondary: Median survival, PFS, DCR | N/A | N/A |
| NCT02944396 | Veliparib in combination with Nivolumab and Platinum doublet Phase 1 | NSCLC | Stage 4 or unresectable Stage 3B Life expectancy > 3 months | Prior therapy with PARPi or checkpoint inhibitor CNS disease | N/A | Primary: PFS, RPTD Secondary: OS, AUC, DOR, Cmax | N/A | N/A |
| NCT02264990 | Veliparib + carboplatin and paclitaxel v investigator’s choice chemotherapy Phase 3 | Non-squamous NSCLC | Stage 3 unresectable, stage 4 Current or former smoker | Peripheral neuropathy, Prior cytotoxic chemo or radiation for NSCLC | EGFR mutation negative ALK fusion negative LP52+ | Primary: Number of participants with DLT Secondary: ORR | mOS 12.1 in both arms (p = 0.846) | [79] |
| NCT01560104 | Veliparib vs. placebo in combination with carboplatin and paclitaxel Phase 2 | NSCLC | Stage 3 unresectable, stage 4 Life expectancy > 3 months | Peripheral neuropathy, Prior cytotoxic chemo or radiation for NSCLC | EGFR mutation negative | Primary: PFS Secondary: OS, ORR, CIPN | mPFS 5.8 months vs. 4.2 months (p = 0.17). mOS 11.7 vs. 9.1 (p = 0.27). ORR: 32.4% vs. 32.1% | [76] |
| NCT012106546 | Veliparib vs. placebo in combination with carboplatin and paclitaxel Phase 3 | Squamous NSCLC | Stage 3 unresectable, stage 4 Life expectancy > 3 months | Peripheral neuropathy, Prior cytotoxic chemo or radiation for NSCLC | EGFR mutation negative ALK fusion negative | Primary: OS (current smokers) Secondary: OS (all subjects), PFS, ORR | mOS 11.9 months vs. 11.1 months (p = 0.266). | [77] |
| NCT01657799 | Veliparib 50 BID with WBRT vs. veliparib 200 BID with WBRT vs. placebo with WBRT for NSCLC Brain metastases Phase 2 | NSCLC | Brain mets on MRI, eligible for WBRT, adequate hematologic, renal, and hepatic function | Dx of brain mets > 28 days prior to day 1, more than 2 sites of metastasis, leptomeningeal spread, prior PARPi therapy | N/A | Primary: OS Secondary: Best tumor response rate, time to intracranial progression | mOS 209 days vs. 209 days vs 185 days p >0.05 | [87] |
| NCT01386385 | Veliparib with or without radiation, carboplatin, and paclitaxel Phase 1 followed by Phase 2 | NSCLC | Stage 3 unresectable disease | Prior cytotoxic chemo or radiation for NSCLC Immune disorder | N/A | Primary: Maximum tolerated dose, PFS Secondary: OS, ORR, incidence of Grade 3+ AE | N/A | Ongoing |
| NCT01282333 | Veliparib, cisplatin, and gemcitabine hydrochloride Phase 1 | NSCLC, (+biliary, pancreatic, urothelial cancer | Stage 3 unresectable, Stage 4 | Prior cytotoxic chemo or radiation | BRCA1 or BRCA 2 germline mutation | Primary: Maximum tolerated dose Secondary: Dose-limiting toxicities, antitumor activity | N/A | N/A (terminated) |
| NCT04173507 | Talazoparib plus avelumab (LUNG-MAP trial) Phase 2 | NSCLC | Stage 4 or recurrent disease Prior treatment with one line of anti-PD-1 or PD-L1 | Prior PARPi, Grade 2/4 cardiac disease, | STK11 gene mutation | Primary: ORR Disease control rate Secondary: Toxicities, PFS, OS, Duration of response | N/A | Ongoing trial |
| NCT03330405 | Avelumab plus Talazoparib Phase 1/2 | NSCLC, Ovarian, urothelial, TNBC | Stage 3 unresectable, Stage 4, ECOG 1, 2 | Prior PARPi, history of brain mets requiring steroid, autoimmune disease | BRCA, ATM mutations | Primary: DLT, OR Secondary: TTR, DOR, PFS, OS | N/A | Ongoing trial |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaddour, K.; Felipe Fernandez, M.; Khabibov, M.; Garifullin, A.; Dressler, D.; Topchu, I.; Patel, J.D.; Weinberg, F.; Boumber, Y. The Prognostic and Therapeutic Potential of DNA Damage Repair Pathway Alterations and Homologous Recombination Deficiency in Lung Cancer. Cancers 2022, 14, 5305. https://doi.org/10.3390/cancers14215305
Khaddour K, Felipe Fernandez M, Khabibov M, Garifullin A, Dressler D, Topchu I, Patel JD, Weinberg F, Boumber Y. The Prognostic and Therapeutic Potential of DNA Damage Repair Pathway Alterations and Homologous Recombination Deficiency in Lung Cancer. Cancers. 2022; 14(21):5305. https://doi.org/10.3390/cancers14215305
Chicago/Turabian StyleKhaddour, Karam, Manuel Felipe Fernandez, Marsel Khabibov, Airat Garifullin, Danielle Dressler, Iuliia Topchu, Jyoti D. Patel, Frank Weinberg, and Yanis Boumber. 2022. "The Prognostic and Therapeutic Potential of DNA Damage Repair Pathway Alterations and Homologous Recombination Deficiency in Lung Cancer" Cancers 14, no. 21: 5305. https://doi.org/10.3390/cancers14215305
APA StyleKhaddour, K., Felipe Fernandez, M., Khabibov, M., Garifullin, A., Dressler, D., Topchu, I., Patel, J. D., Weinberg, F., & Boumber, Y. (2022). The Prognostic and Therapeutic Potential of DNA Damage Repair Pathway Alterations and Homologous Recombination Deficiency in Lung Cancer. Cancers, 14(21), 5305. https://doi.org/10.3390/cancers14215305






