Prognostic Value of [18F]-FDG PET/CT Radiomics Combined with Sarcopenia Status among Patients with Advanced Gastroesophageal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Imaging Acquisition
2.2. Image Analysis and Sarcopenia Measurements
2.3. Image Segmentation and Radiomic Feature Extraction
2.4. Statistical Analysis
3. Results
3.1. Univariable Analysis
3.2. Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Onishi, S.; Tajika, M.; Tanaka, T.; Hirayama, Y.; Hara, K.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Inaba, Y.; Kodaira, T. Prognostic significance of sarcopenia in patients with unresectable advanced esophageal cancer. J. Clin. Med. 2019, 8, 1647. [Google Scholar] [CrossRef] [Green Version]
- Enzinger, P.C.; Mayer, R.J. Esophageal cancer. N. Engl. J. Med. 2003, 349, 2241–2252. [Google Scholar] [CrossRef] [Green Version]
- Puhr, H.C.; Preusser, M.; Ilhan-Mutlu, A. Immunotherapy for Esophageal Cancers: What Is Practice Changing in 2021? Cancers 2021, 13, 4632. [Google Scholar] [CrossRef]
- Huynh, J.; Patel, K.; Gong, J.; Cho, M.; Malla, M.; Parikh, A.; Klempner, S. Immunotherapy in Gastroesophageal Cancers: Current Evidence and Ongoing Trials. Curr. Treat. Options Oncol. 2021, 22, 100. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.M. Radiomics in Oncological PET/CT: Clinical Applications. Nucl. Med. Mol. Imaging 2018, 52, 170–189. [Google Scholar] [CrossRef]
- Han, S.; Kim, Y.J.; Woo, S.; Suh, C.H.; Lee, J.J. Prognostic value of volumetric parameters of pretreatment 18F-FDG PET/CT in esophageal cancer: A systematic review and meta-analysis. Clin. Nucl. Med. 2018, 43, 887–894. [Google Scholar] [CrossRef]
- Kwon, H.R.; Pahk, K.; Park, S.; Kwon, H.W.; Kim, S. Prognostic value of metabolic information in advanced gastric cancer using preoperative 18F-FDG PET/CT. Nucl. Med. Mol. Imaging 2019, 53, 386–395. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef]
- Baessler, B.; Nestler, T.; Pinto dos Santos, D.; Paffenholz, P.; Zeuch, V.; Pfister, D.; Maintz, D.; Heidenreich, A. Radiomics allows for detection of benign and malignant histopathology in patients with metastatic testicular germ cell tumors prior to post-chemotherapy retroperitoneal lymph node dissection. Eur. Radiol. 2020, 30, 2334–2345. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563. [Google Scholar] [CrossRef] [Green Version]
- Sah, B.-R.; Owczarczyk, K.; Siddique, M.; Cook, G.J.; Goh, V. Radiomics in esophageal and gastric cancer. Abdom. Radiol. 2019, 44, 2048–2058. [Google Scholar] [CrossRef] [Green Version]
- Yip, C.; Landau, D.; Kozarski, R.; Ganeshan, B.; Thomas, R.; Michaelidou, A.; Goh, V. Primary esophageal cancer: Heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology 2014, 270, 141–148. [Google Scholar] [CrossRef]
- Van Rossum, P.S.; Xu, C.; Fried, D.V.; Goense, L.; Court, L.E.; Lin, S.H. The emerging field of radiomics in esophageal cancer: Current evidence and future potential. Transl. Cancer Res. 2016, 5, 410. [Google Scholar] [CrossRef] [Green Version]
- Beukinga, R.J.; Hulshoff, J.B.; Mul, V.E.; Noordzij, W.; Kats-Ugurlu, G.; Slart, R.H.; Plukker, J.T. Prediction of response to neoadjuvant chemotherapy and radiation therapy with baseline and restaging 18F-FDG PET imaging biomarkers in patients with esophageal cancer. Radiology 2018, 287, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosisReport of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.-Y.; Zha, P.; Peng, L.; Hou, L.; Huang, K.-L.; Li, X.-Y. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: A comprehensive systematic review and meta-analysis. Dis. Esophagus 2019, 32, doy115. [Google Scholar] [CrossRef] [Green Version]
- Paireder, M.; Asari, R.; Kristo, I.; Rieder, E.; Tamandl, D.; Ba-Ssalamah, A.; Schoppmann, S. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 478–484. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [Green Version]
- Anconina, R.; Ortega, C.; Metser, U.; Liu, Z.A.; Elimova, E.; Allen, M.; Darling, G.E.; Wong, R.; Taylor, K.; Yeung, J.; et al. Combined 18F-FDG PET/CT Radiomics and Sarcopenia Score in Predicting Relapse-Free Survival and Overall Survival in Patients with Esophagogastric Cancer. Clin. Nucl. Med. 2022, 47, 684–691. [Google Scholar] [CrossRef]
- Bunn, A.; Korpela, M. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B. caret: Classification and Regression Training; R Package Version 6.0-86; Astrophysics Source Code Library: Cambridge, MA, USA, 2020. [Google Scholar]
- Venables, W.; Ripley, B.D. Statistics Complements to Modern Applied Statistics with S Plus, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Heagerty, P.J.; Saha-Chaudhuri, P.; Saha-Chaudhuri, M.P. Time-Dependent ROC Curve Estimation from Censored Survival Data, Package ‘survivalROC’, version 1.0.3.; GitHub: San Fracisco, CA, USA, 2013. [Google Scholar]
- Metzger, J.-C.; Wollschläger, D.; Miederer, M.; Vaupel, P.; Moehler, M.; Schmidberger, H.; Mayer, A. Inclusion of PET-CT into planning of primary or neoadjuvant chemoradiotherapy of esophageal cancer improves prognosis. Strahlenther. Onkol. 2017, 193, 791–799. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, J.; Zhang, B.; Mu, D.; Zhang, W.; Li, D.; Han, A.; Song, P.; Li, H.; Yang, G. Using 18F-fluorodeoxyglucose positron emission tomography to estimate the length of gross tumor in patients with squamous cell carcinoma of the esophagus. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 136–141. [Google Scholar] [CrossRef]
- Li, Y.; Zschaeck, S.; Lin, Q.; Chen, S.; Chen, L.; Wu, H. Metabolic parameters of sequential 18F-FDG PET/CT predict overall survival of esophageal cancer patients treated with (chemo-) radiation. Radiat. Oncol. 2019, 14, 35. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Park, G.; Jo, S.; Lee, S.S.; Park, J.; Shim, H.-K. 18F-FDG PET/CT Parameters for predicting prognosis in esophageal cancer patients treated with concurrent chemoradiotherapy. Technol. Cancer Res. Treat. 2021, 20, 15330338211024655. [Google Scholar] [CrossRef]
- Brown, C.; Howes, B.; Jamieson, G.G.; Bartholomeusz, D.; Zingg, U.; Sullivan, T.R.; Thompson, S.K. Accuracy of PET-CT in predicting survival in patients with esophageal cancer. World J. Surg. 2012, 36, 1089–1095. [Google Scholar] [CrossRef]
- Rizk, N.P.; Tang, L.; Adusumilli, P.S.; Bains, M.S.; Akhurst, T.J.; Ilson, D.; Goodman, K.; Rusch, V.W. Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J. Thorac. Oncol. 2009, 4, 875–879. [Google Scholar] [CrossRef] [Green Version]
- Hatt, M.; Tixier, F.; Pierce, L.; Kinahan, P.E.; Le Rest, C.C.; Visvikis, D. Characterization of PET/CT images using texture analysis: The past, the present… any future? Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 151–165. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.P.; Rose, C.J.; Waterton, J.C.; Carano, R.A.; Parker, G.J.; Jackson, A. Imaging intratumor heterogeneity: Role in therapy response, resistance, and clinical outcome. Clin. Cancer Res. 2015, 21, 249–257. [Google Scholar] [CrossRef]
- Campbell, P.J.; Yachida, S.; Mudie, L.J.; Stephens, P.J.; Pleasance, E.D.; Stebbings, L.A.; Morsberger, L.A.; Latimer, C.; McLaren, S.; Lin, M.-L. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010, 467, 1109–1113. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Sun, X.; Zhao, X.; Zhu, W.; Sun, L.; Huang, Y.; Li, W.; Wan, H.; Xing, L.; Yu, J. The impact of intratumoral metabolic heterogeneity on postoperative recurrence and survival in resectable esophageal squamous cell carcinoma. Oncotarget 2017, 8, 14969–14977. [Google Scholar] [CrossRef] [Green Version]
- Tochigi, T.; Shuto, K.; Kono, T.; Ohira, G.; Tohma, T.; Gunji, H.; Hayano, K.; Narushima, K.; Fujishiro, T.; Hanaoka, T. Heterogeneity of glucose metabolism in esophageal cancer measured by fractal analysis of fluorodeoxyglucose positron emission tomography image: Correlation between metabolic heterogeneity and survival. Dig. Surg. 2017, 34, 186–191. [Google Scholar] [CrossRef]
- Yip, S.S.; Coroller, T.P.; Sanford, N.N.; Mamon, H.; Aerts, H.J.; Berbeco, R.I. Relationship between the temporal changes in positron-emission-tomography-imaging-based textural features and pathologic response and survival in esophageal cancer patients. Front. Oncol. 2016, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tan, S.; Chen, W.; Kligerman, S.; Kim, G.; D’Souza, W.D.; Suntharalingam, M.; Lu, W. Modeling pathologic response of esophageal cancer to chemoradiation therapy using spatial-temporal 18F-FDG PET features, clinical parameters, and demographics. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Kligerman, S.; Chen, W.; Lu, M.; Kim, G.; Feigenberg, S.; D’Souza, W.D.; Suntharalingam, M.; Lu, W. Spatial-temporal [18F] FDG-PET features for predicting pathologic response of esophageal cancer to neoadjuvant chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Högner, A.; Moehler, M. Immunotherapy in Gastric Cancer. Curr. Oncol. 2022, 29, 1559–1574. [Google Scholar] [CrossRef]
- Foley, K.G.; Hills, R.K.; Berthon, B.; Marshall, C.; Parkinson, C.; Lewis, W.G.; Crosby, T.D.L.; Spezi, E.; Roberts, S.A. Development and validation of a prognostic model incorporating texture analysis derived from standardised segmentation of PET in patients with oesophageal cancer. Eur. Radiol. 2018, 28, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Nakajo, M.; Jinguji, M.; Nakabeppu, Y.; Nakajo, M.; Higashi, R.; Fukukura, Y.; Sasaki, K.; Uchikado, Y.; Natsugoe, S.; Yoshiura, T. Texture analysis of (18)F-FDG PET/CT to predict tumour response and prognosis of patients with esophageal cancer treated by chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 206–214. [Google Scholar] [CrossRef]
- Xiong, J.; Yu, W.; Ma, J.; Ren, Y.; Fu, X.; Zhao, J. The Role of PET-Based Radiomic Features in Predicting Local Control of Esophageal Cancer Treated with Concurrent Chemoradiotherapy. Sci. Rep. 2018, 8, 9902. [Google Scholar] [CrossRef]
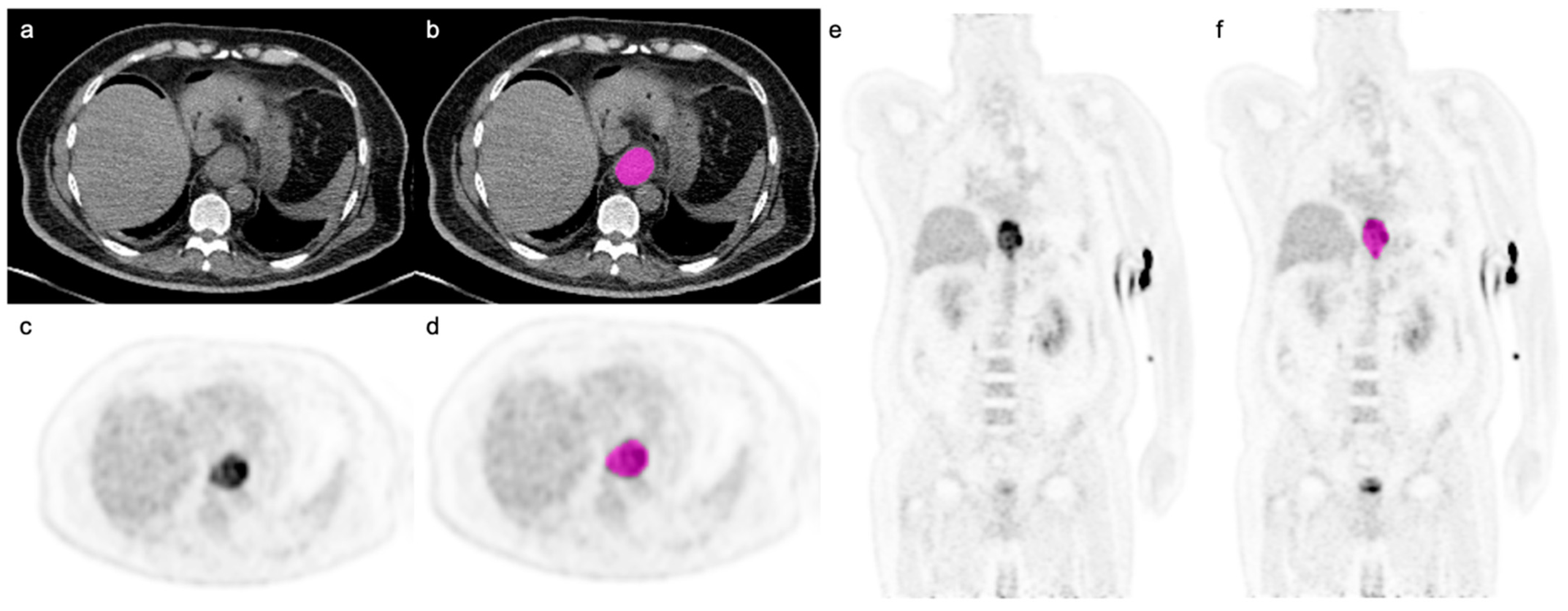
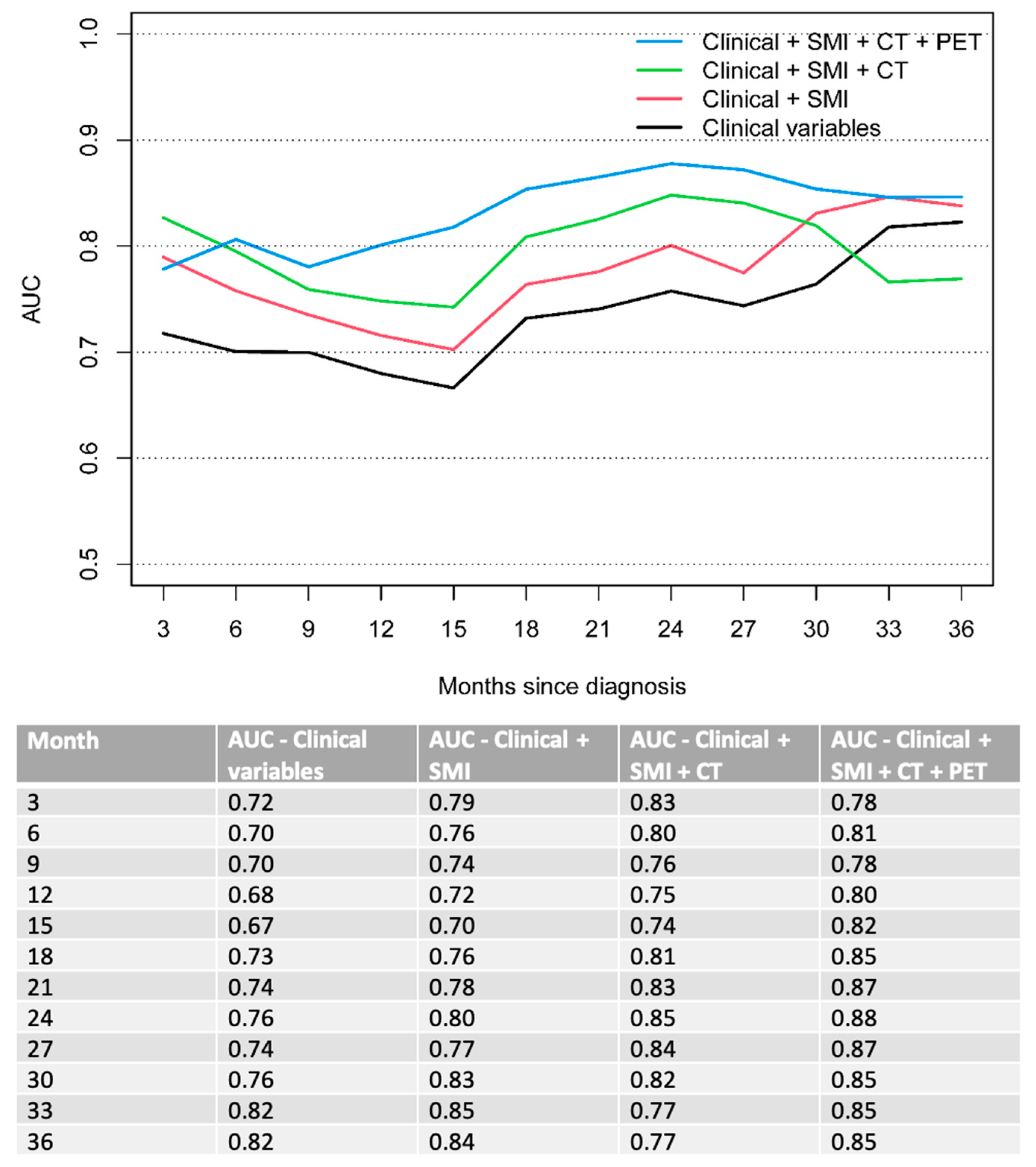
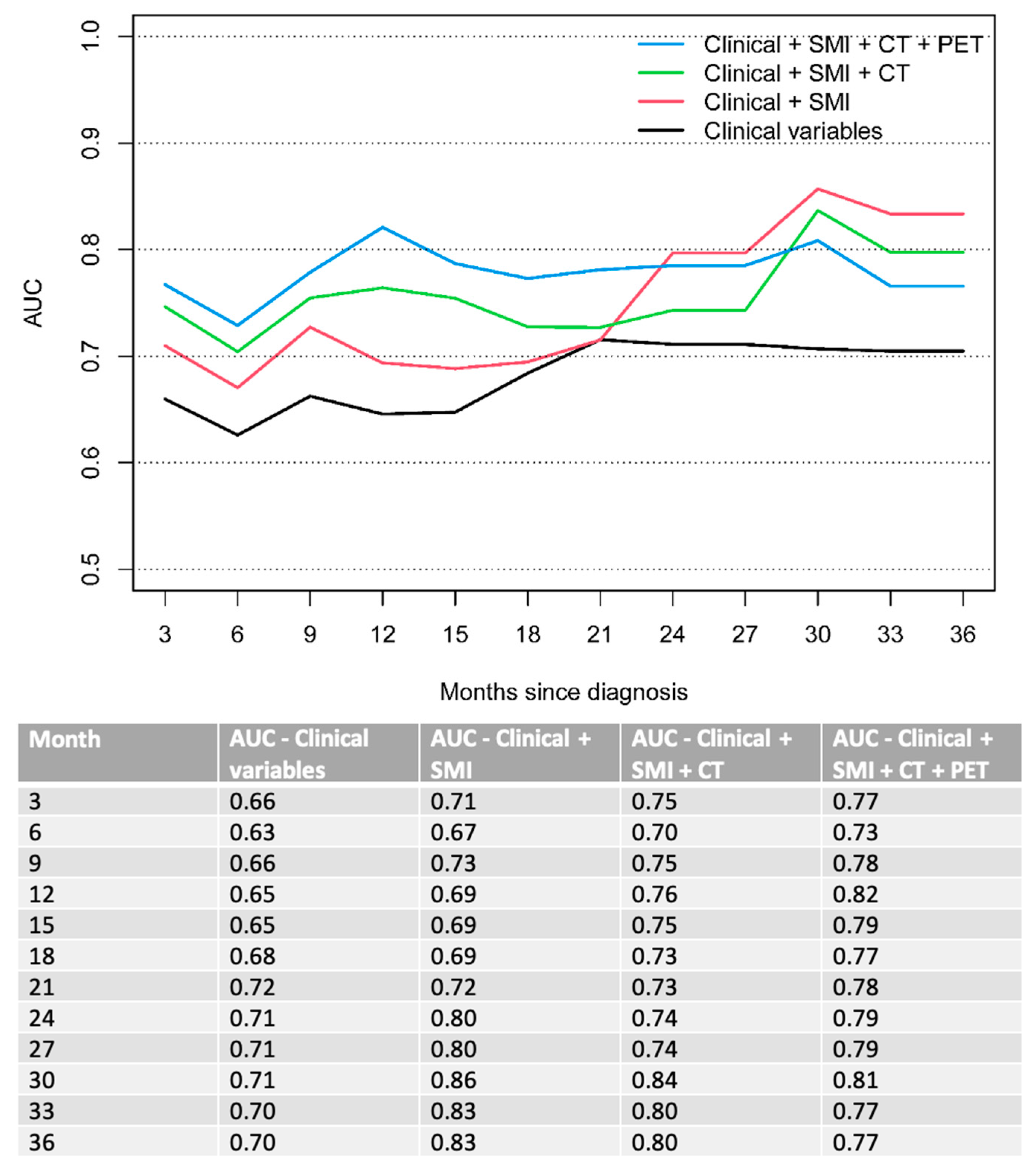
| Characteristics | n = 128 |
|---|---|
| Age (mean ± SD; range) | 63.5 ± 11.7 (29–91) |
| Sex | |
| Females | 26 (20%) |
| Male | 102 (80%) |
| BMI (kg/m2) (mean ± SD) | 24.4 ± 4.9 |
| Race | |
| Asian | 11 (9%) |
| Non-Asian | 117 (91%) |
| Siewert Class | |
| AEG 1: 35–39 cm | 27 (21%) |
| AEG 2: 39–42 cm | 27 (21%) |
| AEG 3: 42–45 cm | 15 (12%) |
| Esophagus: <35 cm | 59 (46%) |
| ECOG | |
| 0 | 28 (22%) |
| 1 | 73 (57%) |
| ≥2 | 27 (21%) |
| Tumor Grade | |
| G1-2 | 47 (37%) |
| G3 | 51 (40%) |
| GX | 30 (23%) |
| T stage | |
| T0-3 | 37 (29%) |
| T4 | 8 (6%) |
| TX | 83 (65%) |
| N stage | |
| N0 | 6 (5%) |
| N1 | 113 (88%) |
| N2 | 4 (3%) |
| NX | 5 (4%) |
| M stage | 128 (100%) |
| Distant Lymph Nodes | 73 (57%) |
| Lung/Pleura | 24 (19%) |
| Liver | 43 (34%) |
| Peritoneum | 16 (12%) |
| Bone | 29 (23%) |
| Brain | 2 (2%) |
| Sarcopenia | 60 (47%; 82% males, 18% females) |
| Covariate | OS | PFS | ||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Age | 1.02 (1.00,1.04) | 0.017 | 1.01 (1.00,1.03) | 0.14 |
| Sex (male) | 0.90 (0.57,1.43) | 0.65 | 1.00 (0.64,1.57) | 0.99 |
| Race (non-asian) | 0.69 (0.36,1.34) | 0.28 | 0.53 (0.27,1.02) | 0.058 |
| ECOG | <0.001 | <0.001 | ||
| 0–1 | Reference | Reference | ||
| 2–3 | 3.13 (1.96,4.98) | 2.30 (1.46,3.62) | ||
| T stage | 0.47 | 0.27 | ||
| T0-3 | Reference | Reference | ||
| T4 | 1.05 (0.44,2.55) | 0.70 (0.29,1.68) | 0.42 | |
| TX | 1.30 (0.84,2.02) | 1.25 (0.83,1.90) | 0.28 | |
| Tumor Histology | 0.68 | 0.69 | ||
| Adenocarcinoma | Reference | Reference | ||
| Squamous cell carcinoma | 0.92 (0.61,1.37) | 1.08 (0.74,1.59) | ||
| Tumor Grade | 0.77 | 0.86 | ||
| G1-2 | Reference | Reference | ||
| G3 | 0.92 (0.60,1.41) | 0.91 (0.60,1.37) | 0.64 | |
| GX | 1.11 (0.67,1.85) | 1.02 (0.63,1.66) | 0.93 | |
| M | 0.44 | 0.45 | ||
| M1 | Reference | Reference | ||
| M1a | 0.70 (0.27,1.79) | 0.69 (0.29,1.65) | 0.4 | |
| M1b | 1.18 (0.77,1.79) | 1.14 (0.76,1.70) | 0.53 | |
| Distant LN | 0.79 (0.54,1.16) | 0.23 | 0.91 (0.63,1.31) | 0.61 |
| Lung/Pleura | 1.06 (0.65,1.73) | 0.8 | 1.09 (0.68,1.73) | 0.73 |
| Liver | 1.27 (0.85,1.89) | 0.25 | 1.15 (0.78,1.70) | 0.47 |
| Peritoneum | 1.34 (0.78,2.32) | 0.29 | 1.01 (0.58,1.73) | 0.98 |
| Bone | 1.67 (1.06,2.63) | 0.028 | 1.61 (1.03,2.51) | 0.038 |
| Brain | 0.49 (0.07,3.53) | 0.48 | 1.63 (0.40,6.63) | 0.5 |
| SUVmax | 0.99 (0.96,1.01) | 0.33 | 1.00 (0.97,1.02) | 0.86 |
| SUVmean | 0.96 (0.91,1.01) | 0.15 | 0.99 (0.94,1.04) | 0.6 |
| SUVpeak | 0.98 (0.95,1.01) | 0.26 | 0.99 (0.96,1.02) | 0.68 |
| SULmax | 0.99 (0.96,1.02) | 0.5 | 1.00 (0.97,1.04) | 0.83 |
| SULmean | 0.96 (0.89,1.03) | 0.23 | 0.99 (0.92,1.07) | 0.86 |
| SULpeak | 0.98 (0.94,1.02) | 0.4 | 1.00 (0.96,1.04) | 0.99 |
| BMI (kg/m2) | 0.97 (0.93,1.02) | 0.21 | 0.98 (0.94,1.02) | 0.3 |
| SMI (cm2/m2) | 0.97 (0.95,0.99) | 0.0075 | 0.97 (0.96,0.99) | 0.011 |
| Sarcopenia (yes) | 1.51 (1.03,2.22) | 0.033 | 1.55 (1.07,2.25) | 0.021 |
| CT features | ||||
| NGLDM_Coarseness | 0.79 (0.65,0.96) | 0.018 | 0.78 (0.64,0.95) | 0.013 |
| NGLDM_Contrast | 0.78 (0.64,0.94) | 0.009 | 0.80 (0.67,0.96) | 0.016 |
| GLZLM_ZP | 0.83 (0.69,0.99) | 0.039 | ||
| PET features | ||||
| SHAPE_Volume_mL | 1.17 (1.00,1.36) | 0.049 | 1.22 (1.04, 1.44) | 0.017 |
| SHAPE_Volume_vx | 1.18 (1.00,1.38) | 0.049 | ||
| SHAPE_Surface_mm2 | 1.18 (1.01,1.39) | 0.043 | ||
| GLZLM_LZE | 1.22 (1.02,1.45) | 0.026 | ||
| GLZLM_LZLGE | 1.21 (1.01,1.46) | 0.044 | 1.20 (1.00,1.44) | 0.046 |
| GLZLM_SZLGE | 1.23 (1.01,150) | 0.043 | ||
| 40_ SHAPE_Volume_mL | 1.20 (1.01,1.42) | 0.038 | ||
| 70_Kurtosis | 1.25 (1.01,1.54) | 0.042 | ||
| 70_Excess Kurtosis | 1.25 (1.01,1.54) | 0.042 |
| Covariate | OS | PFS | ||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Age | 1.01 (1.00,1.03) | 0.13 | ||
| ECOG | <0.001 | <0.001 | ||
| 0–1 | reference | reference | ||
| 2–3 | 2.81 (1.65,4.79) | 2.65 (1.63,4.30) | ||
| Bone | 0.021 | 0.005 | ||
| No | reference | reference | ||
| Yes | 1.93 (1.22,3.04) | 1.71 (1.09,2.69) | ||
| SMI (cm2/m2) | 0.98 (0.96,1.00) | 0.033 | 0.98 (0.96,1.00) | 0.04 |
| CT features | ||||
| NGLDM Coarseness | 0.70 (0.53,0.92) | 0.011 | ||
| NGLDM Contrast | 0.79 (0.65,0.94) | 0.01 | ||
| PET features | ||||
| GLZLM SZLGE | 1.37 (1.12,1.67) | 0.002 | ||
| SHAPE Volume vx | 1.19 (1.01,1.40) | 0.04 | ||
| 70_ Kurtosis | 1.24 (1.00,1.53) | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinzpeter, R.; Mirshahvalad, S.A.; Kulanthaivelu, R.; Ortega, C.; Metser, U.; Liu, Z.A.; Elimova, E.; Wong, R.K.S.; Yeung, J.; Jang, R.W.-J.; et al. Prognostic Value of [18F]-FDG PET/CT Radiomics Combined with Sarcopenia Status among Patients with Advanced Gastroesophageal Cancer. Cancers 2022, 14, 5314. https://doi.org/10.3390/cancers14215314
Hinzpeter R, Mirshahvalad SA, Kulanthaivelu R, Ortega C, Metser U, Liu ZA, Elimova E, Wong RKS, Yeung J, Jang RW-J, et al. Prognostic Value of [18F]-FDG PET/CT Radiomics Combined with Sarcopenia Status among Patients with Advanced Gastroesophageal Cancer. Cancers. 2022; 14(21):5314. https://doi.org/10.3390/cancers14215314
Chicago/Turabian StyleHinzpeter, Ricarda, Seyed Ali Mirshahvalad, Roshini Kulanthaivelu, Claudia Ortega, Ur Metser, Zhihui A. Liu, Elena Elimova, Rebecca K. S. Wong, Jonathan Yeung, Raymond Woo-Jun Jang, and et al. 2022. "Prognostic Value of [18F]-FDG PET/CT Radiomics Combined with Sarcopenia Status among Patients with Advanced Gastroesophageal Cancer" Cancers 14, no. 21: 5314. https://doi.org/10.3390/cancers14215314
APA StyleHinzpeter, R., Mirshahvalad, S. A., Kulanthaivelu, R., Ortega, C., Metser, U., Liu, Z. A., Elimova, E., Wong, R. K. S., Yeung, J., Jang, R. W.-J., & Veit-Haibach, P. (2022). Prognostic Value of [18F]-FDG PET/CT Radiomics Combined with Sarcopenia Status among Patients with Advanced Gastroesophageal Cancer. Cancers, 14(21), 5314. https://doi.org/10.3390/cancers14215314







