The Role of MicroRNAs in HER2-Positive Breast Cancer: Where We Are and Future Prospective
Abstract
Simple Summary
Abstract
1. Introduction
1.1. HER2-Positive Breast Cancer
1.2. Trastuzumab Resistance
1.3. MicroRNAs
1.4. MiRNAs and Breast Cancer
2. MiRNAs and HER2+ Breast Cancer
2.1. Functional Roles of MiRNAs in Tumor Formation, Progression, Metastasis, and Therapy Resistance
2.1.1. MiRNAs Regulated by HER2 and Dysregulated in HER2-Positive Breast Cancer
2.1.2. MiRNAs Involved in Anti-HER2 Therapy Resistance
2.2. MiRNAs as Biomarkers in HER2+ Breast Cancer
2.2.1. MiRNA Signatures for HER2+ Breast Cancer Diagnosis
2.2.2. MiRNAs as Prognostic and Predictive Biomarkers in HER2+ Breast Cancer
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; IARC Press: Lyon, France, 2020; ISBN 978-92-832-0448-0. [Google Scholar]
- Weigelt, B.; Geyer, F.C.; Reis-Filho, J.S. Histological Types of Breast Cancer: How Special Are They? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the Molecular Portraits of Breast Cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.-H.; Sledge, G.; Geyer, C.E.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Planned Joint Analysis of Overall Survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/Neu Oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Albanell, J. Mechanism of Action of Anti-HER2 Monoclonal Antibodies. Ann. Oncol. 2001, 12 (Suppl. S1), S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Sebzari, A.; Kordi-Tamandani, D.M.; Dastjerdi, K. Involvement of the Dysregulation of MiR-23b-3p, MiR-195-5p, MiR-656-5p, and MiR-340-5p in Trastuzumab Resistance of HER2-Positive Breast Cancer Cells and System Biology Approach to Predict Their Targets Involved in Resistance. DNA Cell Biol. 2019, 38, 184–192. [Google Scholar] [CrossRef]
- Pohlmann, P.R.; Mayer, I.A.; Mernaugh, R. Resistance to Trastuzumab in Breast Cancer. Clin. Cancer Res. 2009, 15, 7479–7491. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Claret, F.X. Trastuzumab: Updated Mechanisms of Action and Resistance in Breast Cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef]
- Baselga, J.; Albanell, J.; Molina, M.A.; Arribas, J. Mechanism of Action of Trastuzumab and Scientific Update. Semin Oncol. 2001, 28, 4–11. [Google Scholar] [CrossRef]
- Nagata, Y.; Lan, K.-H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. PTEN Activation Contributes to Tumor Inhibition by Trastuzumab, and Loss of PTEN Predicts Trastuzumab Resistance in Patients. Cancer Cell 2004, 6, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Valabrega, G.; Montemurro, F.; Sarotto, I.; Petrelli, A.; Rubini, P.; Tacchetti, C.; Aglietta, M.; Comoglio, P.M.; Giordano, S. TGFalpha Expression Impairs Trastuzumab-Induced HER2 Downregulation. Oncogene 2005, 24, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Ferracin, M.; Liu, C.-G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA Gene Expression Deregulation in Human Breast Cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef] [PubMed]
- Salim, U.; Kumar, A.; Kulshreshtha, R.; Vivekanandan, P. Biogenesis, Characterization, and Functions of Mirtrons. Wiley Interdiscip Rev. RNA 2022, 13, e1680. [Google Scholar] [CrossRef] [PubMed]
- Medley, J.C.; Panzade, G.; Zinovyeva, A.Y. MicroRNA Strand Selection: Unwinding the Rules. Wiley Interdiscip Rev. RNA 2020, 12, e1627. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A Brief Review on the Mechanisms of MiRNA Regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent Deletions and Down-Regulation of Micro- RNA Genes MiR15 and MiR16 at 13q14 in Chronic Lymphocytic Leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human MicroRNA Genes Are Frequently Located at Fragile Sites and Genomic Regions Involved in Cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. MicroRNA Involvement in Human Cancer. Carcinogenesis 2012, 33, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Ferdin, J.; Kunej, T.; Calin, G.A. Non-Coding RNAs: Identification of Cancer-Associated MicroRNAs by Gene Profiling. Technol. Cancer Res. Treat. 2010, 9, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Sarver, A.L.; Sarver, A.E.; Yuan, C.; Subramanian, S. OMCD: OncomiR Cancer Database. BMC Cancer 2018, 18, 1223. [Google Scholar] [CrossRef] [PubMed]
- Kudela, E.; Samec, M.; Koklesova, L.; Liskova, A.; Kubatka, P.; Kozubik, E.; Rokos, T.; Pribulova, T.; Gabonova, E.; Smolar, M.; et al. MiRNA Expression Profiles in Luminal A Breast Cancer-Implications in Biology, Prognosis, and Prediction of Response to Hormonal Treatment. Int. J. Mol. Sci. 2020, 21, 7691. [Google Scholar] [CrossRef] [PubMed]
- Focke, C.M.; Bürger, H.; van Diest, P.J.; Finsterbusch, K.; Gläser, D.; Korsching, E.; Decker, T. German Breast Screening Pathology Initiative Interlaboratory Variability of Ki67 Staining in Breast Cancer. Eur. J. Cancer 2017, 84, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Søkilde, R.; Persson, H.; Ehinger, A.; Pirona, A.C.; Fernö, M.; Hegardt, C.; Larsson, C.; Loman, N.; Malmberg, M.; Rydén, L.; et al. Refinement of Breast Cancer Molecular Classification by MiRNA Expression Profiles. BMC Genom. 2019, 20, 503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, C.; Xiang, Q.; Xu, L.; Liu, Q.; Pang, X.; Zhang, W.; Zhang, H.; Zhang, S.; et al. Circulating MicroRNAs as Indicators in the Prediction of Neoadjuvant Chemotherapy Response in Luminal B Breast Cancer. Thorac. Cancer 2021, 12, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-K.; Lin, C.-H.; Kuo, Y.-L.; Ger, L.-P.; Cheng, H.-C.; Yao, Y.-C.; Hsiao, M.; Lu, P.-J. MiR-211 Determines Brain Metastasis Specificity through SOX11/NGN2 Axis in Triple-Negative Breast Cancer. Oncogene 2021, 40, 1737–1751. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Salvatore, M.; Incoronato, M. MiRNA-Based Therapeutics in Breast Cancer: A Systematic Review. Front. Oncol. 2021, 11, 668464. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, H.-Y.; Hao, N.-B.; Tang, B.; Guo, H.; Yong, X.; Dong, H.; Yang, S.-M. MicroRNA Inhibitors: Natural and Artificial Sequestration of MicroRNA. Cancer Lett. 2017, 407, 139–147. [Google Scholar] [CrossRef]
- Bahreyni, A.; Alibolandi, M.; Ramezani, M.; Sarafan Sadeghi, A.; Abnous, K.; Taghdisi, S.M. A Novel MUC1 Aptamer-Modified PLGA-Epirubicin-PβAE-Antimir-21 Nanocomplex Platform for Targeted Co-Delivery of Anticancer Agents in Vitro and in Vivo. Colloids Surf. B Biointerfaces 2019, 175, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Devulapally, R.; Sekar, N.M.; Sekar, T.V.; Foygel, K.; Massoud, T.F.; Willmann, J.K.; Paulmurugan, R. Polymer Nanoparticles Mediated Codelivery of AntimiR-10b and AntimiR-21 for Achieving Triple Negative Breast Cancer Therapy. ACS Nano 2015, 9, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Y.; Yang, R.; Mu, J.; Zhou, Z.; Sun, M. Poly-Antioxidants for Enhanced Anti-MiR-155 Delivery and Synergistic Therapy of Metastatic Breast Cancer. Biomater. Sci. 2022, 10, 3637–3646. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Wu, Y.; Wang, Y.; Cai, Y.; Jin, J.; Yang, Z. Dual Antisense Oligonucleotide Targeting MiR-21/MiR-155 Synergize Photodynamic Therapy to Treat Triple-Negative Breast Cancer and Inhibit Metastasis. Biomed. Pharmacother. 2022, 146, 112564. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, Z. Downregulation of MiR-302b Is Associated with Poor Prognosis and Tumor Progression of Breast Cancer. Breast Cancer 2020, 27, 291–298. [Google Scholar] [CrossRef]
- Cataldo, A.; Cheung, D.G.; Balsari, A.; Tagliabue, E.; Coppola, V.; Iorio, M.V.; Palmieri, D.; Croce, C.M. MiR-302b Enhances Breast Cancer Cell Sensitivity to Cisplatin by Regulating E2F1 and the Cellular DNA Damage Response. Oncotarget 2016, 7, 786–797. [Google Scholar] [CrossRef]
- Cataldo, A.; Romero-Cordoba, S.; Plantamura, I.; Cosentino, G.; Hidalgo-Miranda, A.; Tagliabue, E.; Iorio, M.V. MiR-302b as a Combinatorial Therapeutic Approach to Improve Cisplatin Chemotherapy Efficacy in Human Triple-Negative Breast Cancer. Cancers 2020, 12, 2261. [Google Scholar] [CrossRef]
- Lang, L.; Tao, J.; Yang, C.; Li, W. Tumor Suppressive Role of MicroRNA-4731-5p in Breast Cancer through Reduction of PAICS-Induced FAK Phosphorylation. Cell Death Discov. 2022, 8, 154. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; Fang, Z.; Wan, Q.; Du, Z.; Ma, N.; Guo, G.; Lu, W. The Effect of MiR-138 on the Proliferation and Apoptosis of Breast Cancer Cells through the NF-ΚB/VEGF Signaling Pathway. Cell Mol. Biol. 2022, 68, 132–137. [Google Scholar] [CrossRef]
- Gorbatenko, A.; Søkilde, R.; Sorensen, E.E.; Newie, I.; Persson, H.; Morancho, B.; Arribas, J.; Litman, T.; Rovira, C.; Pedersen, S.F. HER2 and P95HER2 Differentially Regulate MiRNA Expression in MCF-7 Breast Cancer Cells and Downregulate MYB Proteins through MiR-221/222 and MiR-503. Sci. Rep. 2019, 9, 3352. [Google Scholar] [CrossRef]
- Galli de Amorim, M.; Branco, G.; Valieris, R.; Tarcitano, E.; Tojal da Silva, I.; Ferreira de Araújo, L.; Noronha Nunes, D.; Dias-Neto, E. The Impact of HER2 Overexpression on the MiRNA and CircRNA Transcriptomes in Two Breast Cell Lines and Their Vesicles. Pharmacogenomics 2019, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ling, Y.; Mehrpour, M.; Saw, P.E.; Liu, Z.; Tan, W.; Tian, Z.; Zhong, W.; Lin, W.; Luo, Q.; et al. Autophagy-Associated CircRNA CircCDYL Augments Autophagy and Promotes Breast Cancer Progression. Mol. Cancer 2020, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ling, Y.; Lin, Q.; Shi, Y.; Luo, Q.; Cen, Y.; Mehrpour, M.; Hamai, A.; Li, J.; Gong, C. MiR-92b-3p Inhibits Proliferation of HER2-Positive Breast Cancer Cell by Targeting CircCDYL. Front. Cell Dev. Biol 2021, 9, 707049. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Z.; Qiu, N.; Ling, L.; Jia, X.; Song, Y.; Li, H.; Li, J.; Lyu, H.; Liu, H.; et al. Disruption of FOXO3a-MiRNA Feedback Inhibition of IGF2/IGF-1R/IRS1 Signaling Confers Herceptin Resistance in HER2-Positive Breast Cancer. Nat. Commun. 2021, 12, 2699. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yuan, Y.; Li, L.; Wang, X.; Quan, Y.; Liu, C.; Yu, M.; Hu, X.; Meng, X.; Zhou, Z.; et al. HER2-Intronic MiR-4728-5p Facilitates HER2 Expression and Accelerates Cell Proliferation and Migration by Targeting EBP1 in Breast Cancer. PLoS ONE 2021, 16, e0245832. [Google Scholar] [CrossRef] [PubMed]
- Rui, T.; Xiang, A.; Guo, J.; Tang, N.; Lin, X.; Jin, X.; Liu, J.; Zhang, X. Mir-4728 Is a Valuable Biomarker for Diagnostic and Prognostic Assessment of HER2-Positive Breast Cancer. Front. Mol. Biosci. 2022, 9, 818493. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, H.; Chen, W.; Zhang, Y.; Hamburger, A.W. The ErbB3 Binding Protein EBP1 Regulates ErbB2 Protein Levels and Tamoxifen Sensitivity in Breast Cancer Cells. Breast Cancer Res. Treat. 2011, 126, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Newie, I.; Søkilde, R.; Persson, H.; Grabau, D.; Rego, N.; Kvist, A.; von Stedingk, K.; Axelson, H.; Borg, Å.; Vallon-Christersson, J.; et al. The HER2-Encoded MiR-4728-3p Regulates ESR1 through a Non-Canonical Internal Seed Interaction. PLoS ONE 2014, 9, e97200. [Google Scholar] [CrossRef]
- Floros, K.V.; Lochmann, T.L.; Hu, B.; Monterrubio, C.; Hughes, M.T.; Wells, J.D.; Morales, C.B.; Ghotra, M.S.; Costa, C.; Souers, A.J.; et al. Coamplification of MiR-4728 Protects HER2-Amplified Breast Cancers from Targeted Therapy. Proc. Natl. Acad. Sci. USA 2018, 115, E2594–E2603. [Google Scholar] [CrossRef]
- Shabaninejad, Z.; Mowla, S.J.; Yousefi, F.; Soltani, B.M. A Novel MiRNA Located in the HER2 Gene Shows an Inhibitory Effect on Wnt Signaling and Cell Cycle Progression. Biomed. Res. Int. 2022, 2022, 7216758. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Normann, L.S.; Aure, M.R.; Leivonen, S.-K.; Haugen, M.H.; Hongisto, V.; Kristensen, V.N.; Mælandsmo, G.M.; Sahlberg, K.K. MicroRNA in Combination with HER2-Targeting Drugs Reduces Breast Cancer Cell Viability in Vitro. Sci. Rep. 2021, 11, 10893. [Google Scholar] [CrossRef] [PubMed]
- Normann, L.S.; Haugen, M.H.; Aure, M.R.; Kristensen, V.N.; Mælandsmo, G.M.; Sahlberg, K.K. MiR-101-5p Acts as a Tumor Suppressor in HER2-Positive Breast Cancer Cells and Improves Targeted Therapy. Breast Cancer 2022, 14, 25–39. [Google Scholar] [CrossRef]
- Han, M.; Hu, J.; Lu, P.; Cao, H.; Yu, C.; Li, X.; Qian, X.; Yang, X.; Yang, Y.; Han, N.; et al. Exosome-Transmitted MiR-567 Reverses Trastuzumab Resistance by Inhibiting ATG5 in Breast Cancer. Cell Death Dis. 2020, 11, 43. [Google Scholar] [CrossRef]
- Pattanayak, B.; Garrido-Cano, I.; Adam-Artigues, A.; Tormo, E.; Pineda, B.; Cabello, P.; Alonso, E.; Bermejo, B.; Hernando, C.; Martínez, M.T.; et al. MicroRNA-33b Suppresses Epithelial-Mesenchymal Transition Repressing the MYC-EZH2 Pathway in HER2+ Breast Carcinoma. Front. Oncol. 2020, 10, 1661. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.P.; Cahill, H.F.; Marcato, P. Breast Cancer Subtype-Specific MiRNAs: Networks, Impacts, and the Potential for Intervention. Biomedicines 2022, 10, 651. [Google Scholar] [CrossRef]
- Wu, X.; Somlo, G.; Yu, Y.; Palomares, M.R.; Li, A.X.; Zhou, W.; Chow, A.; Yen, Y.; Rossi, J.J.; Gao, H.; et al. De Novo Sequencing of Circulating MiRNAs Identifies Novel Markers Predicting Clinical Outcome of Locally Advanced Breast Cancer. J. Transl. Med. 2012, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Souza, K.C.B.; Evangelista, A.F.; Leal, L.F.; Souza, C.P.; Vieira, R.A.; Causin, R.L.; Neuber, A.C.; Pessoa, D.P.; Passos, G.A.S.; Reis, R.M.V.; et al. Identification of Cell-Free Circulating MicroRNAs for the Detection of Early Breast Cancer and Molecular Subtyping. J. Oncol. 2019, 2019, 8393769. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, J.; Chen, J.; Wang, H.; Yang, L.; Chen, F.; Fan, S.; Wang, J.; Shao, B.; Yin, D.; et al. A Serum MicroRNA Signature Predicts Trastuzumab Benefit in HER2-Positive Metastatic Breast Cancer Patients. Nat. Commun. 2018, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Browne, B.C.; Crown, J.; Venkatesan, N.; Duffy, M.J.; Clynes, M.; Slamon, D.; O’Donovan, N. Inhibition of IGF1R Activity Enhances Response to Trastuzumab in HER-2-Positive Breast Cancer Cells. Ann. Oncol. 2011, 22, 68–73. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.-C.; Li, P.; Guo, H.; Poh, S.-B.; Brady, S.W.; Xiong, Y.; Tseng, L.-M.; Li, S.-H.; Ding, Z.; et al. Combating Trastuzumab Resistance by Targeting SRC, a Common Node Downstream of Multiple Resistance Pathways. Nat. Med. 2011, 17, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Bradbury, I.; Eidtmann, H.; Di Cosimo, S.; de Azambuja, E.; Aura, C.; Gómez, H.; Dinh, P.; Fauria, K.; Van Dooren, V.; et al. Lapatinib with Trastuzumab for HER2-Positive Early Breast Cancer (NeoALTTO): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2012, 379, 633–640. [Google Scholar] [CrossRef]
- Di Cosimo, S.; Appierto, V.; Pizzamiglio, S.; Tiberio, P.; Iorio, M.V.; Hilbers, F.; de Azambuja, E.; de la Peña, L.; Izquierdo, M.; Huober, J.; et al. Plasma MiRNA Levels for Predicting Therapeutic Response to Neoadjuvant Treatment in HER2-Positive Breast Cancer: Results from the NeoALTTO Trial. Clin. Cancer Res. 2019, 25, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, S.; Appierto, V.; Pizzamiglio, S.; Silvestri, M.; Baselga, J.; Piccart, M.; Huober, J.; Izquierdo, M.; de la Pena, L.; Hilbers, F.S.; et al. Early Modulation of Circulating MicroRNAs Levels in HER2-Positive Breast Cancer Patients Treated with Trastuzumab-Based Neoadjuvant Therapy. Int. J. Mol. Sci. 2020, 21, 1386. [Google Scholar] [CrossRef]
- Pizzamiglio, S.; Cosentino, G.; Ciniselli, C.M.; De Cecco, L.; Cataldo, A.; Plantamura, I.; Triulzi, T.; El-Abed, S.; Wang, Y.; Bajji, M.; et al. What If the Future of HER2-Positive Breast Cancer Patients Was Written in MiRNAs? An Exploratory Analysis from NeoALTTO Study. Cancer Med. 2022, 11, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Stevic, I.; Müller, V.; Weber, K.; Fasching, P.A.; Karn, T.; Marmé, F.; Schem, C.; Stickeler, E.; Denkert, C.; van Mackelenbergh, M.; et al. Specific MicroRNA Signatures in Exosomes of Triple-Negative and HER2-Positive Breast Cancer Patients Undergoing Neoadjuvant Therapy within the GeparSixto Trial. BMC Med. 2018, 16, 179. [Google Scholar] [CrossRef]
- Liu, B.; Su, F.; Lv, X.; Zhang, W.; Shang, X.; Zhang, Y.; Zhang, J. Serum MicroRNA-21 Predicted Treatment Outcome and Survival in HER2-Positive Breast Cancer Patients Receiving Neoadjuvant Chemotherapy Combined with Trastuzumab. Cancer Chemother. Pharmacol. 2019, 84, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Wang, Y.; Peng, J.; Yuan, C.; Zhou, L.; Xu, S.; Lin, Y.; Du, Y.; Yang, F.; et al. Serum MiR-222-3p as a Double-Edged Sword in Predicting Efficacy and Trastuzumab-Induced Cardiotoxicity for HER2-Positive Breast Cancer Patients Receiving Neoadjuvant Target Therapy. Front. Oncol. 2020, 10, 631. [Google Scholar] [CrossRef]
- Ding, S.; Huang, H.; Xu, Y.; Zhu, H.; Zhong, C. MiR-222 in Cardiovascular Diseases: Physiology and Pathology. Biomed. Res. Int. 2017, 2017, 4962426. [Google Scholar] [CrossRef]
- Iorio, M.V.; Casalini, P.; Piovan, C.; Di Leva, G.; Merlo, A.; Triulzi, T.; Ménard, S.; Croce, C.M.; Tagliabue, E. MicroRNA-205 Regulates HER3 in Human Breast Cancer. Cancer Res. 2009, 69, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.; Piovan, C.; Plantamura, I.; D’Ippolito, E.; Camelliti, S.; Casalini, P.; Giussani, M.; Déas, O.; Cairo, S.; Judde, J.-G.; et al. MiR-205 as Predictive Biomarker and Adjuvant Therapeutic Tool in Combination with Trastuzumab. Oncotarget 2018, 9, 27920–27928. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Müller, V.; Oliveira-Ferrer, L.; Steinbach, B.; Pantel, K.; Schwarzenbach, H. Interplay of LncRNA H19/MiR-675 and LncRNA NEAT1/MiR-204 in Breast Cancer. Mol. Oncol. 2019, 13, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
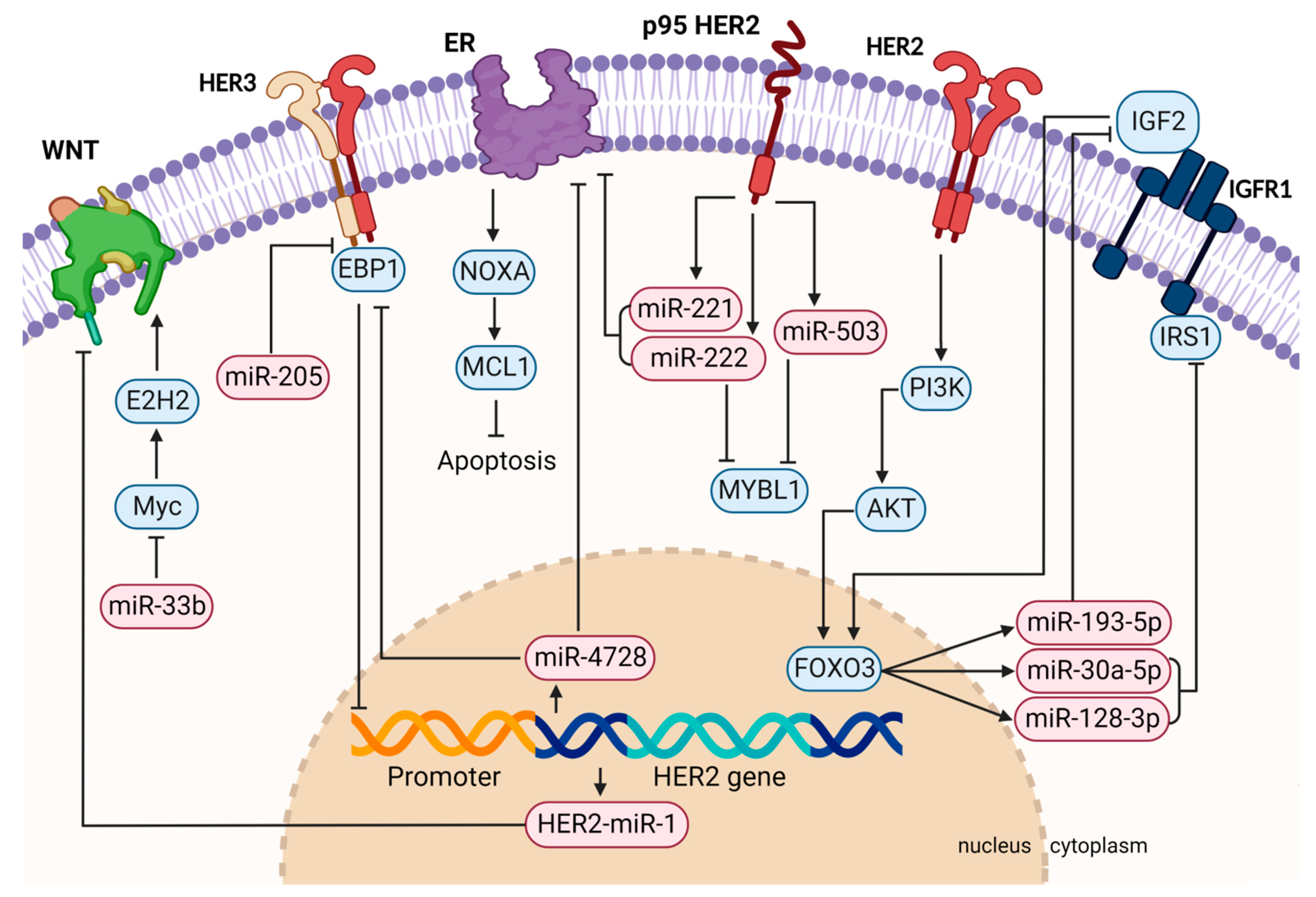
| miRNA | Reference | Target | Functional Role |
|---|---|---|---|
| miR-128-3p, miR-30a-5p, miR-193-5p | Luo et al. (2021) [45] | IRS1 and IGF2 | Regulation of IGFR1 pathway |
| miR-33b | Pattanayak et al. (2020) [56] | MYC | Promotion of apoptosis and reduction of invasion and migration |
| HER2-miR1 | Shabaninejad et al. (2022) [51] | Wnt pathway | Oncosuppressive intronic miRNA |
| miR-4728 | Zhou et al. (2021), Rui et al. (2022), Lu et al. (2011), Floros et al. (2018) [46,47,48,49] | EBP1 and ESR1 | Promotion of cell survival and response to anti-HER2 therapy; reduction of sensitivity to hormonal therapy |
| miR-221, miR-222, miR-503 | Gorbatenko et al. (2019) [41] | ESR1 and MYBL1 | Reduction of response to endocrine therapy and increase of cell mobility |
| miR-101-5p, miR-518a-5p, miR-19b-2-5p, miR-1237-3p, miR-29a-3p, miR-29c-3p, miR-106a-5p, miR-744-3p | Normann et al. (2021) [53] | Promotion of response to anti-HER2 therapy and prognostic role | |
| miR-101-5p | Normann et al. (2022) [54] | Downregulated in HER2+ breast cancer patients | |
| miR-567 | Han et al. (2020) [55] | ATG5 | Promotion of response to anti-HER2 therapy |
| miR-23b-3p, miR-195-5p, miR-656-5p, miR-340-5p | Rezaei et al. (2019) [9] | Drug resistance pathways | Dysregulated in resistant cells |
| miR-92b-3p | Liang et al. (2021) [44] | circCDYL | Reduction of cell proliferation and prognostic role |
| miRNA | Reference | Value in HER2+ Breast Cancer |
|---|---|---|
| miR-451a, miR-16-5p, miR-17-3p, miR-940 | Li H et al. (2018) [60] | Predictor of response and prognostic role |
| miR-27b, miR-433, miR-16, miR-328, miR-660, miR-422a | Stevic et al. (2018) [67] | Predictive role, association with N-status, distinction between TNBC and HER2+ BC |
| miR-21 | Liu et al. (2019) [68] | Predictor of response |
| miR-222-3p | Zhang et al. (2020) [69] | Predictive and prognostic role and association with cardiotoxicity and anemia, side effects of trastuzumab therapy |
| miR-205 | Cataldo et al. (2018) [72] | Predictor of response and prognostic role |
| miR-675, miR-204 | Müller et al. (2019) [73] | Indicators of disease aggressiveness |
| miR-100-5p, miR-374a-5p, miR-574-3p, miR-140-5p, miR-328-3p, miR-145-5p, miR-34a-5p, miR-98-5p, miR-100-5p, miR-144-3p, miR-362-3p, miR-197-3p, miR-320c, miR-100-5p, miR-376c-3p, miR-874-3p, | Di Cosimo et al. (2019) [64] | Predictive and prognostic role |
| miR-148a-3p, miR-374a-5p | Di Cosimo et al. (2020) [65] | Predictive role |
| miR-215-5p, miR-30c-2-3p, miR-153-3p, miR-219a-5p, miR-31-3p, miR-382-3p | Pizzamiglio and Cosentino et al. (2021) [66] | Predictive and prognostic role |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogazzi, V.; Kapahnke, M.; Cataldo, A.; Plantamura, I.; Tagliabue, E.; Di Cosimo, S.; Cosentino, G.; Iorio, M.V. The Role of MicroRNAs in HER2-Positive Breast Cancer: Where We Are and Future Prospective. Cancers 2022, 14, 5326. https://doi.org/10.3390/cancers14215326
Fogazzi V, Kapahnke M, Cataldo A, Plantamura I, Tagliabue E, Di Cosimo S, Cosentino G, Iorio MV. The Role of MicroRNAs in HER2-Positive Breast Cancer: Where We Are and Future Prospective. Cancers. 2022; 14(21):5326. https://doi.org/10.3390/cancers14215326
Chicago/Turabian StyleFogazzi, Valentina, Marcel Kapahnke, Alessandra Cataldo, Ilaria Plantamura, Elda Tagliabue, Serena Di Cosimo, Giulia Cosentino, and Marilena V. Iorio. 2022. "The Role of MicroRNAs in HER2-Positive Breast Cancer: Where We Are and Future Prospective" Cancers 14, no. 21: 5326. https://doi.org/10.3390/cancers14215326
APA StyleFogazzi, V., Kapahnke, M., Cataldo, A., Plantamura, I., Tagliabue, E., Di Cosimo, S., Cosentino, G., & Iorio, M. V. (2022). The Role of MicroRNAs in HER2-Positive Breast Cancer: Where We Are and Future Prospective. Cancers, 14(21), 5326. https://doi.org/10.3390/cancers14215326









