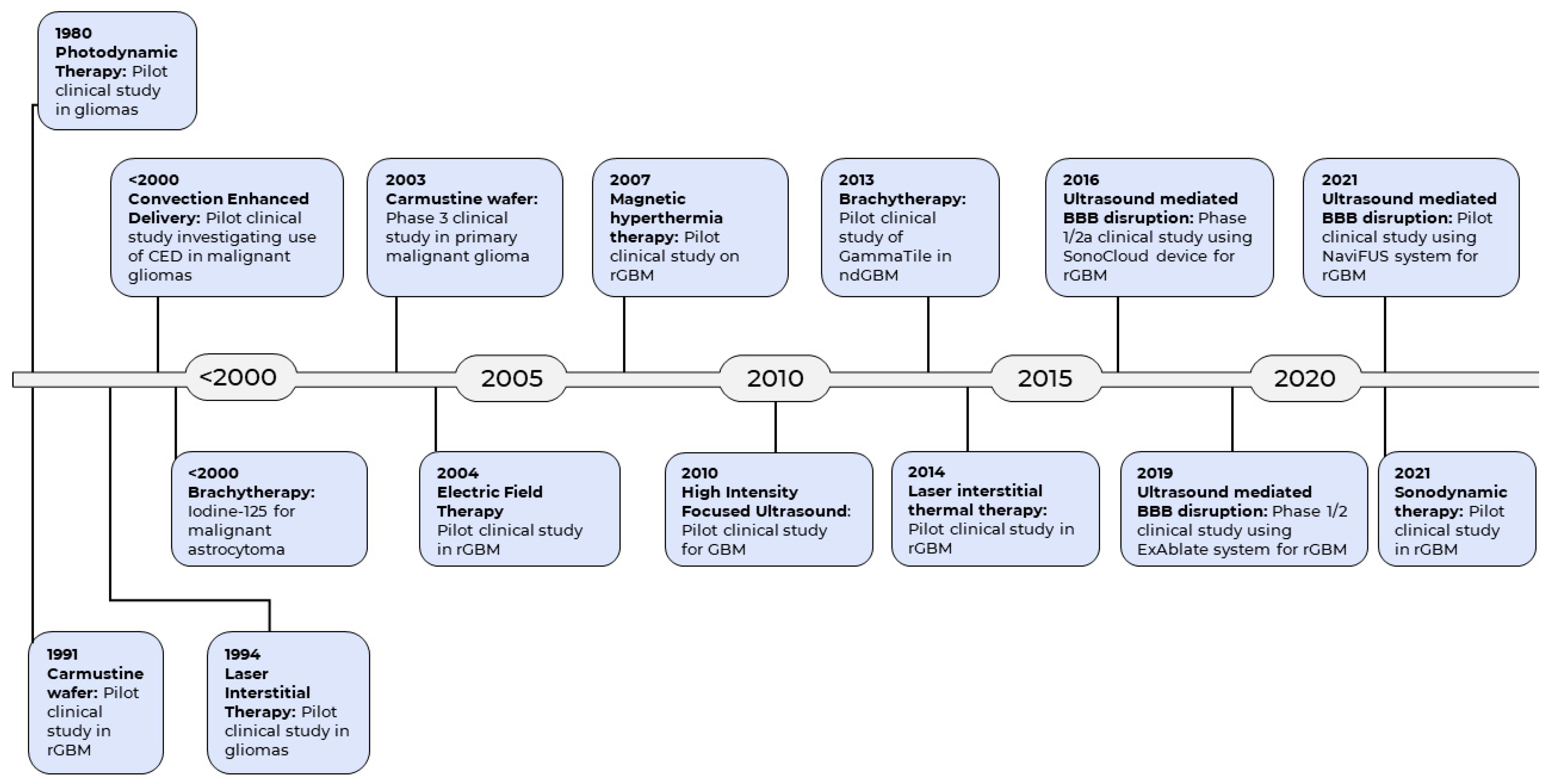
Medical Device Advances in the Treatment of Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
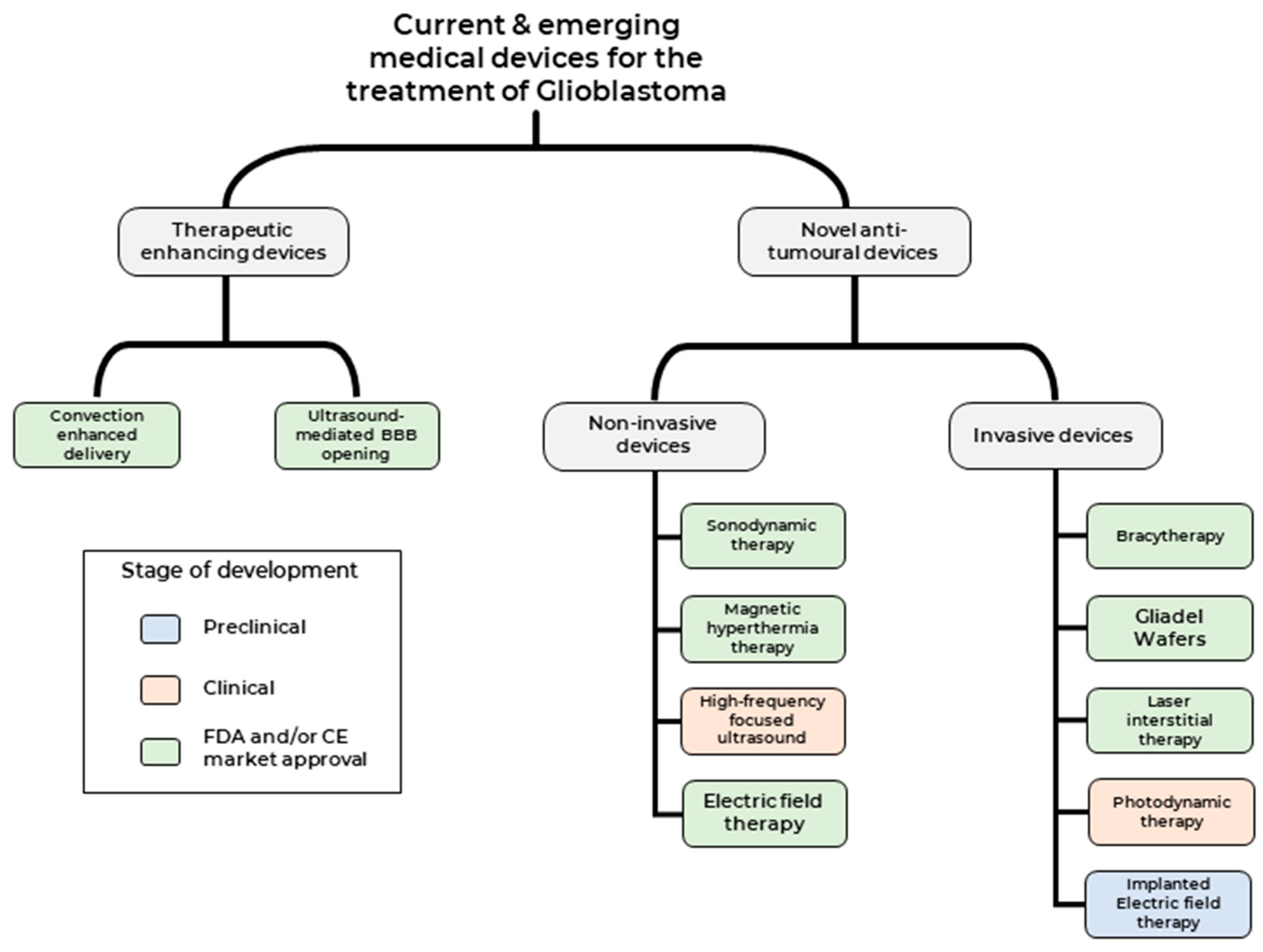
2. Medical Devices Used to Enhance Current Therapeutic Modalities
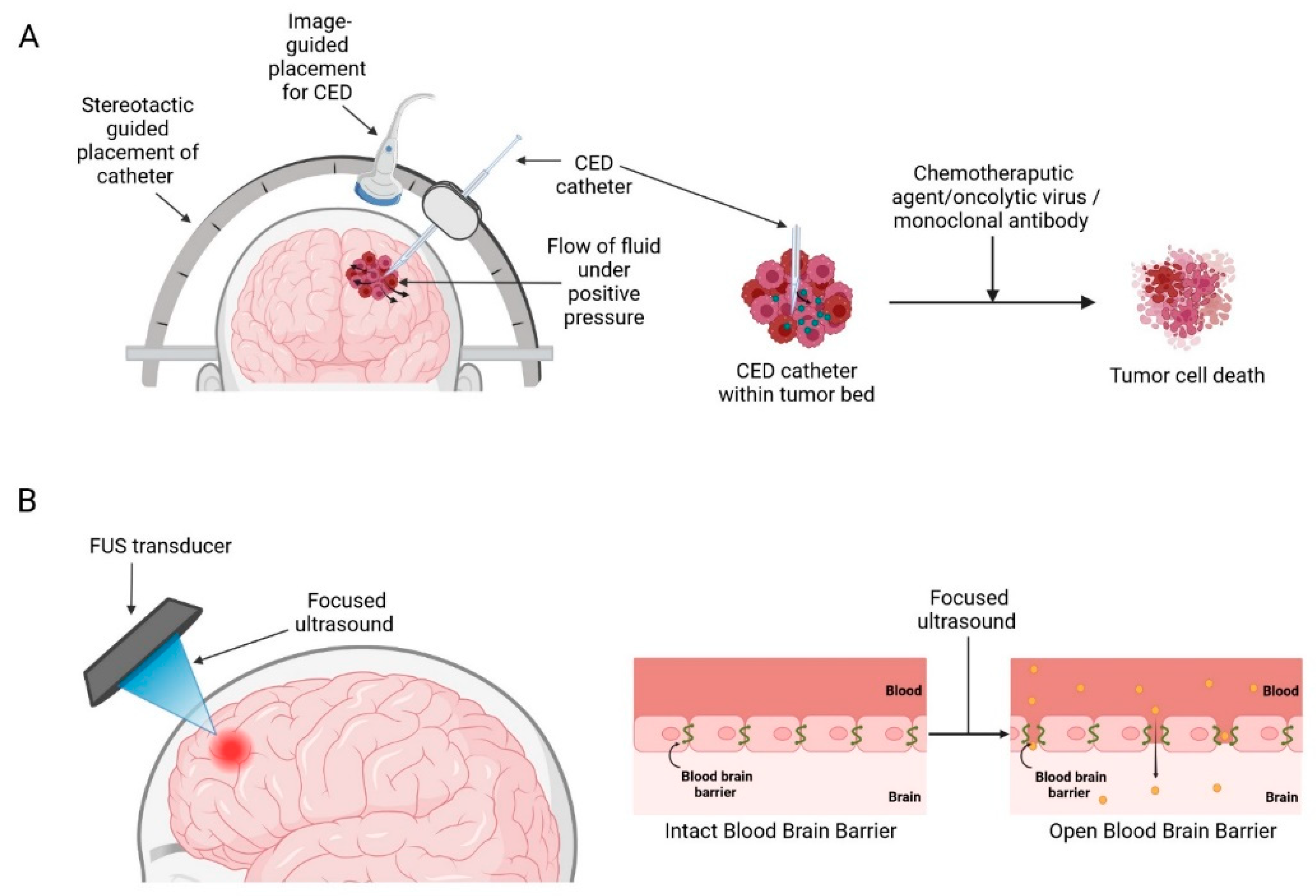
2.1. Convection-Enhanced Delivery
2.2. CED with Chemotherapeutic Agents
2.3. CED with Viral Vectors
2.4. CED with Monoclonal Antibody
2.5. Ultrasound-Mediated BBB Opening
3. Medical Devices Used to Deliver Novel Anti-Tumour Therapeutic Modalities
3.1. Non-Invasive Medical Devices
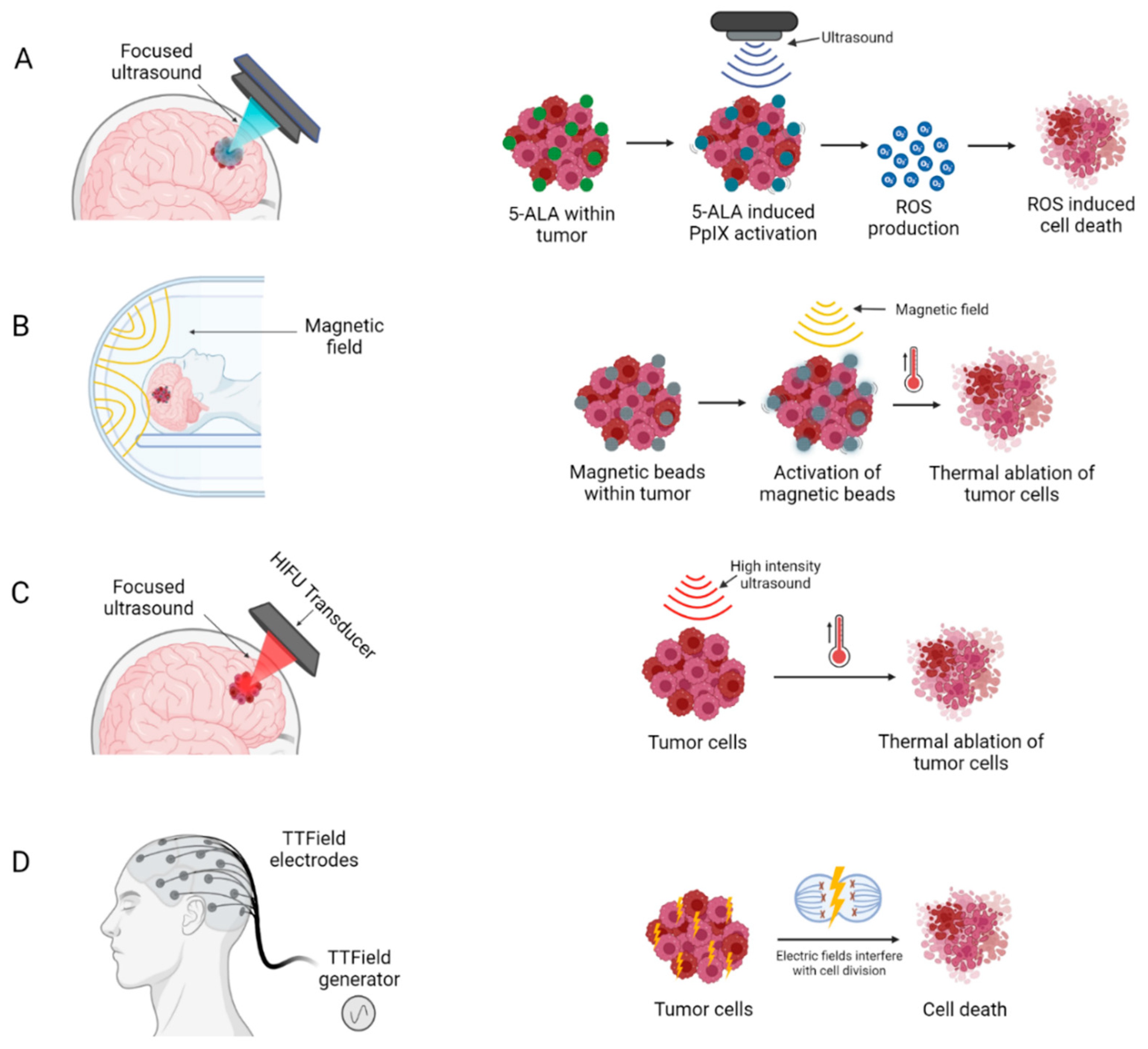
3.1.1. Sonodynamic Therapy
3.1.2. Magnetic Hyperthermia Therapy
3.1.3. High-Intensity Focused Ultrasound
3.1.4. Electric Field Therapy
3.2. Invasive Medical Devices
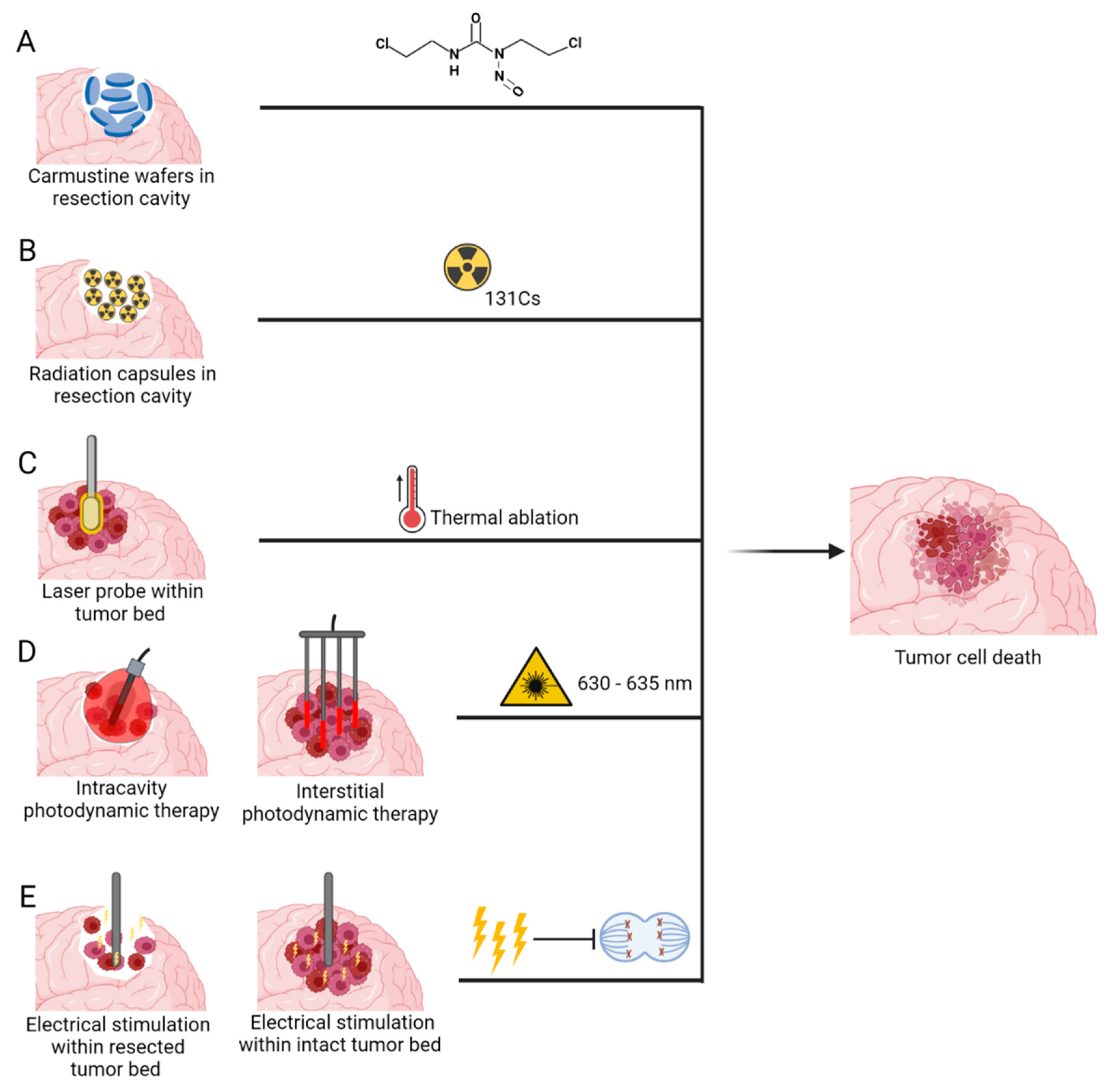
3.2.1. Carmustine Wafers
3.2.2. Brachytherapy
3.2.3. Laser Interstitial Thermal Therapy
3.2.4. Photodynamic Therapy
3.2.5. Implantable Electric Field Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Poon, M.T.C.; Sudlow, C.L.M.; Figueroa, J.D.; Brennan, P.M. Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11622. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumours Diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20 (Suppl. S4), iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, A.; Proietti, G.; Sica, G.; Scicchitano, B.M. Pathological and Molecular Features of Glioblastoma and Its Peritumoural Tissue. Cancers 2019, 11, 469. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Hill, R.; Pilkington, G.J.; Madureira, P.A. The Role of Hypoxia in Glioblastoma Invasion. Cells 2017, 6, 45. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Glioblastoma multiforme: Effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol. Lett. 2016, 12, 2283–2288. [Google Scholar] [CrossRef]
- Murat, A.; Migliavacca, E.; Gorlia, T.; Lambiv, W.L.; Shay, T.; Hamou, M.-F.; De Tribolet, N.; Regli, L.; Wick, W.; Kouwenhoven, M.; et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J. Clin. Oncol. 2008, 26, 3015–3024. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Kumaria, A. Stem cell-based therapies and glioblastoma: A seminal matter. Hematol. Oncol. Stem Cell Ther. 2021, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; DeCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumour Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e6. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neuro-Oncol. 2016, 130, 269–282. [Google Scholar] [CrossRef]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar]
- Showalter, T.N.; Andrel, J.; Andrews, D.W.; Curran, W.J.; Daskalakis, C.; Werner-Wasik, M. Multifocal glioblastoma multiforme: Prognostic factors and patterns of progression. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 820–824. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, W.; Sun, T.; Wang, L.; Du, C.; Hu, Y.; Liu, W.; Feng, F.; Chen, Y.; Sun, H. Therapeutic strategies of glioblastoma (GBM): The current advances in the molecular targets and bioactive small molecule compounds. Acta Pharm. Sin. B 2022, 12, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- Niedbała, M.; Malarz, K.; Sharma, G.; Kramer-Marek, G.; Kaspera, W. Glioblastoma: Pitfalls and Opportunities of Immunotherapeutic Combinations. Onco Targets Ther. 2022, 15, 437–468. [Google Scholar] [CrossRef]
- Rong, L.; Ni Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.; Di Nunno, V.; Franceschi, E.; Tosoni, A.; Bartolini, S.; Brandes, A.A. Pharmacotherapeutic Treatment of Glioblastoma: Where Are We to Date? Drugs 2022, 82, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Cloughesy, T.F.; Maus, M.V.; Prins, R.M.; A Reardon, D.; Sonabend, A.M. Emerging immunotherapies for malignant glioma: From immunogenomics to cell therapy. Neuro-Oncology 2020, 22, 1425–1438. [Google Scholar] [CrossRef]
- Sun, R.; Cuthbert, H.; Watts, C. Fluorescence-Guided Surgery in the Surgical Treatment of Gliomas: Past, Present and Future. Cancers 2021, 13, 3508. [Google Scholar] [CrossRef]
- Behbahani, M.; Keisham, B.; Rosinski, C.L.; Berry, V.; Mehta, A.I. Intraoperative imaging device for glioblastoma multiforme surgery: Review of Raman-based intraoperative imaging and introduction of a novel handheld probe technology. J. Raman Spectrosc. 2021, 52, 1228–1236. [Google Scholar] [CrossRef]
- McCrorie, P.; Vasey, C.E.; Smith, S.J.; Marlow, M.; Alexander, C.; Rahman, R. Biomedical engineering approaches to enhance therapeutic delivery for malignant glioma. J. Control. Release 2020, 328, 917–931. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; John, J.A.S.; Ekberg, J.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood-Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Brady, M.L.; Rodríguez-Ponce, M.I.; Hartlep, A.; Pedain, C.; Sampson, J.H. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg. Focus 2006, 20, E12. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brewer, C.; Barnett, G.H.; Mohammadi, A.M.; Peereboom, D.M.; Ahluwalia, M.S.; Gao, S. First-in-human evaluation of the Cleveland Multiport Catheter for convection-enhanced delivery of topotecan in recurrent high-grade glioma: Results of pilot trial 1. J Neurosurg. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Wang, J.L.; Barth, R.F.; Cavaliere, R.; Puduvalli, V.K.; Giglio, P.; Lonser, R.R.; Elder, J.B. Phase I trial of intracerebral convection-enhanced delivery of carboplatin for treatment of recurrent high-grade gliomas. PLoS ONE 2020, 15, e0244383. [Google Scholar] [CrossRef]
- Bruce, J.N.; Fine, R.L.; Canoll, P.; Yun, J.; Kennedy, B.C.; Rosenfeld, S.S.; Sands, S.A.; Surapaneni, K.; Lai, R.; Yanes, C.L.; et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 2011, 69, 1272–1279; discussion 1279–1280. [Google Scholar] [CrossRef]
- Kicielinski, K.P.; Chiocca, E.A.; Yu, J.S.; Gill, G.M.; Coffey, M.; Markert, J.M. Phase 1 clinical trial of intratumoural reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol. Ther. 2014, 22, 1056–1062. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, E.H.P.; Kleijn, A.; Van Beusechem, V.W.; Noske, D.; Lamers, C.H.; De Goede, A.L.; Idema, S.; Hoefnagel, D.; Kloezeman, J.J.; Fueyo, J.; et al. Convection Enhanced Delivery of the Oncolytic Adenovirus Delta24-RGD in Patients with Recurrent GBM: A Phase I Clinical Trial Including Correlative Studies. Clin. Cancer Res. 2022, 28, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Nwagwu, C.D.; Immidisetti, A.V.; Bukanowska, G.; Vogelbaum, M.A.; Carbonell, A.-M. Convection-Enhanced Delivery of a First-in-Class Anti-β1 Integrin Antibody for the Treatment of High-Grade Glioma Utilizing Real-Time Imaging. Pharmaceutics 2020, 13, 40. [Google Scholar] [CrossRef]
- Yung, W.K.; Mechtler, L.; Gleason, M.J. Intravenous carboplatin for recurrent malignant glioma: A phase II study. J. Clin. Oncol. 1991, 9, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.D.; Warnick, R.; Mack, E.E.; Chandler, K.L.; Rabbitt, J.; Page, M.; Malec, M. Intravenous carboplatin for recurrent gliomas. A dose-escalating phase II trial. Am. J. Clin. Oncol. 1996, 19, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Poisson, M.; Chiras, J.; Delattre, J.Y. Treatment of recurrent malignant supratentorial gliomas with carboplatin (CBDCA). J. Neuro-Oncol. 1991, 10, 139–144. [Google Scholar] [CrossRef]
- Upadhyayula, P.S.; Spinazzi, E.F.; Argenziano, M.G.; Canoll, P.; Bruce, J.N. Convection Enhanced Delivery of Topotecan for Gliomas: A Single-Center Experience. Pharmaceutics 2020, 13, 39. [Google Scholar] [CrossRef]
- Everson, R.G.; Gromeier, M.; Sampson, J. Viruses in the treatment of malignant glioma. Expert Rev. Neurother. 2007, 7, 321–324. [Google Scholar] [CrossRef]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.-X.; Levin, A.V.; Yung, W.K.A.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gomez-Manzano, C.; Bekele, B.N.; Yung, W.A.; Fueyo, J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007, 67, 11499–11504. [Google Scholar] [CrossRef]
- Alonso, M.M.; Jiang, H.; Yokoyama, T.; Xu, J.; Bekele, N.B.; Lang, F.F.; Kondo, S.; Gomez-Manzano, C.; Fueyo, J. Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol. Ther. 2008, 16, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Lamfers, M.L.; Idema, S.; Bosscher, L.; Heukelom, S.; Moeniralm, S.; Van Der Meulen-Muileman, I.H.; Overmeer, R.M.; Van Der Valk, P.; Van Beusechem, V.W.; Gerritsen, W.R.; et al. Differential effects of combined Ad5- delta 24RGD and radiation therapy in in vitro versus in vivo models of malignant glioma. Clin. Cancer Res. 2007, 13, 7451–7458. [Google Scholar] [CrossRef]
- Jiang, H.; Clise-Dwyer, K.; Ruisaard, K.E.; Fan, X.; Tian, W.; Gumin, J.; Lamfers, M.L.; Kleijn, A.; Lang, F.F.; Yung, W.-K.A.; et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE 2014, 9, e97407. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, W.S.; DeLay, M.; Jahangiri, A.; Park, C.C.; Aghi, M.K. β1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 2013, 73, 3145–3154. [Google Scholar] [CrossRef]
- Jahangiri, A.; Aghi, M.K.; Carbonell, W.S. Carbonell, β1 integrin: Critical path to antiangiogenic therapy resistance and beyond. Cancer Res. 2014, 74, 3–7. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neuro-Oncol. 2021, 151, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Horodyckid, C.; Canney, M.; Vignot, A.; Boisgard, R.; Drier, A.; Huberfeld, G.; François, C.; Prigent, A.; Santin, M.D.; Adam, C.; et al. Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: A multiparametric study in a primate model. J. Neurosurg. 2017, 126, 1351–1361. [Google Scholar] [CrossRef]
- Beccaria, K.; Canney, M.; Goldwirt, L.; Fernandez, C.; Adam, C.; Piquet, J.; Autret, G.; Clément, O.; Lafon, C.; Chapelon, J.-Y.; et al. Opening of the blood-brain barrier with an unfocused ultrasound device in rabbits. J. Neurosurg. 2013, 119, 887–898. [Google Scholar] [CrossRef]
- Dréan, A.; Lemaire, N.; Bouchoux, G.; Goldwirt, L.; Canney, M.; Goli, L.; Bouzidi, A.; Schmitt, C.; Guehennec, J.; Verreault, M.; et al. Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J. Neuro-Oncol. 2019, 144, 33–41. [Google Scholar] [CrossRef]
- Liu, H.-L.; Hua, M.-Y.; Chen, P.-Y.; Chu, P.-C.; Pan, C.-H.; Yang, H.-W.; Huang, C.-Y.; Wang, J.-J.; Yen, T.-C.; Wei, K.-C. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010, 255, 415–425. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumours with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.J.; Jung, H.H.; Chang, W.S.; Choi, H.S.; Rachmilevitch, I.; Zadicario, E.; Chang, J.W. One-Year Outcome of Multiple Blood-Brain Barrier Disruptions With Temozolomide for the Treatment of Glioblastoma. Front. Oncol. 2020, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-T.; Chai, W.-Y.; Lin, Y.-J.; Lin, C.-J.; Chen, P.-Y.; Tsai, H.-C.; Huang, C.-Y.; Kuo, J.S.; Liu, H.-L.; Wei, K.-C. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumours. Sci. Adv. 2021, 7, eabd0772. [Google Scholar] [CrossRef] [PubMed]
- Jameel, A.; Bain, P.; Nandi, D.; Jones, B.; Gedroyc, W. Device profile of exAblate Neuro 4000, the leading system for brain magnetic resonance guided focused ultrasound technology: An overview of its safety and efficacy in the treatment of medically refractory essential tremor. Expert Rev. Med. Devices 2021, 18, 429–437. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Samiotaki, G.; Konofagou, E.E. Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 2257–2265. [Google Scholar] [CrossRef]
- Murray, L.J.; Bridgewater, C.; Levy, D. Carboplatin chemotherapy in patients with recurrent high-grade glioma. Clin. Oncol. (R. Coll. Radiol.) 2011, 23, 55–61. [Google Scholar] [CrossRef]
- Roci, E.; Cakani, B.; Brace, G.; Bushati, T.; Rroji, A.; Petrela, M.; Kaloshi, G. Platinum-based chemotherapy in recurrent high-grade glioma patients: Retrospective study. Med. Arch. 2014, 68, 140–143. [Google Scholar] [CrossRef]
- Chen, K.T.; Wei, K.C.; Liu, H.L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.-C.; Tsai, H.-C.; Lu, Y.-J.; Yang, H.-W.; Hua, M.-Y.; Wu, M.-F.; Chen, P.-Y.; Huang, C.-Y.; Yen, T.-C.; Liu, H.-L. Neuronavigation-guided focused ultrasound-induced blood-brain barrier opening: A preliminary study in swine. AJNR Am. J. Neuroradiol. 2013, 34, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Wei, K.C.; Liu, H.L. Focused Ultrasound Combined with Microbubbles in Central Nervous System Applications. Pharmaceutics 2021, 13, 1084. [Google Scholar] [CrossRef] [PubMed]
- McHale, A.P.; Callan, J.F.; Nomikou, N.; Fowley, C.; Callan, J.F. Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. Adv. Exp. Med. Biol. 2016, 880, 429–450. [Google Scholar] [PubMed]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Ishizuka, M.; Abe, F.; Sano, Y.; Takahashi, K.; Inoue, K.; Nakajima, M.; Kohda, T.; Komatsu, N.; Ogura, S.-I.; Tanaka, T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int. Immunopharmacol. 2011, 11, 358–365. [Google Scholar] [CrossRef]
- Ohmura, T.; Fukushima, T.; Shibaguchi, H.; Yoshizawa, S.; Inoue, T.; Kuroki, M.; Sasaki, K.; Umemura, S.-I. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res. 2011, 31, 2527–2533. [Google Scholar]
- Jeong, E.-J.; Seo, S.-J.; Ahn, Y.-J.; Choi, K.-H.; Kim, K.-H.; Kim, J.-K. Sonodynamically induced antitumour effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic rat glioma model. Ultrasound Med. Biol. 2012, 38, 2143–2150. [Google Scholar] [CrossRef]
- Bilmin, K.; Kujawska, T.; Secomski, W.; Nowicki, A.; Grieb, P. 5-Aminolevulinic acid-mediated sonosensitization of rat RG2 glioma cells in vitro. Folia Neuropathol. 2016, 54, 234–240. [Google Scholar] [CrossRef]
- Folaron, M.; Strawbridge, R.; Samkoe, K.S.; Filan, C.; Roberts, D.W.; Davis, S.C. Elucidating the kinetics of sodium fluorescein for fluorescence-guided surgery of glioma. J. Neurosurg. 2018, 131, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Zhang, L.; Wang, J.; Wang, Q.; Gao, J.; Fan, P. Investigation on interaction and sonodynamic damage of fluorescein derivants to bovine serum albumin (BSA) under ultrasonic irradiation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 110, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Sheybani, N.; Franzini, A.; Moore, D.; Cordeiro, D.; Sheehan, J.; Timbie, K.; Xu, Z. Fluorescein-mediated sonodynamic therapy in a rat glioma model. J. Neuro-Oncol. 2020, 148, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Raspagliesi, L.; D’Ammando, A.; Gionso, M.; Sheybani, N.D.; Lopes, M.-B.; Moore, D.; Allen, S.; Gatesman, J.; Porto, E.; Timbie, K.; et al. Intracranial Sonodynamic Therapy With 5-Aminolevulinic Acid and Sodium Fluorescein: Safety Study in a Porcine Model. Front. Oncol. 2021, 11, 679989. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; Pikis, S.; Padilla, F.; Prada, F.; Sheehan, J. Sonodynamic therapy for gliomas. J. Neuro-Oncol. 2022, 156, 1–10. [Google Scholar] [CrossRef]
- Gneveckow, U.; Jordan, A.; Scholz, R.; Brüß, V.; Waldöfner, N.; Ricke, J.; Feussner, A.; Hildebrandt, B.; Rau, B.; Wust, P. Description and characterization of the novel hyperthermia- and thermoablation-system MFH 300F for clinical magnetic fluid hyperthermia. Med. Phys. 2004, 31, 1444–1451. [Google Scholar] [CrossRef]
- AG, M. European CE Certificate (“European Certification”) and thus Official Approval of NanoTherm® Therapy for the Treatment of Brain Tumours. 2011. Available online: https://www.magforce.com/en/home/for_patients/ (accessed on 3 October 2022).
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; Von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neuro-Oncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoural thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neuro-Oncol. 2019, 141, 83–94. [Google Scholar] [CrossRef]
- Edison Investment Research Institute. MagForce Nanotechnologies Interim Results. 2019. Available online: https://www.magforce.com/fileadmin/user_upload/MagForce-The-land-of-opportunity-awaits-NanoTherm-2.pdf (accessed on 3 October 2022).
- Meng, Y.; Suppiah, S.; Mithani, K.; Solomon, B.; Schwartz, M.L.; Lipsman, N. Current and emerging brain applications of MR-guided focused ultrasound. J. Ther. Ultrasound 2017, 5, 26. [Google Scholar] [CrossRef]
- Wang, T.R.; Dallapiazza, R.; Elias, W.J. Neurological applications of transcranial high intensity focused ultrasound. Int. J. Hyperth. 2015, 31, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jung, S.E.; Kim, H.L.; Hahn, S.T.; Park, G.S.; Park, W.C. The potential role of dynamic MRI in assessing the effectiveness of high-intensity focused ultrasound ablation of breast cancer. Int. J. Hyperth. 2010, 26, 594–603. [Google Scholar] [CrossRef]
- Ram, Z.; Cohen, Z.R.; Harnof, S.; Tal, S.; Faibel, M.; Nass, D.; Maier, S.E.; Hadani, M.; Mardor, Y. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumour therapy. Neurosurgery 2006, 59, 949–955; discussion 955–956. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Clement, G.T.; Black, P.; Jolesz, F.; Hynynen, K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumours: Initial findings in 3 patients. Neurosurgery 2010, 66, 323–332; discussion 332. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Fandino, J.; Schwyzer, L.; O’Gorman, R.; Remonda, L.; Anon, J.; Martin, E.; Werner, B. First noninvasive thermal ablation of a brain tumour with MR-guided focused ultrasound. J. Ther. Ultrasound 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumour-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.U.; Bihari, F.; Whitehead, S.; Wong, E.; Schmid, S.; Hebb, O.M. In Vitro Validation of Intratumoural Modulation Therapy for Glioblastoma. Anticancer Res. 2016, 36, 71–80. [Google Scholar]
- Di Sebastiano, A.R.; Deweyert, A.; Benoît, S.; Iredale, E.; Xu, H.; De Oliveira, C.; Wong, E.; Schmid, S.; Hebb, M.O. Preclinical outcomes of Intratumoural Modulation Therapy for glioblastoma. Sci. Rep. 2018, 8, 7301. [Google Scholar] [CrossRef]
- Kumaria, A. Observations on the anti-glioma potential of electrical fields: Is there a role for surgical neuromodulation? Br. J. Neurosurg. 2021, 36, 564–568. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Liu, Y.; Ouyang, B.; Zhang, J.; Jin, H.; Yu, Z.; Liu, R.; Li, Z.; Jiang, L.; et al. An implantable ultrasound-powered device for the treatment of brain cancer using electromagnetic fields. Sci. Adv. 2022, 8, eabm5023. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef]
- Kirson, E.D.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumour models and human brain tumours. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef]
- Bokstein, F.; Blumenthal, D.; Limon, D.; Ben Harosh, C.; Ram, Z.; Grossman, R. Concurrent Tumour Treating Fields (TTFields) and Radiation Therapy for Newly Diagnosed Glioblastoma: A Prospective Safety and Feasibility Study. Front. Oncol. 2020, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Chaudhary, R.T.; Rogers, L.R.; Wei, W.; Brewer, C.J.; Peereboom, D.M.; Ahluwalia, M.S. Clinical outcomes of the combination of bevacizumab and ttfields in patients with recurrent glioblastoma: Results of a phase II clinical trial. J. Clin. Oncol. 2020, 38 (Suppl. S15), 2537. [Google Scholar] [CrossRef]
- Giladi, M.; Schneiderman, R.S.; Porat, Y.; Munster, M.; Itzhaki, A.; Mordechovich, D.; Cahal, S.; Kirson, E.D.; Weinberg, U.; Palti, Y. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumour treating fields. Pancreatology 2014, 14, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Munster, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Blat, R.; Zielinska-Chomej, K.; Hååg, P.; Bomzon, Z.; Kirson, E.D.; et al. Tumour treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat. Oncol. 2017, 12, 206. [Google Scholar] [CrossRef]
- Karanam, N.K.; Ding, L.; Aroumougame, A.; Story, M.D. Tumour treating fields cause replication stress and interfere with DNA replication fork maintenance: Implications for cancer therapy. Transl. Res. 2020, 217, 33–46. [Google Scholar] [CrossRef]
- Chen, D.; Le, S.B.; Hutchinson, T.E.; Calinescu, A.-A.; Sebastian, M.; Jin, D.; Liu, T.; Ghiaseddin, A.; Rahman, M.; Tran, D.D. Tumour Treating Fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J. Clin. Investig. 2022, 132, e149258. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Kumaria, A. Tumour Treating Fields: Additional Mechanisms and Additional Applications. J. Korean Neurosurg. Soc. 2021, 64, 469–471. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.; Shen, V.; Loudon, W.; Bota, A.D. First report of tumour treating fields use in combination with bevacizumab in a pediatric patient: A case report. CNS Oncol. 2017, 6, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Makimoto, A.; Nishikawa, R.; Terashima, K.; Kurihara, J.; Fujisaki, H.; Ihara, S.; Morikawa, Y.; Yuza, Y. Tumour-Treating Fields Therapy for Pediatric Brain Tumours. Neurol. Int. 2021, 13, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Mrugala, M.M.; Engelhard, H.; Tran, D.D.; Kew, Y.; Cavaliere, R.; Villano, J.L.; Bota, D.A.; Rudnick, J.; Sumrall, A.L.; Zhu, J.-J.; et al. Clinical practice experience with NovoTTF-100A™ system for glioblastoma: The Patient Registry Dataset (PRiDe). Semin. Oncol. 2014, 41 (Suppl. S6), S4–S13. [Google Scholar] [CrossRef]
- Wick, W. TTFields: Where does all the skepticism come from? Neuro-Oncology 2016, 18, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Chavez, G.; Furnback, W.; Chuang, P.-Y.; Wang, B.; Proescholdt, C.; Tang, C.-H. Health-Related Quality of Life for Patients Receiving Tumour Treating Fields for Glioblastoma. Front. Oncol. 2021, 11, 772261. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhao, Q.; Bai, L.; Chen, X.; Zhou, Z. Management of dermatologic adverse events associated with tumour treating fields in patients with glioblastoma multiforme: A 27-case series. Asia-Pac. J. Oncol. Nurs. 2022, 9, 100095. [Google Scholar] [CrossRef]
- Taphoorn, M.J.B.; Dirven, L.; Kanner, A.A.; Lavy-Shahaf, G.; Weinberg, U.; Taillibert, S.; Toms, S.A.; Honnorat, J.; Chen, T.C.; Sroubek, J.; et al. Influence of Treatment With Tumour-Treating Fields on Health-Related Quality of Life of Patients With Newly Diagnosed Glioblastoma: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 495–504. [Google Scholar] [CrossRef]
- Henriksson, R.; Asklund, T.; Poulsen, H.S. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: A review. J. Neuro-Oncol. 2011, 104, 639–646. [Google Scholar] [CrossRef]
- Olubajo, F.; Thorpe, A.; Davis, C.; Sinha, R.; Crofton, A.; Mills, S.J.; Williams, M.; Jenkinson, M.D.; Price, S.J.; Watts, C.; et al. Tumour treating fields in glioblastoma: Is the treatment tolerable, effective, and practical in UK patients? Br. J. Neurosurg. 2022, in press. [CrossRef]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Novocure. Novocure, inc 10-k Filings 2022. 2022. Available online: https://www.novocure.com/sec-filings/ (accessed on 26 August 2022).
- Bernard-Arnoux, F.; Lamure, M.; Ducray, F.; Aulagner, G.; Honnorat, J.; Armoiry, X. The cost-effectiveness of tumour-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro-Oncology 2016, 18, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Lassman, A.B. NovoTTF: Where to go from here? Neuro-Oncology 2017, 19, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Blumenthal, D.T.; Bush, N.A.O.; Kebir, S.; Lukas, R.V.; Muragaki, Y.; Zhu, J.-J.; Glas, M. Global post-marketing safety surveillance of Tumour Treating Fields (TTFields) in patients with high-grade glioma in clinical practice. J. Neuro-Oncol. 2020, 148, 489–500. [Google Scholar] [CrossRef]
- Lazaridis, L.; Schäfer, N.; Teuber-Hanselmann, S.; Blau, T.; Schmidt, T.; Oster, C.; Weller, J.; Tzaridis, T.; Pierscianek, D.; Keyvani, K.; et al. Tumour Treating Fields (TTFields) in combination with lomustine and temozolomide in patients with newly diagnosed glioblastoma. J. Cancer Res. Clin. Oncol. 2020, 146, 787–792. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Wafers, G. Gliadel (Polifeprosan 20 with Carmustine): Uses, Dosage, Side Effects, Interactions, Warning. Available online: https://www.rxlist.com/gliadel-drug.htm#indications (accessed on 26 August 2022).
- Bota, D.A.; Desjardins, A.; A Quinn, J.; Affronti, M.L.; Friedman, H.S. Interstitial chemotherapy with biodegradable BCNU (Gliadel) wafers in the treatment of malignant gliomas. Ther. Clin. Risk Manag. 2007, 3, 707–715. [Google Scholar]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology 2003, 5, 79–88. [Google Scholar] [CrossRef]
- Pallud, J.; Audureau, E.; Noel, G.; Corns, R.; Lechapt-Zalcman, E.; Duntze, J.; Pavlov, V.; Guyotat, J.; Hieu, P.D.; Le Reste, P.-J.; et al. Long-term results of carmustine wafer implantation for newly diagnosed glioblastomas: A controlled propensity-matched analysis of a French multicenter cohort. Neuro-Oncology 2015, 17, 1609–1619. [Google Scholar] [CrossRef]
- Price, S.J.; Whittle, I.R.; Ashkan, K.; Grundy, P.; Cruickshank, G.; UK-HGG Study Group NICE. guidance on the use of carmustine wafers in high grade gliomas: A national study on variation in practice. Br. J. Neurosurg. 2012, 26, 331–335. [Google Scholar] [CrossRef]
- Bregy, A.; Shah, A.; Diaz, M.V.; E Pierce, H.; Ames, P.L.; Diaz, D.; Komotar, R.J. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 2013, 13, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Pirzkall, A.; McGue, C.; Saraswathy, S.; Cha, S.; Liu, R.; Vandenberg, S.; Lamborn, K.R.; Berger, M.S.; Chang, S.M.; Nelson, S.J. Tumour regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro-Oncology 2009, 11, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Mizowaki, T.; Arakawa, Y.; Ogura, M.; Sakanaka, K.; Miyamoto, S.; Hiraoka, M. Initial and cumulative recurrence patterns of glioblastoma after temozolomide-based chemoradiotherapy and salvage treatment: A retrospective cohort study in a single institution. Radiat. Oncol. 2013, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Petrecca, K.; Guiot, M.-C.; Panet-Raymond, V.; Souhami, L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J. Neuro-Oncol. 2013, 111, 19–23. [Google Scholar] [CrossRef]
- Buszek, S.M.; Al Feghali, K.A.; ElHalawani, H.; Chevli, N.; Allen, P.K.; Chung, C. Optimal Timing of Radiotherapy Following Gross Total or Subtotal Resection of Glioblastoma: A Real-World Assessment using the National Cancer Database. Sci. Rep. 2020, 10, 4926. [Google Scholar] [CrossRef]
- Bartek, J.; Alattar, A.A.; Dhawan, S.; Ma, J.; Koga, T.; Nakaji, P.; Dusenbery, K.E.; Chen, C.C. Receipt of brachytherapy is an independent predictor of survival in glioblastoma in the Surveillance, Epidemiology, and End Results database. J. Neuro-Oncol. 2019, 145, 75–83. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Yondorf, M.Z.; Peng, L.; Trichter, S.; Nedialkova, L.; Sabbas, A.; Kulidzhanov, F.; Parashar, B.; Nori, D.; Chao, K.S.C.; et al. Phase I/II study of resection and intraoperative cesium-131 radioisotope brachytherapy in patients with newly diagnosed brain metastases. J. Neurosurg. 2014, 121, 338–348. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Smith, A.W.; Taube, S.; Yondorf, M.Z.; Parashar, B.; Trichter, S.; Nedialkova, L.; Sabbas, A.; Christos, P.; Ramakrishna, R.; et al. Cesium-131 brachytherapy for recurrent brain metastases: Durable salvage treatment for previously irradiated metastatic disease. J. Neurosurg. 2017, 126, 1212–1219. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Yondorf, M.Z.; Parashar, B.; Nori, D.; Chao, K.S.C.; Boockvar, J.A.; Pannullo, S.; Stieg, P.; Schwartz, T.H. The cost-effectiveness of surgical resection and cesium-131 intraoperative brachytherapy versus surgical resection and stereotactic radiosurgery in the treatment of metastatic brain tumours. J. Neuro-Oncol. 2016, 127, 145–153. [Google Scholar] [CrossRef]
- Gessler, D.J.; Ferreira, C.; Dusenbery, K.; Chen, C.C. GammaTile. Future Oncol. 2020, 16, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Gessler, D.J.; Neil, E.C.; Shah, R.; Levine, J.; Shanks, J.; Wilke, C.; Reynolds, M.; Zhang, S.; Özütemiz, C.; Gencturk, M.; et al. GammaTile® brachytherapy in the treatment of recurrent glioblastomas. Neuro-Oncol. Adv. 2022, 4, vdab185. [Google Scholar] [CrossRef]
- Budnick, H.C.; Richardson, A.M.; Shiue, K.; Watson, G.; Ng, S.K.; Le, Y.; Shah, M.V. GammaTile for Gliomas: A Single-Center Case Series. Cureus 2021, 13, e19390. [Google Scholar] [CrossRef] [PubMed]
- Wernicke, A.G.; Hirschfeld, C.B.; Smith, A.W.; Taube, S.; Yondorf, M.Z.; Parashar, B.; Nedialkova, L.; Kulidzhanov, F.; Trichter, S.; Sabbas, A.; et al. Clinical Outcomes of Large Brain Metastases Treated With Neurosurgical Resection and Intraoperative Cesium-131 Brachytherapy: Results of a Prospective Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1059–1068. [Google Scholar] [CrossRef]
- Rivard, M.J.; Ballester, F.; Butler, W.M.; DeWerd, L.A.; Ibbott, G.S.; Meigooni, A.S.; Melhus, C.S.; Mitch, M.G.; Nath, R.; Papagiannis, P. Supplement 2 for the 2004 update of the AAPM Task Group No. 43 Report: Joint recommendations by the AAPM and GEC-ESTRO. Med. Phys. 2017, 44, e297–e338. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Sterling, D.; Reynolds, M.; Dusenbery, K.; Chen, C.; Alaei, P. First clinical implementation of GammaTile permanent brain implants after FDA clearance. Brachytherapy 2021, 20, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Vitaz, T.W.; Warnke, P.C.; Tabar, V.; Gutin, P.H. Brachytherapy for brain tumours. J. Neuro-Oncol. 2005, 73, 71–86. [Google Scholar] [CrossRef]
- Missios, S.; Bekelis, K.; Barnett, G.H. Renaissance of laser interstitial thermal ablation. Neurosurg. Focus 2015, 38, E13. [Google Scholar] [CrossRef]
- Rahmathulla, G.; Recinos, P.F.; Kamian, K.; Mohammadi, A.M.; Ahluwalia, M.S.; Barnett, G.H. MRI-guided laser interstitial thermal therapy in neuro-oncology: A review of its current clinical applications. Oncology 2014, 87, 67–82. [Google Scholar] [CrossRef]
- Stafford, R.J.; Fuentes, D.; Elliott, A.A.; Weinberg, J.S.; Ahrar, K. Laser-induced thermal therapy for tumour ablation. Crit. Rev. Biomed. Eng. 2010, 38, 79–100. [Google Scholar] [CrossRef]
- Sloan, A.E.; Ahluwalia, M.S.; Valerio-Pascua, J.; Manjila, S.; Torchia, M.G.; Jones, S.E.; Sunshine, J.L.; Phillips, M.; Griswold, M.A.; Clampitt, M.; et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: Clinical article. J. Neurosurg. 2013, 118, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, N.V.; Danish, S.F. Intracranial MR-guided laser-induced thermal therapy: Single-center experience with the Visualase thermal therapy system. J. Neurosurg. 2016, 125, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.A.; Friedman, D.D.; Akbari, S.H.; Kim, A.H.; Tao, Y.; Luo, J.; Leuthardt, E.C. Glioblastoma Treated With Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy: Safety, Efficacy, and Outcomes. Neurosurgery 2019, 84, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.M.; Sharma, M.; Beaumont, T.L.; O Juarez, K.; Kemeny, H.; Dechant, C.; Seas, A.; Sarmey, N.; Lee, B.S.; Jia, X.; et al. Upfront Magnetic Resonance Imaging-Guided Stereotactic Laser-Ablation in Newly Diagnosed Glioblastoma: A Multicenter Review of Survival Outcomes Compared to a Matched Cohort of Biopsy-Only Patients. Neurosurgery 2019, 85, 762–772. [Google Scholar] [CrossRef]
- Beaumont, T.L.; Mohammadi, A.M.; Kim, A.H.; Barnett, G.H.; Leuthardt, E.C. Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy for Glioblastoma of the Corpus Callosum. Neurosurgery 2018, 83, 556–565. [Google Scholar] [CrossRef]
- Hawasli, A.H.; Kim, A.H.; Dunn, G.P.; Tran, D.D.; Leuthardt, E.C. Stereotactic laser ablation of high-grade gliomas. Neurosurg. Focus 2014, 37, E1. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Chugh, J.; Wright, C.H.; Alonso, F.; Hdeib, A.; Gittleman, H.; Barnholtz-Sloan, J.; Sloan, A.E. Laser interstitial thermal therapy followed by minimal-access transsulcal resection for the treatment of large and difficult to access brain tumors. Neurosurg. Focus 2016, 41, E14. [Google Scholar] [CrossRef]
- Kahn, T.; Bettag, M.; Ulrich, F.; Schwarzmaier, H.-J.; Schober, R.; Fürst, G.; Mödder, U. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J. Comput. Assist. Tomogr. 1994, 18, 519–532. [Google Scholar] [CrossRef]
- Carpentier, A.; McNichols, R.J.; Stafford, R.J.; Itzcovitz, J.; Guichard, J.-P.; Reizine, D.; Delaloge, S.; Vicaut, E.; Payen, D.; Gowda, A.; et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumours. Neurosurgery 2008, 63 (Suppl. S1), ONS21-8; discussion ONS28-9. [Google Scholar]
- Chen, C.; Lee, I.; Tatsui, C.; Elder, T.; Sloan, A.E. Laser interstitial thermotherapy (LITT) for the treatment of tumours of the brain and spine: A brief review. J. Neuro-Oncol. 2021, 151, 429–442. [Google Scholar] [CrossRef]
- Carpentier, A.; McNichols, R.J.; Stafford, R.J.; Guichard, J.-P.; Reizine, D.; Delaloge, S.; Vicaut, E.; Payen, D.; Gowda, A.; George, B. Laser thermal therapy: Real-time MRI-guided and computer-controlled procedures for metastatic brain tumours. Lasers Surg. Med. 2011, 43, 943–950. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumours: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.G.; Chang, S.M.; Gutin, P.H.; Malec, M.K.; McDermott, M.W.; Prados, M.D.; Wilson, C.B. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 1998, 42, 709–720; discussion 720–723. [Google Scholar] [CrossRef]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.A.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Reardon, D.A.; Coan, A.; Marcello, J.; Herndon, J.E.; Bailey, L.; Peters, K.B.; Friedman, H.S.; Vredenburgh, J.J. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer 2012, 118, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E., 2nd; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef] [PubMed]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.; Camphausen, K.; et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumour progression in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Lemos, A.M.; Gokaslan, A.; Cabrera-Aldana, E.E.; Ashary, A.; Olivi, A.; Quinones-Hinojosa, A. The butterfly effect on glioblastoma: Is volumetric extent of resection more effective than biopsy for these tumours? J. Neurooncol. 2014, 120, 625–634. [Google Scholar] [CrossRef]
- Shah, A.H.; Burks, J.D.; Buttrick, S.S.; Debs, L.; E Ivan, M.; Komotar, R.J. Laser Interstitial Thermal Therapy as a Primary Treatment for Deep Inaccessible Gliomas. Neurosurgery 2019, 84, 768–777. [Google Scholar] [CrossRef]
- Jethwa, P.R.; Barrese, J.C.; Gowda, A.; Shetty, A.; Danish, S.F. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: Initial experience. Neurosurgery 2012, 71 (Suppl. S1), 133–145. [Google Scholar] [CrossRef]
- Pruitt, R.; Gamble, A.; Black, K.; Schulder, M.; Mehta, A.D. Complication avoidance in laser interstitial thermal therapy: Lessons learned. J. Neurosurg. 2017, 126, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.D.; Barnett, G. The value of using a brain laser interstitial thermal therapy (LITT) system in patients presenting with high grade gliomas where maximal safe resection may not be feasible. Cost Eff. Resour. Alloc. 2016, 14, 6. [Google Scholar] [CrossRef]
- Raab, O. Ueber die wirkung fluoreszierenden stoffen. Infusoria Z Biol. 1900, 39, 524. [Google Scholar]
- Von Tappeiner, H. Uber die wirkung der photodynamischen (fluorescierenden) stoffe auf protozoen und enzyme. Dtsch. Arch. Klin. Med. 1904, 80, 427–487. [Google Scholar]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F.; Snell, M.E. Hematoporphyrin derivative: A possible aid in the diagnosis and therapy of carcinoma of the bladder. J. Urol. 1976, 115, 150–151. [Google Scholar] [CrossRef]
- Perria, C.; Capuzzo, T.; Cavagnaro, G.; Datti, R.; Francaviglia, N.; Rivano, C.; E Tercero, V. Fast attempts at the photodynamic treatment of human gliomas. J. Neurosurg. Sci. 1980, 24, 119–129. [Google Scholar]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2019, 6, 81. [Google Scholar] [CrossRef]
- Leroy, H.A.; Guérin, L.; Lecomte, F.; Baert, G.; Vignion, A.-S.; Mordon, S.; Reyns, N. Is interstitial photodynamic therapy for brain tumours ready for clinical practice? A systematic review. Photodiagn. Photodyn. Ther. 2021, 36, 102492. [Google Scholar] [CrossRef]
- Baran, T.M.; Foster, T.H. Comparison of flat cleaved and cylindrical diffusing fibers as treatment sources for interstitial photodynamic therapy. Med. Phys. 2014, 41, 022701. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Lietke, S.; Schmutzer, M.; Schwartz, C.; Weller, J.; Siller, S.; Aumiller, M.; Heckl, C.; Forbrig, R.; Niyazi, M.; Egensperger, R.; et al. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 2021, 13, 1767. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Fujimoto, S.; Yamaguchi, H.; Yamauchi, T.; Yoshimoto, T.; Tokuda, K. Photodynamic Therapy of Malignant Gliomas. Prog. Neurol. Surg. 2018, 32, 1–13. [Google Scholar] [PubMed]
- Kaneko, S.; Okura, I.; Tanaka, T. (Eds.) Photodynamic Applications (PDD, PDT) Using Aminolevulinic Acid in Neurosurgery; Aminolevulinic Acid: Science, Technology and Applications. Tokyo, SBI ALApromo; Tokyo Institute of Technology Press: Tokyo, Japan, 2011; pp. 119–140. (In Japanese) [Google Scholar]
- Krishnamurthy, S.; Powers, S.K.; Witmer, P.; Brown, T. Optimal light dose for interstitial photodynamic therapy in treatment for malignant brain tumours. Lasers Surg. Med. 2000, 27, 224–234. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.-A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neuro-Oncol. 2021, 152, 501–514. [Google Scholar]
- Vermandel, M.; Quidet, M.; Vignion-Dewalle, A.-S.; Leroy, H.-A.; Leroux, B.; Mordon, S.; Reyns, N. Comparison of different treatment schemes in 5-ALA interstitial photodynamic therapy for high-grade glioma in a preclinical model: An MRI study. Photodiagn. Photodyn. Ther. 2019, 25, 166–176. [Google Scholar] [CrossRef]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Guzauskas, G.F.; Pollom, E.L.; Stieber, V.W.; Wang, B.C.M.; Garrison, L.P., Jr. Tumour treating fields and maintenance temozolomide for newly-diagnosed glioblastoma: A cost-effectiveness study. J. Med. Econ. 2019, 22, 1006–1013. [Google Scholar] [CrossRef]
- Kumaria, A. Tumour treating fields in pediatric brain tumours: Overcoming challenges. Childs Nerv. Syst. 2022, 38, 1847–1848. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.P.W.; Finch, A.; Gerigk, M.; Triantis, I.F.; Watts, C.; Malliaras, G.G. Electrotherapies for Glioblastoma. Adv. Sci. 2021, 8, e2100978. [Google Scholar] [CrossRef] [PubMed]
- Iredale, E.; Deweyert, A.; Hoover, D.A.; Chen, J.Z.; Schmid, S.; Hebb, M.O.; Peters, T.M.; Wong, E. Optimization of multi-electrode implant configurations and programming for the delivery of non-ablative electric fields in intratumoural modulation therapy. Med. Phys. 2020, 47, 5441–5454. [Google Scholar] [CrossRef]
- Aryal, M.; Fischer, K.; Gentile, C.; Gitto, S.; Zhang, Y.-Z.; McDannold, N. Effects on P-Glycoprotein Expression after Blood-Brain Barrier Disruption Using Focused Ultrasound and Microbubbles. PLoS ONE 2017, 12, e0166061. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.L.; Raghavan, R.; Alexander, A.; Kubota, K.; Sillay, K.; Emborg, M.E. Pathways of infusate loss during convection-enhanced delivery into the putamen nucleus. Stereotact. Funct. Neurosurg. 2013, 91, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumours of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
| Study Design and Trial Ref | Indication | Therapeutic Agent | No. of Patients | Catheter | Infusion Rate (mL/h) | Volume Infused (mL) | Duration (h) | Median OS (Months) |
|---|---|---|---|---|---|---|---|---|
| Chemotherapeutic Agent | ||||||||
| Pilot trial NCT02278510 [37] | Recurrent HGG | Topotecan | 3 | 2 Cleveland Multiport catheters | 0.396 | 38 | 96 | n/a |
| Pilot trial NCT01644955 [38] | Grade III/IV gliomas | Carboplatin | 10 | 1–4 barium-impregnated CSF-ventricular catheters | 0.75 | 54 | 72 | 9.6 |
| Phase 1b dose-escalation study [39] | GBM | Topotecan | 10 | Implantable chronic infusion pump (Synchromed II, Medtronic) | 0.2 | n/a | 100 | n/a |
| Oncolytic Virus | ||||||||
| Phase 1 trial [40] | Grade III/IV gliomas | Purified reovirus | 18 | 1–4 CED catheters (Phoenix Biomedical) | 0.4 | n/a | 72 | 4.6 |
| Phase 1 trial NCT01491893 [41] | Grade IV malignant glioma | PVSRIPO | 61 | Medfusion 3500 or 3010 infusion pump and catheter | 0.5 | 3.25 | 6.5 | 12.5 |
| Phase 1 trial [42] | Recurrent GBM | Delta24-RGD | 20 | 2 CED catheters | 0.2 to 0.3 | n/a | 44 to 66.7 | 4.24 |
| Monoclonal Antibody | ||||||||
| Phase 1 trial NCT04608812 [43] | Grade III/IV gliomas | OS2966 | n/a | Infuseon Cleveland Multiport Catheter | Max 0.3 | Up to 4.8 | Up to 4 | n/a |
| Study Design and Trial Ref | Indication | Device | No. of Patients | Drug | FUS Parameters | Main Findings |
|---|---|---|---|---|---|---|
| Phase 1, single-arm trial NCT02343991 [61] | Grade III/IV gliomas | ExAblate Neuro 4000 (220 kHz) | 5 | Temozolomide or liposomal doxorubicin | 4–15 W, 0.74% DC for 50s | Safe and effective opening of BBB, with immediate 15–50% increase in contrast enhancement, and resolution 20 h later |
| Prospective single arm trial NCT03712293 [62] | GBM | ExAblate Neuro 4000 (220 kHz) | 6 | Temozolomide | 210s per target | Median survival has increased up to 14.6 months, the 2-year survival rate up to 27.2%, and the 5-year survival up to 10% |
| Phase 1/2a single arm trial NCT02253212 [63] | Recurrent GBM | SonoCloud (1.05 MHz) | 17 | Carboplatin | 0.5–1.1 MPa | BBB was disrupted at acoustic pressure levels up to 1.1 MPa without detectable adverse effects |
| Single arm pilot trial NCT02253212 [64] | Recurrent GBM | SonoCloud (1.05 MHz) | 21 | Carboplatin | 0.41–1.15 MPa | Patients with clear BBB disruption had an increased median OS of 12.94 months. |
| Phase 1, single-arm trial NCT03626896 [65] | Recurrent GBM | NaviFUS system (500 kHz) | 6 | n/a | Energy doses: 0.48, 0.58, 0.68 MI; total exposure time: 120s | Safe and reversible BBB opening using NaviFUS in patients with rGBM. |
| Study Design and Trial Ref | Indication | Intervention | No. of Patients | Median OS (Months) | Median PFS (Months) | Main Findings |
|---|---|---|---|---|---|---|
| Phase 3 randomised trial (EF-14) NCT00916409 [98] | ndGBM | NovoTTF-100A device, chemotherapy | 695 (466 TTFields, 229 chemo) | 6.7 vs. 4.0 | 20.9 vs. 16.0 | TTField improved OS with no further toxicity and negative effect on quality of life vs. chemo |
| Phase 3 randomised trial (EF-11) NCT00379470 [104] | rGBM | NovoTTF-100A device, chemotherapy | 237 (120 TTFields, 117 chemo) | 6.6 vs. 6.0 | 2.2 vs. 2.1 | TTField just as effective as chemo but with reduced severe adverse reactions |
| Single arm pilot trial (EF-07) [105] | rGBM | NovoTTF-100A device, chemotherapy | 10 | 14.3 (62.2 weeks) | 4.9 (26.1 weeks) | Mild to moderate contact dermatitis. No device related serious adverse events |
| Single arm pilot trial, NCT03780569 [106] | ndGBM | NovoTTF-200A, Radiotherapy (60Gy), temozolomide | 10 | - | 8.9 | Grade 1–2 TTFields related skin toxicity. |
| Single arm Phase 2 trial, NCT01894061 [107] | rGBM | NovoTTF-100A device, Bevacizumab | 23 | 10.5 | 4.1 |
| Study Design and Trial Ref | Indication | No. of Patients | Local FFP (%) | Median OS (Months) | Complications (Total %) |
|---|---|---|---|---|---|
| Prospective case series NCT04427384 [146] | Recurrent GBM | 22 | 81 | 24.4 | CSF leak, DVT, seizure (13.6%) |
| [147] | Recurrent gliomas | 7 | n/a | n/a | Radiation necrosis (7.6%) |
| Study Design and Trial Ref | LITT System | No. of Patients | Median Tumour Volume (cm3) | Median OS (Months) | Median PFS (Months) | Complications |
|---|---|---|---|---|---|---|
| [153] | NeuroBlate | 34 | 10.13 | Not reached | 5.1 | Neurological deficits (7), seizure (1), DVT (1), infection (2) |
| Phase 1 trial NCT00747253 [155] | NeuroBlate | 10 | 6.8 | 10.4 | n/a | Neurological deficits (2), ICH (1), DVT (2), PE (1) neutropenia (1), |
| Retrospective analysis [156] | Visualase | 87 | n/a | n/a | n/a | Neurological deficits (14), haemorrhage (3), refractory edema (5), infection (2), deaths (3) |
| Retrospective descriptive study [157] | NeuroBlate | 54 | 12.5 | 11.5 | 6.6 | cerebral oedema (3), seizures (3), hydrocephalus (1), infection (1), death (2) |
| Retrospective study [158] | NeuroBlate | 28 | 9.3 | 14.4 | 4.3 | Neurological deficits (6), ICH (2), DVT (1) |
| Retrospective analysis [159] | NeuroBlate | 15 | 18.7 | 7.2 | 3.4 | Neurological deficits (4), hydrocephalus (1), ventriculitis (1) |
| Pilot trial [160] | NeuroBlate | 17 | 11.6 | 10.9 | 7.6 | Transient aphasia (3), transient hemiparesis (3), DVT (1), meningitis (1) |
| Retrospective analysis [161] (Wright et al., 2016) | NeuroBlate | 10 | 38 | 16.1 | 9.3 | Neurological deficits (2), infection (3), hydrocephalus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foo, C.Y.; Munir, N.; Kumaria, A.; Akhtar, Q.; Bullock, C.J.; Narayanan, A.; Fu, R.Z. Medical Device Advances in the Treatment of Glioblastoma. Cancers 2022, 14, 5341. https://doi.org/10.3390/cancers14215341
Foo CY, Munir N, Kumaria A, Akhtar Q, Bullock CJ, Narayanan A, Fu RZ. Medical Device Advances in the Treatment of Glioblastoma. Cancers. 2022; 14(21):5341. https://doi.org/10.3390/cancers14215341
Chicago/Turabian StyleFoo, Cher Ying, Nimrah Munir, Ashwin Kumaria, Qasim Akhtar, Christopher J. Bullock, Ashwin Narayanan, and Richard Z. Fu. 2022. "Medical Device Advances in the Treatment of Glioblastoma" Cancers 14, no. 21: 5341. https://doi.org/10.3390/cancers14215341
APA StyleFoo, C. Y., Munir, N., Kumaria, A., Akhtar, Q., Bullock, C. J., Narayanan, A., & Fu, R. Z. (2022). Medical Device Advances in the Treatment of Glioblastoma. Cancers, 14(21), 5341. https://doi.org/10.3390/cancers14215341






