The Validity of Surrogate Endpoints in Sub Groups of Metastatic Colorectal Cancer Patients Defined by Treatment Class and KRAS Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
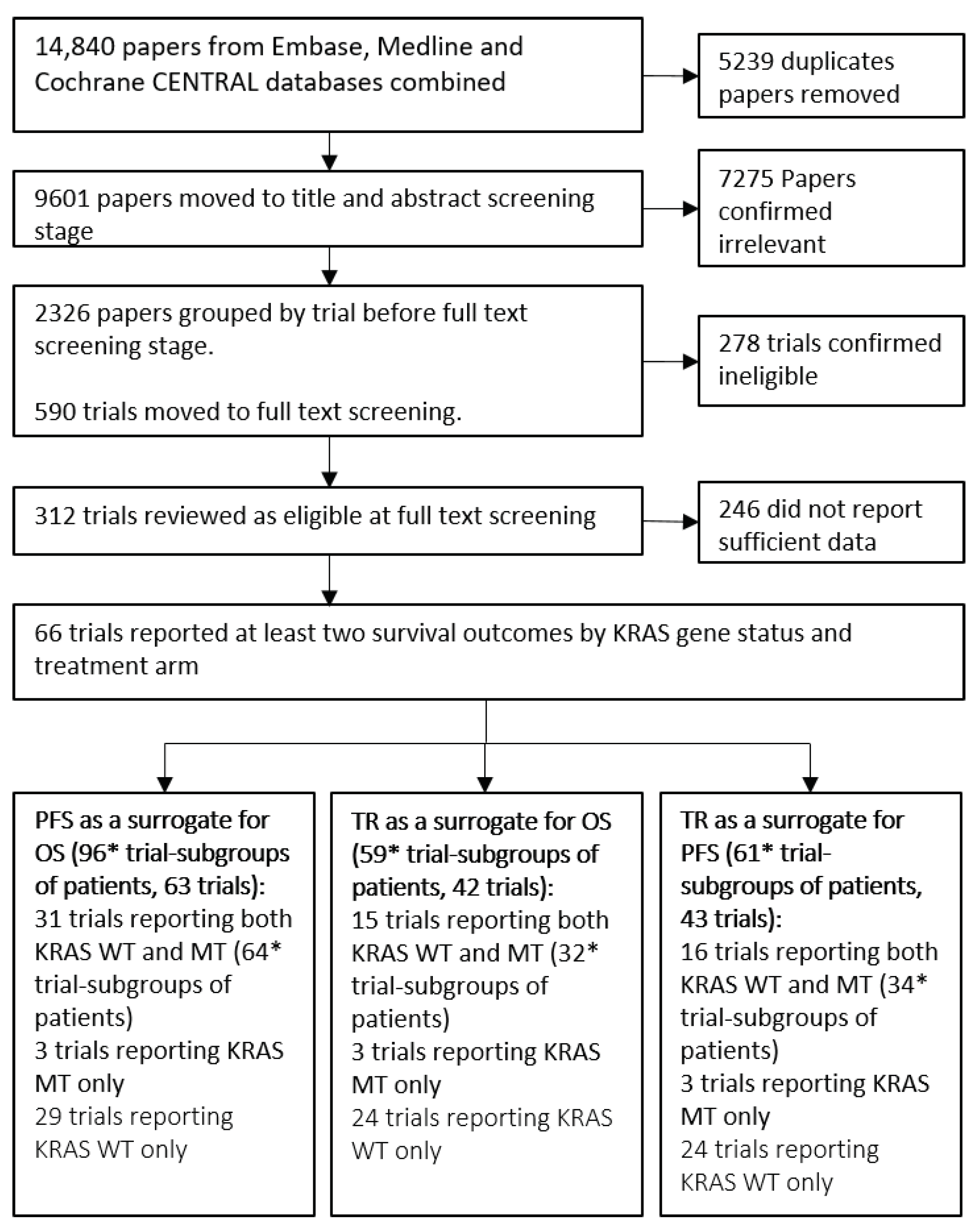
2.1. Trial Identification
2.2. Data Extraction
2.3. Statistical Methods
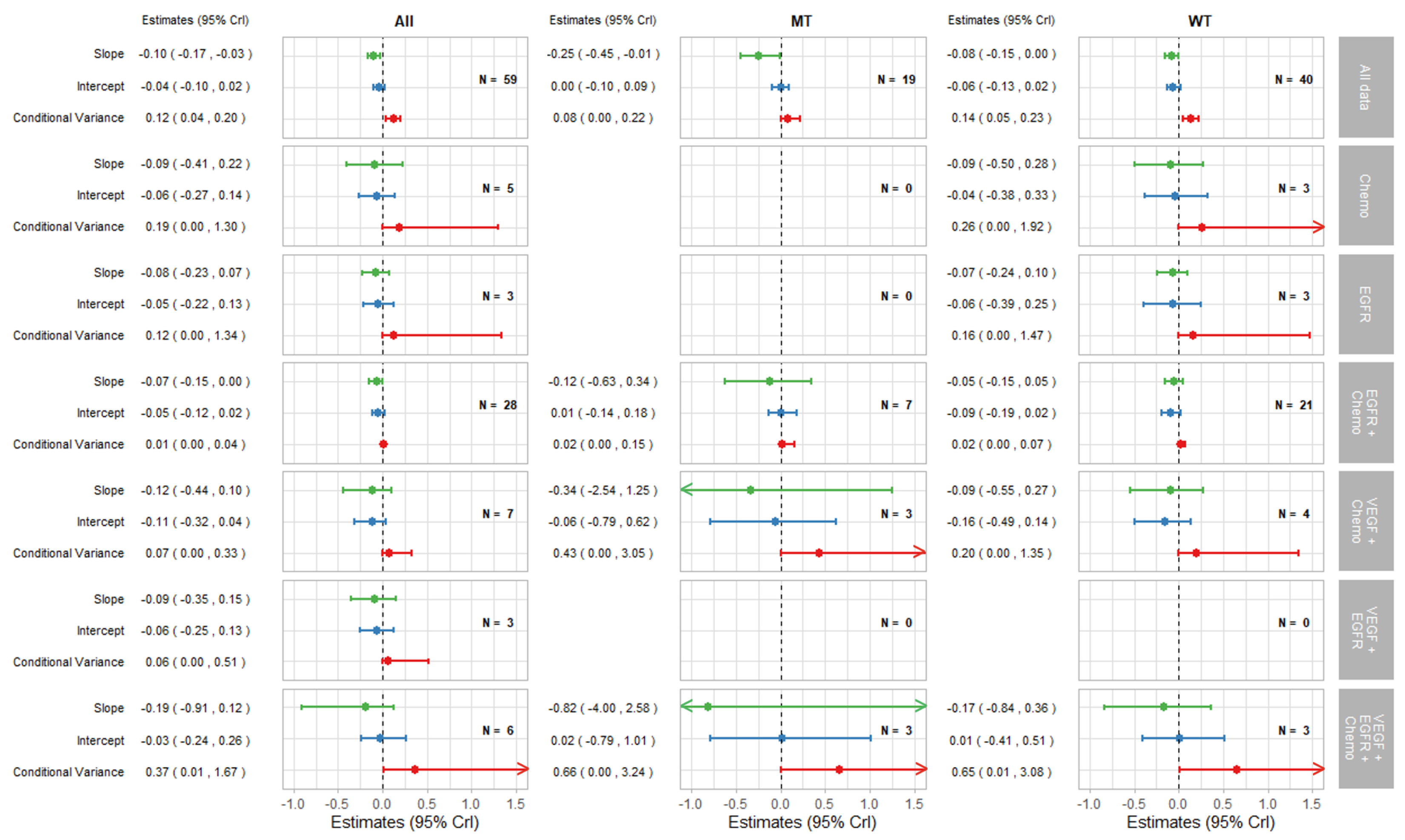
3. Results
3.1. Summary of Included Trials
3.2. Exploration of Surrogate Relationships
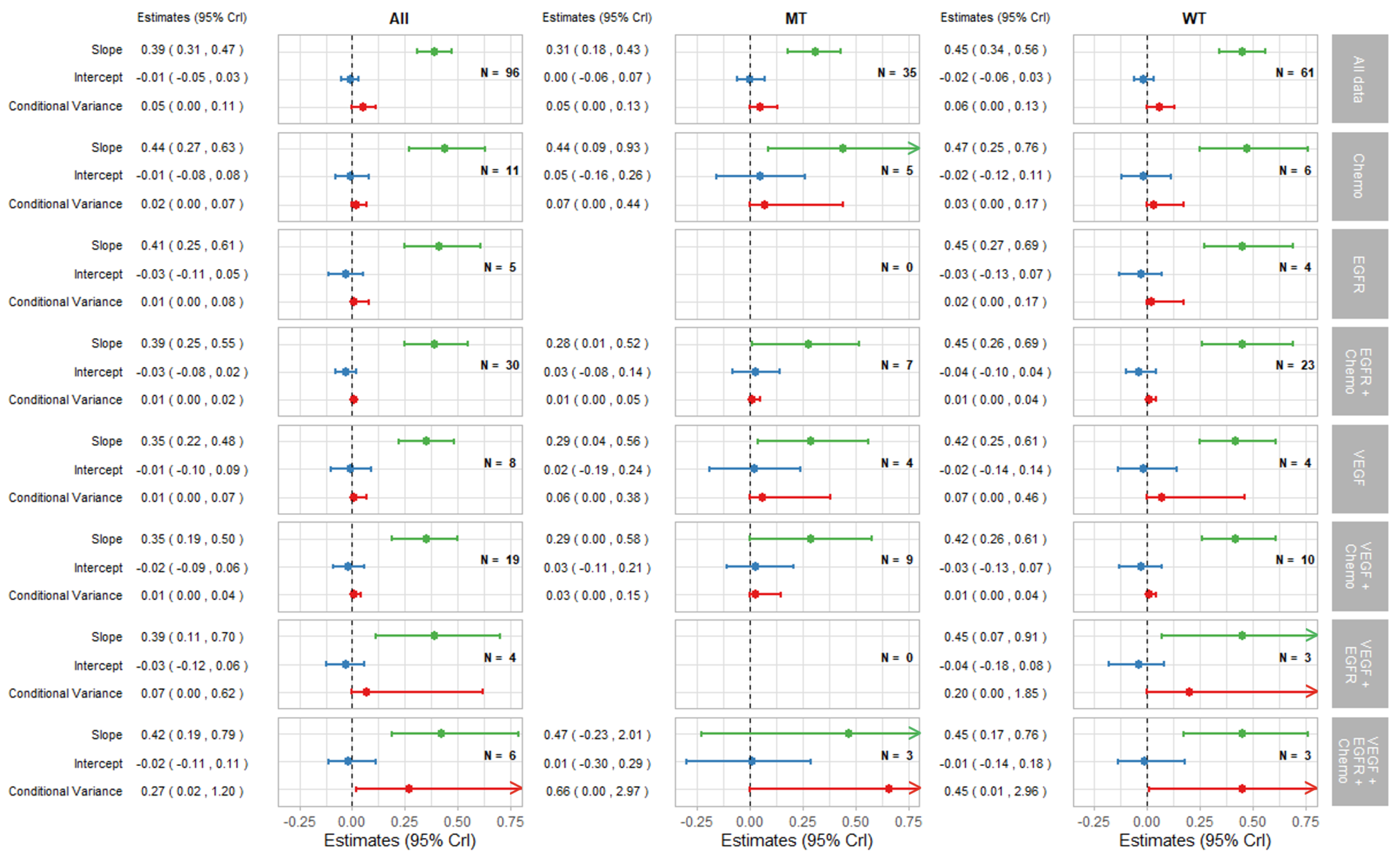
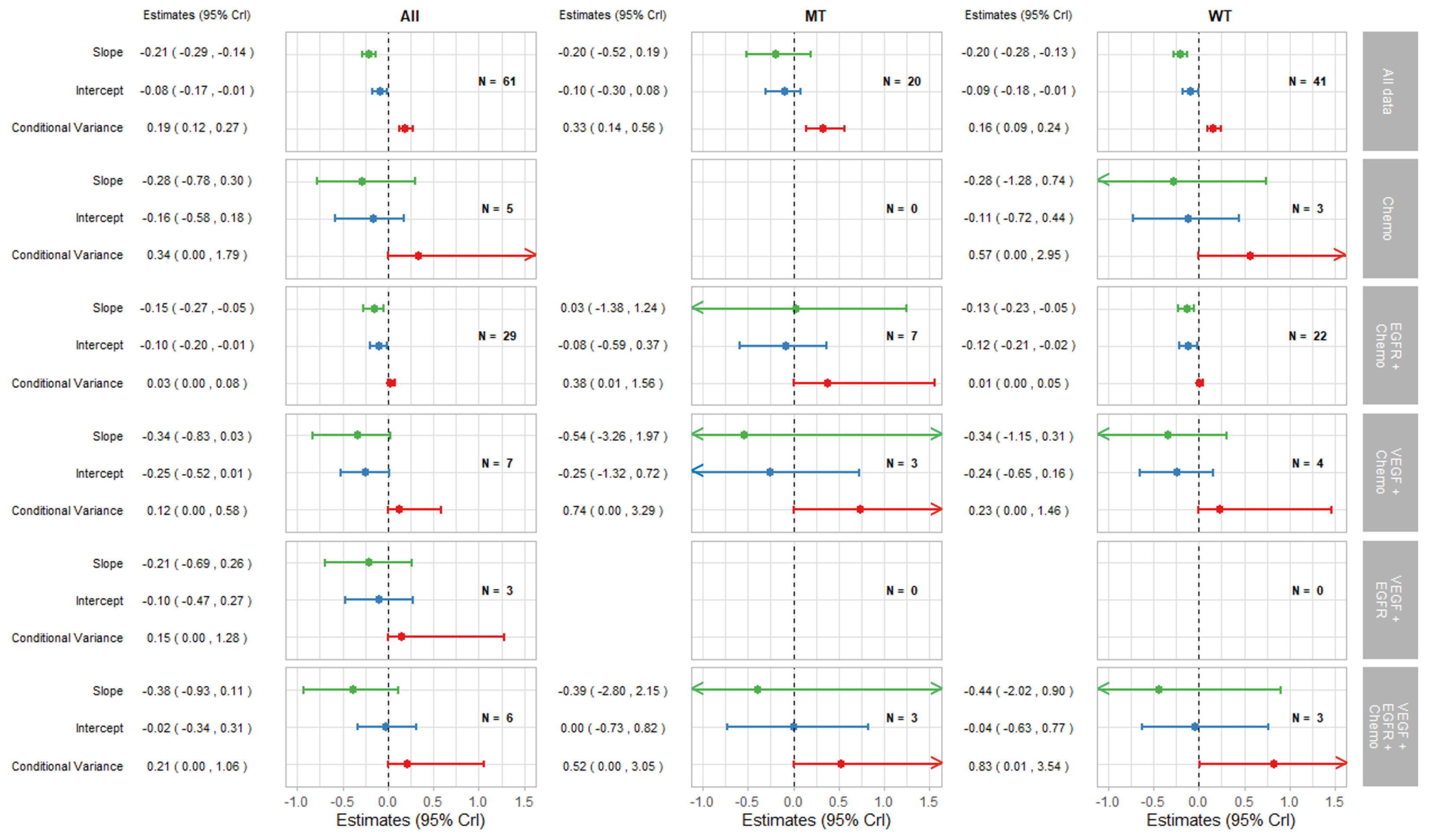
3.2.1. Surrogate Relationships Overall and by KRAS Status
3.2.2. Surrogate Relationships by Treatment Class: Overall and in KRAS Subgroups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Further Methods
Appendix A.1. Trial Identification: Search Strategies
Appendix A.1.1. Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
| #1 | MeSH descriptor: [Immunotherapy] explode all trees | 7760 |
| #2 | MeSH descriptor: [Angiogenesis Modulating Agents] explode all trees | 1132 |
| #3 | MeSH descriptor: [Antibodies, Monoclonal] explode all trees | 12,528 |
| #4 | MeSH descriptor: [Antineoplastic Agents] explode all trees | 11,805 |
| #5 | MeSH descriptor: [Drug Therapy] explode all trees | 137,825 |
| #6 | MeSH descriptor: [Antigens, Neoplasm] explode all trees | 2149 |
| #7 | chemotherap* | 78,829 |
| #8 | immunotherap* | 10,758 |
| #9 | (antitum?r or "anti tum?r" or anti-tum?r) | 5131 |
| #10 | inhibitor | 52,242 |
| #11 | cytotoxic | 4112 |
| #12 | cytostatic | 501 |
| #13 | (target* next (treatment or agent or therapy or administration or drug)) | 3166 |
| #14 | (hormone* next (treatment or agent or therapy or administration or drug)) | 5148 |
| #15 | (drug next (treatment or agent or therapy or administration)) | 405,830 |
| #16 | (antineoplas* or anti neoplas* or anti-neoplas*) | 29,472 |
| #17 | (anticancer* or anti cancer* or anti-cancer*) | 14,743 |
| #18 | (antiangiogen* or anti-angiogen* or anti angiogen*) | 2049 |
| #19 | infusion | 58,181 |
| #20 | immune response | 15,989 |
| #21 | (pharmacologic* next (treatment or agent or therapy or administration)) | 5686 |
| #22 | antigen | 19,362 |
| #23 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 | 556,231 |
| #24 | MeSH descriptor: [Colorectal Neoplasms] explode all trees | 7912 |
| #25 | MeSH descriptor: [Neoplasm Metastasis] explode all trees | 4995 |
| #26 | (((colorect* or colon* or rect* or anal* or intestin* or bowel* or sigmoid*) near/3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or adenoma*)) near/4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or "stage IV" or "stage 4" or "stage four" or irresectable or unresectable or palliati*)) | 7801 |
| #27 | (CRC near/4 (metastas* or metastatic* or micrometastas* ir micrometastatic* or advance* or "stage IV" or "stage 4" or "stage four" or irresectable or unresectable or palliati*)) | 1783 |
| #28 | aCRC or mCRC | 1662 |
| #29 | #24 and #25 | 706 |
| #30 | #26 or #27 or #28 or #29 | 8148 |
| #31 | #30 and #23 with Publication Year from 2003 to 2020, in Trials | 5047 |
Appendix A.1.2. Ovid MEDLINE
| 1 | exp Immunotherapy/ | 271,523 |
| 2 | exp Angiogenesis Modulating Agents/ | 62,615 |
| 3 | exp Antibodies, Monoclonal/ | 232,456 |
| 4 | exp Antineoplastic Agents/ | 1,085,139 |
| 5 | exp Drug Therapy/ | 1,341,564 |
| 6 | exp Antigens, Neoplasm/ | 116,062 |
| 7 | chemotherap*.mp. | 477,700 |
| 8 | immunotherap*.mp. | 111,265 |
| 9 | (antitum?r or “anti tum?r” or anti-tum?r).mp. | 166,008 |
| 10 | inhibitor.mp. | 652,707 |
| 11 | cytotoxic.mp. | 190,318 |
| 12 | cytostatic.mp. | 13,996 |
| 13 | (target* adj (treatment or agent or therapy or administration or drug)).mp. | 65,873 |
| 14 | (hormone* adj (treatment or agent or therapy or administration or drug)).mp. | 22,054 |
| 15 | (drug adj (treatment or agent or therapy or administration)).mp. | 2,373,269 |
| 16 | (antineoplas* or anti neoplas* or anti-neoplas*).mp. | 499,852 |
| 17 | (anticancer* or anti cancer* or anti-cancer*).mp. | 120,875 |
| 18 | (antiangiogen* or anti-angiogen* or anti angiogen*).mp. | 25,384 |
| 19 | infusion.mp. | 226,908 |
| 20 | immune response.mp. | 155,377 |
| 21 | (pharmacologic* adj (treatment or agent or therapy or administration)).mp. | 28,789 |
| 22 | antigen.mp. | 628,662 |
| 23 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 | 5,271,893 |
| 24 | randomized controlled trial.pt. | 503,142 |
| 25 | controlled clinical trial.pt. | 93,595 |
| 26 | randomized.ti,ab. | 513,184 |
| 27 | placebo.ti,ab. | 212,175 |
| 28 | clinical trials as topic.sh. | 190,619 |
| 29 | randomly.ti,ab. | 331,224 |
| 30 | trial.ti. | 215,880 |
| 31 | 24 or 25 or 26 or 27 or 28 or 29 or 30 | 1,287,975 |
| 32 | exp animals not humans.sh. | 4,685,426 |
| 33 | 31 not 32 | 1,185,372 |
| 34 | (((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel* or sigmoid*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or adenoma*) adj4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or “stage IV” or “stage 4” or “stage four” or irresectable or unresectable or palliati*)) or "aCRC" or “mCRC”).mp. | 40,955 |
| 35 | (CRC adj4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or “stage IV” or “stage 4” or “stage four” or irresectable or unresectable or palliati*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 5279 |
| 36 | exp colorectal neoplasms/ | 197,894 |
| 37 | exp neoplasm metastasis/ | 200,932 |
| 38 | 36 and 37 | 17,461 |
| 39 | 34 or 35 or 38 | 52,675 |
| 40 | 33 and 39 | 5701 |
| 41 | 23 and 40 | 4736 |
| 42 | limit 41 to yr="2003 -Current" | 3413 |
Appendix A.1.3. Ovid EMBASE
| 1 | exp drug therapy/ | 2,759,981 |
| 2 | exp immunotherapy/ | 212,046 |
| 3 | exp drug activity/ | 2,336,899 |
| 4 | exp monoclonal antibody/ | 563,094 |
| 5 | exp cancer therapy/ | 802,327 |
| 6 | exp antigen/ | 1,561,070 |
| 7 | chemotherap*.mp. | 843,036 |
| 8 | immunotherap*.mp. | 197,910 |
| 9 | (antitum?r or “anti tum?r” or anti-tum?r).mp. | 169,575 |
| 10 | inhibitor.mp. | 1,419,535 |
| 11 | cytotoxic.mp. | 273,532 |
| 12 | cytostatic.mp. | 21,483 |
| 13 | (target* adj (treatment or agent or therapy or administration or drug)).mp. | 93,739 |
| 14 | (hormone* adj (treatment or agent or therapy or administration or drug)).mp. | 47,449 |
| 15 | (drug adj (treatment or agent or therapy or administration)).mp. | 5,633,269 |
| 16 | (antineoplas* or anti neoplas* or anti-neoplas*).mp. | 460,857 |
| 17 | (anticancer* or anti cancer* or anti-cancer*).mp. | 163,990 |
| 18 | (antiangiogen* or anti-angiogen* or anti angiogen*).mp. | 45,610 |
| 19 | infusion.mp. | 368,696 |
| 20 | immune response.mp. | 356,585 |
| 21 | (pharmacologic* adj (treatment or agent or therapy or administration)).mp. | 44,297 |
| 22 | antigen.mp. | 1,339,922 |
| 23 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 | 9,964,936 |
| 24 | random*.ab,ti. or placebo*.de,ab,ti. or (double adj1 blind*).ab,ti. | 1,772,713 |
| 25 | (exp animal/ or exp nonhuman/ or exp animal experiment/ or exp experimental animal/) not exp human/ | 6,440,460 |
| 26 | 24 not 25 | 1,566,673 |
| 27 | exp “metastatic colorectal cancer”/ | 10,749 |
| 28 | (((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel* or sigmoid*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or adenoma*) adj4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or “stage IV” or “stage 4” or “stage four” or irresectable or unresectable or palliati*)) or “aCRC” or “mCRC”).mp. | 67,297 |
| 29 | (CRC adj4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or “stage IV” or “stage 4” or “stage four” or irresectable or unresectable or palliati*)).mp. | 9236 |
| 30 | 27 or 28 or 29 | 70,548 |
| 31 | 23 and 26 and 30 | 7155 |
| 32 | limit 31 to yr = “2003 -Current” | 6380 |
Appendix A.2. Data Extraction: Definitions of Treatment Effects
Appendix A.3. Statistical Methods
Appendix A.3.1. Cross Validation
Appendix A.3.2. Further Statistical Methods
Appendix B. Further Results
Appendix B.1. Results and Conclusions for TR as a Surrogate for OS
Appendix B.1.1. Results
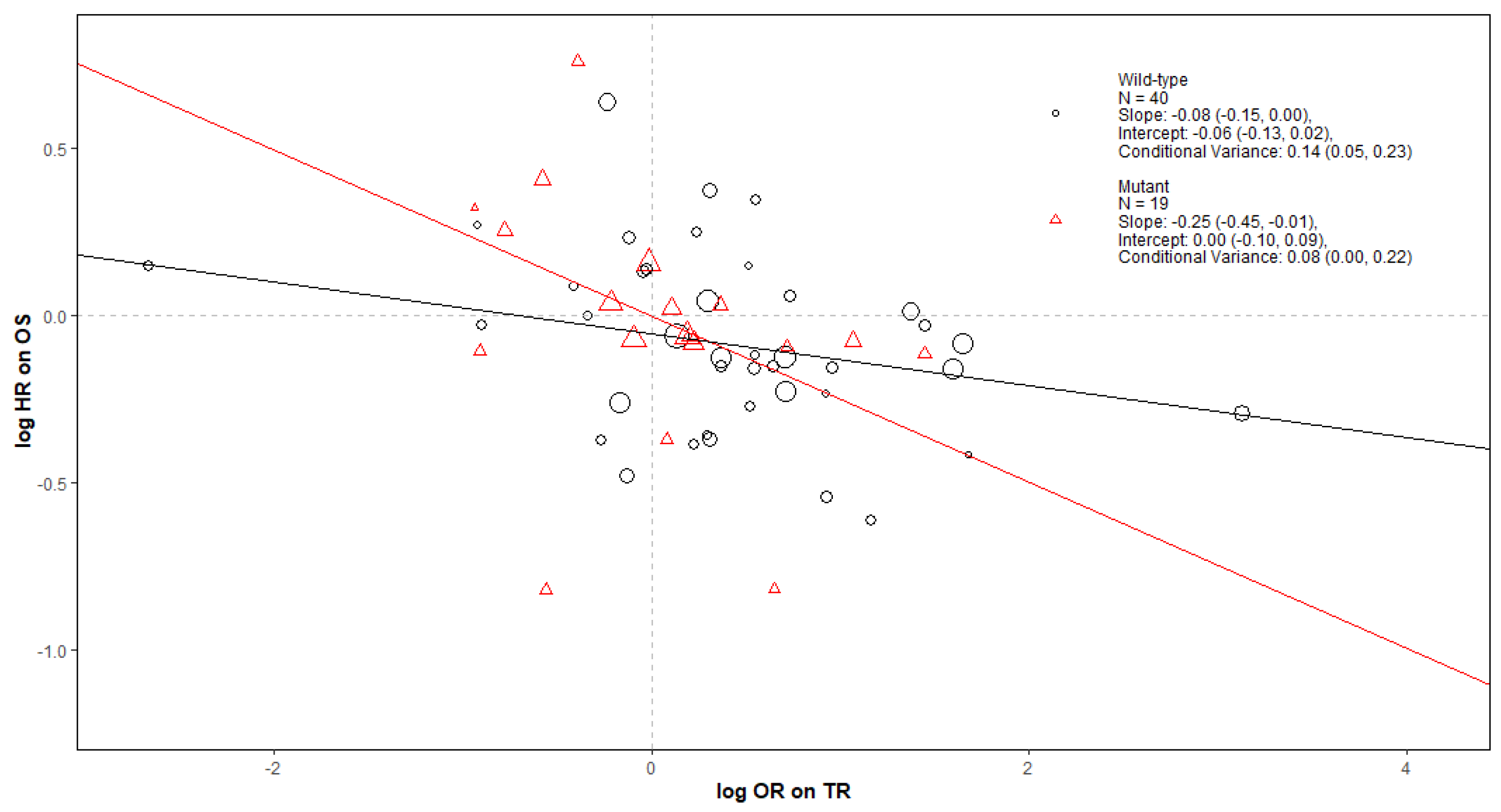
Appendix B.1.2. Conclusions
Appendix B.2. Cross Validation and Predictions
| Trial-Subgroups | All | MT | WT | |||
|---|---|---|---|---|---|---|
| Model | Daniels and Hughes | Hierarchical | Daniels and Hughes | Hierarchical | Daniels and Hughes | Hierarchical |
| Percentage of observed effect estimates within 95% predicted interval | 94.79% | 96.39% | 94.29% | 100.00% | 95.08% | 100.00% |
| Average absolute difference between the observed and predicted effect estimates | 0.16 | 0.16 | 0.17 | 0.20 | 0.15 | 0.16 |
| Average ratio of the width of intervals between the predicted and observer treatment effects | 1.14 | 1.51 | 1.13 | 2.14 | 1.21 | 2.02 |
References
- Managing Metastatic Colorectal Cancer—NICE Pathways. Available online: https://pathways.nice.org.uk/pathways/colorectal-cancer/managing-metastatic-colorectal-cancer (accessed on 3 August 2020).
- Bujkiewicz, S.; Achana, F.; Papanikos, T.; Riley, R.D.; Abrams, K.R. NICE DSU Technical Support Document 20: Multivariate Meta-Analysis of Summary Data for Combining Treatment Effects on Correlated Outcomes and Evaluating Surrogate Endpoints. 2019. Available online: http://www.nicedsu.org.uk (accessed on 5 September 2022).
- Ciani, O.; Buyse, M.; Drummond, M.; Rasi, G.; Saad, E.D.; Taylor, R.S. Time to Review the Role of Surrogate End Points in Health Policy: State of the Art and the Way Forward. Value Health. 2017, 20, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Downing, N.S.; Aminawung, J.A.; Shah, N.D.; Krumholz, H.M.; Ross, J.S. Clinical Trial Evidence Supporting FDA Approval of Novel Therapeutic Agents, 2005–2012. JAMA 2014, 311, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, D.; Naci, H.; Ciani, O.; Bujkiewicz, S. Raising the bar for using surrogate endpoints in drug regulation and health technology assessment. BMJ 2021, 374, n2191. [Google Scholar] [CrossRef]
- Buyse, M.; Burzykowski, T.; Carroll, K.; Michiels, S.; Sargent, D.J.; Miller, L.L.; Elfring, G.L.; Pignon, J.P.; Piedbois, P. Progression-Free Survival Is a Surrogate for Survival in Advanced Colorectal Cancer. J. Clin. Oncol. 2007, 25, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Giessen, C.; Laubender, R.P.; Ankerst, D.P.; Stintzing, S.; Modest, D.P.; Mansmann, U.; Heinemann, V. Progression-Free Survival as a Surrogate Endpoint for Median Overall Survival in Metastatic Colorectal Cancer: Literature-Based Analysis from 50 Randomized First-Line Trials. Clin. Cancer Res. 2013, 19, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ciani, O.; Buyse, M.; Garside, R.; Peters, J.; Saad, E.D.; Stein, K.; Taylor, R.S. Meta-analyses of randomized controlled trials show suboptimal validity of surrogate outcomes for overall survival in advanced colorectal cancer. J. Clin. Epidemiol. 2015, 68, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- Cox, A.D.; Der, C.J. Ras history. Small GTPases 2010, 1, 2–27. [Google Scholar] [CrossRef]
- Bylsma, L.C.; Gillezeau, C.; Garawin, T.A.; Kelsh, M.A.; Fryzek, J.P.; Sangaré, L.; Lowe, K.A. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2020, 9, 1044–1057. [Google Scholar] [CrossRef]
- PROSPERO, International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 22 July 2020).
- Embase (Excerpta Medica Database). Available online: https://www.embase.com/landing?status=grey (accessed on 3 December 2021).
- MEDLINE®: National Library of Medicine (United States). Available online: https://www.nlm.nih.gov/bsd/medline.html (accessed on 22 July 2020).
- Cochrane Controlled Register of Trials (CENTRAL) | Cochrane Library. Available online: https://www.cochranelibrary.com/central/about-central (accessed on 22 July 2020).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Daniels, M.J.; Hughes, M.D. Meta-analysis for the evaluation of potential surrogate markers. In Statistics in Medicine; John Wiley & Sons Ltd.: New York, NY, USA, 1997; Volume 16, pp. 1965–1982. [Google Scholar]
- Papanikos, T.; Thompson, J.R.; Abrams, K.R.; Städler, N.; Ciani, O.; Taylor, R.; Bujkiewicz, S. Bayesian hierarchical meta-analytic methods for modeling surrogate relationships that vary across treatment classes using aggregate data. Stat. Med. 2020, 39, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Hagman, H.; Frödin, J.E.; Berglund, Å.; Sundberg, J.; Vestermark, L.W.; Albertsson, M.; Fernebro, E.; Johnsson, A. A randomized study of KRAS-guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first-line induction treatment of metastatic colorectal cancer: The Nordic ACT2 trial. Ann. Oncol. 2016, 27, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Philip, P.; Saunders, M.; Kolevska, T.; Mukherjee, K.; Samuel, L.; Bondarde, S.; Dobbs, T.; Tagliaferri, M.; Hoch, U.; et al. Randomized study of etirinotecan pegol versus irinotecan as second-line treatment for metastatic colorectal cancer. Cancer Chemother. Pharmacol. 2017, 80, 1161–1169. [Google Scholar] [CrossRef]
- Maughan, T.S.; Adams, R.A.; Smith, C.G.; Meade, A.M.; Seymour, M.T.; Wilson, R.H.; Idziaszczyk, S.; Harris, R.; Fisher, D.; Kenny, S.L.; et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet 2011, 377, 2103–2114. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Passardi, A.; Scarpi, E.; Gelsomino, F.; Palladino, M.A.; Casadei Gardini, A.; Turci, D.; Chiuri, V.E.; Mucciarini, C.; Tassinari, D.; Ragazzini, A.; et al. Impact of second-line cetuximab-containing therapy in patients with KRAS wild-type metastatic colorectal cancer: Results from the ITACa randomized clinical trial. Sci. Rep. 2017, 7, 10426. [Google Scholar] [CrossRef]
- Reinacher-Schick, A.; Schulmann, K.; Modest, D.P.; Bruns, N.; Graeven, U.; Jaworska, M.; Greil, R.; Porschen, R.; Arnold, D.; Schmiegel, W.; et al. Effect of KRAS codon13 mutations in patients with advanced colorectal cancer (advanced CRC) under oxaliplatin containing chemotherapy. Results from a translational study of the AIO colorectal study group. BMC Cancer 2012, 12, 349. [Google Scholar] [CrossRef]
- Richman, S.D.; Seymour, M.T.; Chambers, P.; Elliott, F.; Daly, C.L.; Meade, A.M.; Taylor, G.; Barrett, J.H.; Quirke, P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: Results from the MRC FOCUS trial. J. Clin. Oncol. 2009, 27, 5931–5937. [Google Scholar] [CrossRef]
- Yoshino, T.; Mizunuma, N.; Yamazaki, K.; Nishina, T.; Komatsu, Y.; Baba, H.; Tsuji, A.; Yamaguchi, K.; Muro, K.; Sugimoto, N.; et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012, 13, 993–1001. [Google Scholar] [CrossRef]
- Aranda, E.; Garcia Alfonso, P.; Benavides, M.; Sanchez Ruiz, A.; Guillen-Ponce, C.; Safont, M.J.; Alcaide, J.; Gomez, A.; Lopez, R.; Manzano, J.L.; et al. First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: Phase II randomised MACRO2 TTD study. Eur. J. Cancer 2018, 101, 263–272. [Google Scholar] [CrossRef]
- Harbison, C.T.; Horak, C.E.; Ledeine, J.M.; Mukhopadhyay, P.; Malone, D.P.; O’Callaghan, C.; Jonker, D.J.; Karapetis, C.S.; Khambata-Ford, S.; Gustafson, N.; et al. Validation of companion diagnostic for detection of mutations in codons 12 and 13 of the KRAS gene in patients with metastatic colorectal cancer: Analysis of the NCIC CTG CO.17 trial. Arch. Pathol. Lab. Med. 2013, 137, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Elme, A.; Park, J.O.; Udrea, A.A.; Kim, S.Y.; Ahn, J.B.; Valencia, R.V.; Krishnan, S.; Manojlovic, N.; Guan, X.; et al. Final Analysis of Outcomes and RAS/BRAF Status in a Randomized Phase 3 Study of Panitumumab and Best Supportive Care in Chemorefractory Wild Type KRAS Metastatic Colorectal Cancer. Clin. Colorectal. Cancer 2018, 17, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Montagut, C.; Argilés, G.; Ciardiello, F.; Poulsen, T.T.; Dienstmann, R.; Kragh, M.; Kopetz, S.; Lindsted, T.; Ding, C.; Vidal, J.; et al. Efficacy of Sym004 in Patients With Metastatic Colorectal Cancer With Acquired Resistance to Anti-EGFR Therapy and Molecularly Selected by Circulating Tumor DNA Analyses: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e175245. [Google Scholar] [CrossRef] [PubMed]
- Price, T.; Kim, T.W.; Li, J.; Cascinu, S.; Ruff, P.; Suresh, A.S.; Thomas, A.; Tjulandin, S.; Guan, X.; Peeters, M. Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: Randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur. J. Cancer 2016, 68, 51–59. [Google Scholar] [CrossRef][Green Version]
- Bokemeyer, C.; Bondarenko, I.; Hartmann, J.T.; de Braud, F.; Schuch, G.; Zubel, A.; Celik, I.; Schlichting, M.; Koralewski, P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann. Oncol. 2011, 22, 1535–1546. [Google Scholar] [CrossRef]
- Bridgewater, J.; Pugh, S.; Whitehead, A.; Stanton, L.; Eminton, Z.; Mellor, J.; Allen, A.; Finch-Jones, M.; Falk, S.; Iveson, T.; et al. Perioperative chemotherapy with or without cetuximab in patients (pts) with resectable colorectal liver metastasis (CRLM): Mature analysis of overall survival (OS) in the New EPOC randomised controlled trial. Ann. Oncol. 2017, 28, v162. [Google Scholar] [CrossRef]
- Brodowicz, T.; Ciuleanu, T.E.; Radosavljevic, D.; Shacham-Shmueli, E.; Vrbanec, D.; Plate, S.; Mrsic-Krmpotic, Z.; Dank, M.; Purkalne, G.; Messinger, D.; et al. Folfox4 plus cetuximab administered weekly or every second week in the first-line treatment of patients with kras wild-type metastatic colorectal cancer: A randomized phase ii cecog study. Ann. Oncol. 2013, 24, 1769–1777. [Google Scholar] [CrossRef]
- Carrato, A.; Abad, A.; Massuti, B.; Gravalos, C.; Escudero, P.; Longo-Munoz, F.; Manzano, J.L.; Gomez, A.; Safont, M.J.; Gallego, J.; et al. First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: A randomised, phase II trial (PLANET-TTD). Eur. J. Cancer 2017, 81, 191–202. [Google Scholar] [CrossRef]
- Cascinu, S.; Rosati, G.; Nasti, G.; Lonardi, S.; Zaniboni, A.; Marchetti, P.; Leone, F.; Bilancia, D.; Iaffaioli, R.V.; Zagonel, V.; et al. Treatment sequence with either irinotecan/cetuximab followed by FOLFOX-4 or the reverse strategy in metastatic colorectal cancer patients progressing after first-line FOLFIRI/bevacizumab: An Italian Group for the Study of Gastrointestinal Cancer phase III, randomised trial comparing two sequences of therapy in colorectal metastatic patients. Eur. J. Cancer 2017, 83, 106–115. [Google Scholar] [CrossRef]
- Ciardiello, F.; Normanno, N.; Martinelli, E.; Troiani, T.; Pisconti, S.; Cardone, C.; Nappi, A.; Bordonaro, A.R.; Rachiglio, M.; Lambiase, M.; et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): A randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann. Oncol. 2016, 27, 1055–1061. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014, 25, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Zemelka, T.; Fountzilas, G.; Barone, C.; Schlichting, M.; Heighway, J.; Eggleton, S.P.; Srimuninnimit, V. FOLFOX4 with cetuximab vs. UFOX with cetuximab as first-line therapy in metastatic colorectal cancer: The randomized phase II FUTURE study. Clin. Colorectal. Cancer 2014, 13, 14–26.e1. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Sakai, D.; Takano, T.; Shinozaki, K.; Goto, M.; Taniguchi, H.; Kishimoto, J.; Boku, N.; Hyodo, I.; Muro, K. WJOG6510G: Randomized phase II of Pmab + IRI vs. Cmab + IRI for KRAS WT mCRC previously treated after FU, IRI, and L-OHP. Ann. Oncol. 2017, 28, 74–75. [Google Scholar] [CrossRef]
- Hecht, J.R.; Cohn, A.; Dakhil, S.; Saleh, M.; Piperdi, B.; Cline-Burkhardt, M.; Tian, Y.; Go, W.Y. SPIRITT: A randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin. Colorectal. Cancer 2015, 14, 72–80. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmuller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Maughan, T.S.; Meade, A.M.; Adams, R.A.; Richman, S.D.; Butler, R.; Fisher, D.; Wilson, R.H.; Jasani, B.; Taylor, G.R.; Williams, G.T.; et al. A feasibility study testing four hypotheses with phase II outcomes in advanced colorectal cancer (MRC FOCUS3): A model for randomised controlled trials in the era of personalised medicine? Br. J. Cancer 2014, 110, 2178–2186. [Google Scholar] [CrossRef]
- Munemoto, Y.; Nakamura, M.; Takahashi, M.; Kotaka, M.; Kuroda, H.; Kato, T.; Minagawa, N.; Noura, S.; Fukunaga, M.; Kuramochi, H.; et al. SAPPHIRE: A randomised phase II study of planned discontinuation or continuous treatment of oxaliplatin after six cycles of modified FOLFOX6 plus panitumumab in patients with colorectal cancer. Eur. J. Cancer 2019, 119, 158–167. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; Andre, T.; Chan, E.; Lordick, F.; Punt, C.J.A.; et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 4706–4713. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; Andre, T.; Chan, E.; Lordick, F.; Punt, C.J.A.; et al. Final results from a randomized phase 3 study of FOLFIRI +/- panitumumab for second-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014, 25, 107–116. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Wang, L.; Xu, J.; Cheng, Y.; Bai, Y.; Li, W.; Xu, N.; Lin, L.Z.; Wu, Q.; et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J. Clin. Oncol. 2018, 36, 3031–3039. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 2014, 32, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Seymour, M.T.; Brown, S.R.; Middleton, G.; Maughan, T.; Richman, S.; Gwyther, S.; Lowe, C.; Seligmann, J.F.; Wadsley, J.; Maisey, N.; et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): A prospectively stratified randomised trial. Lancet Oncol. 2013, 14, 749–759. [Google Scholar] [CrossRef]
- Shapiro, J.D.; Thavaneswaran, S.; Underhill, C.R.; Robledo, K.P.; Karapetis, C.S.; Day, F.L.; Nott, L.M.; Jefford, M.; Chantrill, L.A.; Pavlakis, N.; et al. Cetuximab Alone or With Irinotecan for Resistant KRAS-, NRAS-, BRAF- and PIK3CA-wild-type Metastatic Colorectal Cancer: The AGITG Randomized Phase II ICECREAM Study. Clin. Colorectal. Cancer 2018, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Yonesaka, K.; Denda, T.; Yamazaki, K.; Moriwaki, T.; Tsuda, M.; Takano, T.; Okuda, H.; Nishina, T.; Sakai, K.; et al. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci. 2016, 107, 1843–1850. [Google Scholar] [CrossRef]
- Tveit, K.M.; Guren, T.; Glimelius, B.; Pfeiffer, P.; Sorbye, H.; Pyrhonen, S.; Sigurdsson, F.; Kure, E.; Ikdahl, T.; Skovlund, E.; et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J. Clin. Oncol. 2012, 30, 1755–1762. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Kohne, C.H.; Lang, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Ye, L.C.; Liu, T.S.; Ren, L.; Wei, Y.; Zhu, D.X.; Zai, S.Y.; Ye, Q.H.; Yu, Y.; Xu, B.; Qin, X.Y.; et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J. Clin. Oncol. 2013, 31, 1931–1938. [Google Scholar] [CrossRef]
- Garcia, E.D.R.; Gomez, A.; Yuste, A.; Puente, J.; Lopez-Lopez, C.; Safont, M.J.; Layos, L.; Reboredo, M.; Benavides, M.; Aranda, E. Role of kras status in patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab—A TTD Spanish group cooperative study. PLoS ONE 2012, 47, S391–S392. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.H.; Shen, L.; Xu, J.; Bai, Y.; Yang, L.; Deng, Y.; Chen, Z.D.; Zhong, H.; et al. Effect of Fruquintinib vs. Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018, 319, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Lenz, H.J.; Siena, S.; Sobrero, A.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouche, O.; Mineur, L.; Barone, C.; et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: A retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015, 16, 937–948. [Google Scholar] [CrossRef]
- Bennouna, J.; Sastre, J.; Arnold, D.; Osterlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Vieitez, J.M.; Bouche, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef]
- Bennouna, J.; Hiret, S.; Bertaut, A.; Bouche, O.; Deplanque, G.; Borel, C.; Francois, E.; Conroy, T.; Ghiringhelli, F.; Des Guetz, G.; et al. Continuation of Bevacizumab vs. Cetuximab Plus Chemotherapy after First Progression in KRAS Wild-Type Metastatic Colorectal Cancer: The UNICANCER PRODIGE18 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 83–90. [Google Scholar] [CrossRef]
- Goey, K.K.H.; Elias, S.G.; van Tinteren, H.; Lacle, M.M.; Willems, S.M.; De Leng, W.W.J.; Strengman, E.; Vreuls, C.; Creemers, G.J.; Van Der Velden, A.; et al. Predictive value of KRAS mutation status in metastatic colorectal cancer (mCRC) patients treated with capecitabine and bevacizumab (CAP-B) maintenance treatment vs. observation in the phase III CAIRO3 study. J. Clin. Oncol. 2016, 34, 3525. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Yi, J.; Ince, W.; Novotny, W.F.; Rosen, O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: Analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 2009, 14, 22–28. [Google Scholar] [CrossRef]
- Nakayama, G.; Mitsuma, A.; Sunagawa, Y.; Ishigure, K.; Yokoyama, H.; Matsui, T.; Nakayama, H.; Nakata, K.; Ishiyama, A.; Asada, T.; et al. Randomized Phase II Trial of CapOX plus Bevacizumab and CapIRI plus Bevacizumab as First-Line Treatment for Japanese Patients with Metastatic Colorectal Cancer (CCOG-1201 Study). Oncologist 2018, 23, 919–927. [Google Scholar] [CrossRef]
- Price, T.J.; Hardingham, J.E.; Lee, C.K.; Weickhardt, A.; Townsend, A.R.; Wrin, J.W.; Chua, A.; Shivasami, A.; Cummins, M.M.; Murone, C.; et al. Impact of KRAS and BRAF Gene Mutation Status on Outcomes From the Phase III AGITG MAX Trial of Capecitabine Alone or in Combination With Bevacizumab and Mitomycin in Advanced Colorectal Cancer. J. Clin. Oncol. 2011, 29, 2675–2682. [Google Scholar] [CrossRef]
- Smith, J.C.; Brooks, L.; Hoff, P.M.; McWalter, G.; Dearden, S.; Morgan, S.R.; Wilson, D.; Robertson, J.D.; Jurgensmeier, J.M. KRAS mutations are associated with inferior clinical outcome in patients with metastatic colorectal cancer, but are not predictive for benefit with cediranib. Eur. J. Cancer 2013, 49, 2424–2432. [Google Scholar] [CrossRef]
- Tabernero, J.; Garcia-Carbonero, R.; Cassidy, J.; Sobrero, A.; Van Cutsem, E.; Kohne, C.H.; Tejpar, S.; Gladkov, O.; Davidenko, I.; Salazar, R.; et al. Sorafenib in combination with oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as first-line treatment of metastatic colorectal cancer: The RESPECT trial. Clin. Cancer Res. 2013, 19, 2541–2550. [Google Scholar] [CrossRef]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Maria Vieitez, J.; Bouche, O.; Osterlund, P.; Bennouna, J.; Andre, T.; Sastre, J.; Alonso-Orduna, V.; Kubicka, S.; Greil, R.; et al. Randomised phase III study of bevacizumab + chemotherapy beyond progression in bevacizumab-treated patients with metastatic colorectal cancer: TML study KRAS subgroup findings. Ann. Oncol. 2012, 23 (Suppl. 4). [Google Scholar] [CrossRef]
- Siu, L.L.; Shapiro, J.D.; Jonker, D.J.; Karapetis, C.S.; Zalcberg, J.R.; Simes, J.; Couture, F.; Moore, M.J.; Price, T.J.; Siddiqui, J.; et al. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: The NCIC Clinical Trials Group and AGITG CO.20 trial. J. Clin. Oncol. 2013, 31, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Tournigand, C.; Chibaudel, B.; Samson, B.; Scheithauer, W.; Vernerey, D.; Mesange, P.; Lledo, G.; Viret, F.; Ramee, J.F.; Tubiana-Mathieu, N.; et al. Bevacizumab with or without erlotinib as maintenance therapy in patients with metastatic colorectal cancer (GERCOR DREAM; OPTIMOX3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 1493–1505. [Google Scholar] [CrossRef]
- Liu, Y.; Luan, L.; Wang, X. A randomized Phase II clinical study of combining panitumumab and bevacizumab, plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for patients with metastatic colorectal cancer and KRAS mutation. Onco. Targets Ther. 2015, 8, 1061–1068. [Google Scholar] [CrossRef]
- Hecht, J.R.; Mitchell, E.; Chidiac, T.; Scroggin, C.; Hagenstad, C.; Spigel, D.; Marshall, J.; Cohn, A.; McCollum, D.; Stella, P.; et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J. Clin. Oncol. 2009, 27, 672–680. [Google Scholar] [CrossRef]
- Bendell, J.C.; Hochster, H.; Hart, L.L.; Firdaus, I.; Mace, J.R.; McFarlane, J.J.; Kozloff, M.; Catenacci, D.; Hsu, J.J.; Hack, S.P.; et al. A Phase II Randomized Trial (GO27827) of First-Line FOLFOX Plus Bevacizumab with or Without the MET Inhibitor Onartuzumab in Patients with Metastatic Colorectal Cancer. Oncologist 2017, 22, 264–271. [Google Scholar] [CrossRef]
- Cohn, A.L.; Tabernero, J.; Maurel, J.; Nowara, E.; Sastre, J.; Chuah, B.Y.S.; Kopp, M.V.; Sakaeva, D.D.; Mitchell, E.P.; Dubey, S.; et al. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann. Oncol. 2013, 24, 1777–1785. [Google Scholar] [CrossRef]
- Elez, E.; Kocakova, I.; Hohler, T.; Martens, U.M.; Bokemeyer, C.; Van cutsem, E.; Melichar, B.; Smakal, M.; Csoszi, T.; Topuzov, E.; et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: The randomised phase I/II POSEIDON trial. Ann. Oncol. 2015, 26, 132–140. [Google Scholar] [CrossRef]
- Randolph Hecht, J.; Benson, A.B.; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A phase II, randomized, double-blind, placebo-controlled study of simtuzumab in combination with FOLFIRI for the Second-Line treatment of metastatic KRAS mutant colorectal adenocarcinoma. Oncologist 2017, 22, 243-e23. [Google Scholar] [CrossRef]
- Hill, A.G.; Findlay, M.P.; Burge, M.E.; Jackson, C.; Alfonso, P.G.; Samuel, L.; Ganju, V.; Karthaus, M.; Amatu, A.; Jeffery, M.; et al. Phase II study of the dual EGFR/her3 inhibitor duligotuzumab (mehd7945a) versus cetuximab in combination with folfiri in second-line ras wild-type metastatic colorectal cancer. Clin. Cancer Res. 2018, 24, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Strickland, A.H.; Lichinitser, M.; Suresh, A.V.S.; Manikhas, G.; Shapiro, J.; Rogowski, W.; Huang, X.; Wu, B.; Warner, D.; et al. A randomised, double-blind, placebo-controlled phase 2 study of trebananib (AMG 386) in combination with FOLFIRI in patients with previously treated metastatic colorectal carcinoma. Br. J. Cancer 2013, 108, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Oliner, K.; Siena, S.; Van Cutsem, E.; Humblet, Y.; Van Laethem, J.L.; Andre, T.; Tian, Y.; Sidhu, R.; Patterson, S. Comprehensive kras and nras mutation analysis: Predictive biomarkers in a phase 3 panitumumab monotherapy study of metastatic colorectal cancer (MCRC). Ann. Oncol. 2013, 24. [Google Scholar] [CrossRef]
- Sclafani, F.; Kim, T.Y.; Cunningham, D.; Kim, T.W.; Tabernero, J.; Schmoll, H.J.; Roh, J.K.; Kim, S.Y.; Park, Y.S.; Guren, T.K.; et al. A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2015, 107, djv258. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Eng, C.; Nowara, E.; Swieboda-Sadlej, A.; Tebbutt, N.C.; Mitchell, E.; Davidenko, I.; Stephenson, J.; Elez, E.; Prenen, H.; et al. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin. Cancer Res. 2014, 20, 4240–4250. [Google Scholar] [CrossRef]
- Watkins, D.J.; Ayers, M.; Cunningham, D.; Tabernero, J.; Tejpar, S.; Kim, T.Y.; Kim, T.W.; Kim, S.Y.; Roh, J.K.; Beale, P.J.; et al. Molecular analysis of the randomized phase II/III study of the anti-IGF-1R antibody dalotuzumab (MK-0646) in combination with cetuximab (Cx) and irinotecan (Ir) in the treatment of chemorefractory KRAS wild-type metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2012, 30, 3531. [Google Scholar] [CrossRef]
- Xu, R.H.; Muro, K.; Morita, S.; Iwasa, S.; Han, S.W.; Wang, W.; Kotaka, M.; Nakamura, M.; Ahn, J.B.; Deng, Y.H.; et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018, 19, 660–671. [Google Scholar] [CrossRef]
- Cicero, G.; De Luca, R.; Dieli, F. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic colorectal cancer. Onco. Targets Ther. 2018, 11, 3059. [Google Scholar] [CrossRef]
- Cremolini, C.; Antoniotti, C.; Pietrantonio, F.; Berenato, R.; Tampellini, M.; Baratelli, C.; Salvatore, L.; Marmorino, F.; Borelli, B.; Nichetti, F.; et al. Surrogate endpoints in second-line trials of targeted agents in metastatic colorectal cancer: A literature-based systematic review and meta-analysis. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2017, 49, 834–845. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System; World Health Organization: Geneva, Switzerland, 2019.
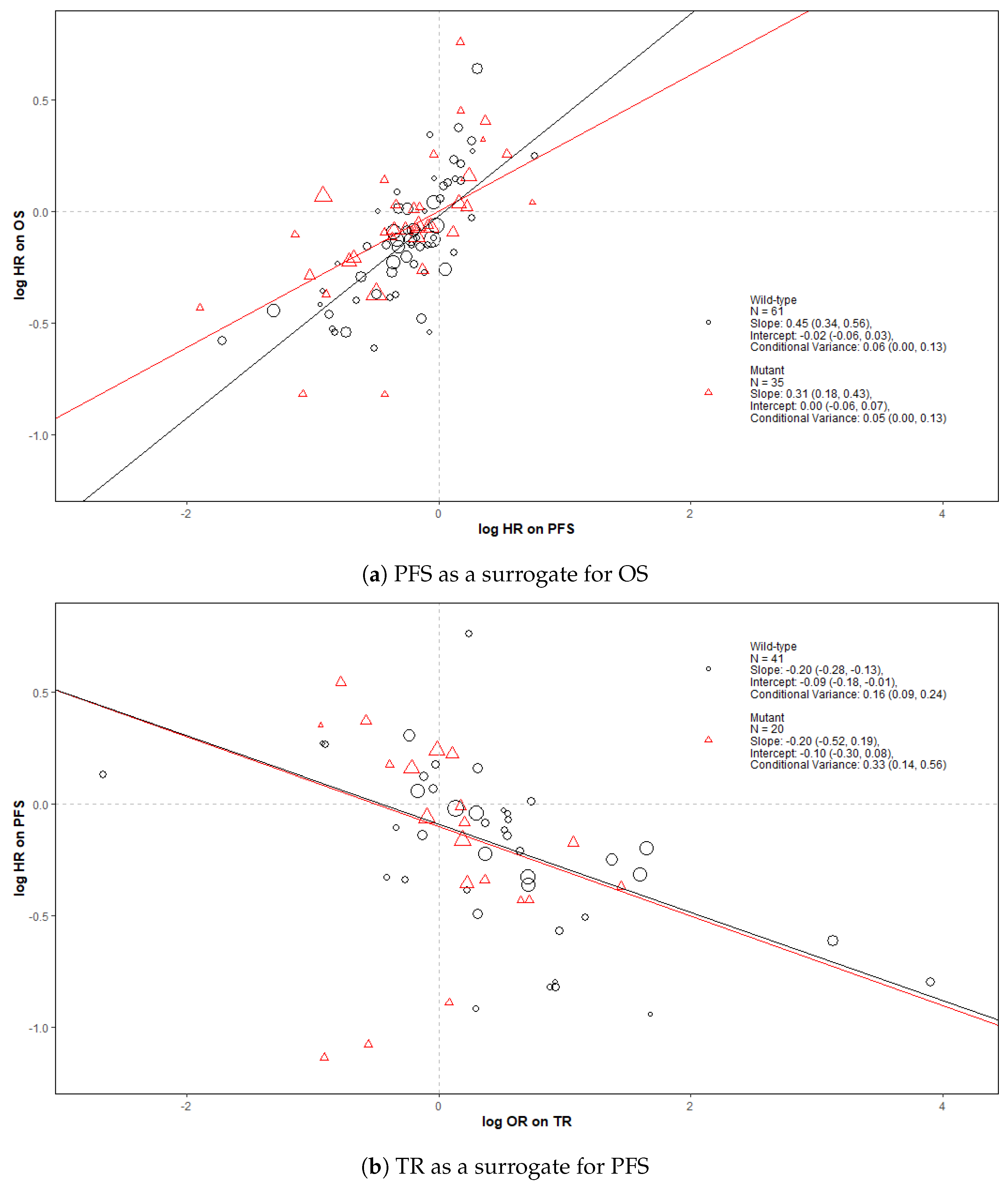
| Treatment Class (Total Trial-Subgroups) | Treatment Arm Combination | Number of Trial-Subgroups |
|---|---|---|
| Chemo (11) | Chemo + BSC vs. Placebo + BSC | 2 |
| Chemo vs. Chemo | 5 | |
| Chemo vs. Placebo | 2 | |
| Chemo vs. VEGF | 1 | |
| Chemo vs. VEGF + Chemo | 1 | |
| EGFR (6) | EGFR + BSC vs. BSC | 3 |
| EGFR vs. BSC | 1 | |
| EGFR vs. EGFR | 1 | |
| EGFR vs. EGFR + Chemo | 1 | |
| EGFR + Chemo (31) | EGFR + Chemo vs. Chemo | 18 |
| EGFR + Chemo vs. EGFR | 1 | |
| EGFR + Chemo vs. EGFR + Chemo | 7 | |
| EGFR + Chemo vs. VEGF + Chemo | 5 | |
| VEGF (8) | VEGF + BSC vs. Placebo + BSC | 2 |
| VEGF vs. Placebo | 4 | |
| VEGF vs. VEGF + Chemo | 2 | |
| VEGF + Chemo (19) | VEGF + Chemo vs. Chemo | 14 |
| VEGF + Chemo vs. EGFR + Chemo | 1 | |
| VEGF + Chemo vs. Observation | 2 | |
| VEGF + Chemo vs. VEGF + Chemo | 2 | |
| VEGF + EGFR (4) | VEGF + EGFR vs. EGFR | 1 |
| VEGF + EGFR vs. VEGF | 3 | |
| VEGF + EGFR + Chemo (6) | VEGF + EGFR + Chemo vs. Chemo | 2 |
| VEGF + EGFR + Chemo vs. VEGF + Chemo | 4 | |
| Studies that could not be separated into a treatment class (15) | ANG1/2/TIE2 + Chemo vs. Chemo | 2 |
| BSC vs. EGFR + BSC | 2 | |
| Chemo ± VEGF vs. Chemo ± VEGF | 2 | |
| C-met + Chemo vs. Chemo | 2 | |
| EGFR + CD51 + Chemo vs. EGFR + Chemo | 1 | |
| EGFR + HGF vs. EGFR | 1 | |
| EGFR + IGF-1 + Chemo vs. EGFR + Chemo | 2 | |
| HER3 + Chemo vs. EGFR + Chemo | 1 | |
| LOXL2 + Chemo vs. Chemo | 1 | |
| TRAIL + Chemo vs. Chemo | 1 |
| Surrogate Relationship | |||||
|---|---|---|---|---|---|
| Treatment Class | Study ID | KRAS Status | PFS for OS | TR for PFS | TR for OS |
| Chemo | Hagman 2016 [19] | MT | ✓ | X | X |
| Lenz 2017 [20] | MT | ✓ | ✓ | ✓ | |
| Maughan 2011 [21] | WT | ✓ | ✓ | ✓ | |
| Mayer 2015 [22] | WT | ✓ | X | X | |
| MT | ✓ | X | X | ||
| Passardi 2017 [23] | WT | ✓ | ✓ | ✓ | |
| Reinacher-Schick 2012 [24] | WT | ✓ | X | X | |
| Richman 2009 [25] | WT | ✓ | X | X | |
| MT | ✓ | X | X | ||
| Yoshino 2012 [26] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| EGFR | Aranda 2018 [27] | WT | ✓ | X | X |
| Harbison 2013 [28] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
| Kim 2018 [29] | WT | ✓ | ✓ | ✓ | |
| Montagut 2018 [30] | WT | X | X | ✓ | |
| Price 2016 [31] | WT | ✓ | ✓ | ✓ | |
| EGFR + Chemo | Bokemeyer 20011 [32] | MT | ✓ | ✓ | ✓ |
| WT | ✓ | ✓ | ✓ | ||
| Bridgewater 2017 [33] | WT | ✓ | ✓ | ✓ | |
| Brodowicz 2013 [34] | WT | ✓ | ✓ | ✓ | |
| Carrato 2017 [35] | WT | ✓ | ✓ | ✓ | |
| Cascinu 2017 [36] | WT | ✓ | ✓ | ✓ | |
| Ciardiello 2016 [37] | WT | ✓ | ✓ | ✓ | |
| Douillard 2014 [38] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| Douillard 2014 (2) [39] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| Hara 2017 [40] | WT | ✓ | ✓ | ✓ | |
| Hecht 2015 [41] | WT | ✓ | ✓ | ✓ | |
| Heinemann 2014 [42] | WT | ✓ | ✓ | ✓ | |
| Maughan 2014 [43] | WT | X | ✓ | X | |
| Munemoto 2019 [44] | WT | ✓ | ✓ | ✓ | |
| Peeters 2010 [45] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| Peeters 2014 [46] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| Qin 2018 [47] | WT | ✓ | X | X | |
| Schwartzberg 2014 [48] | WT | ✓ | ✓ | ✓ | |
| Seymour 2013 [49] | WT | ✓ | ✓ | ✓ | |
| Shapiro 2018 [50] | WT | ✓ | ✓ | ✓ | |
| Shitara 2016 [51] | WT | ✓ | ✓ | ✓ | |
| Tveit 2012 [52] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| VanCutsem 2011 [53] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| Venook 2017 [54] | WT | ✓ | X | X | |
| Ye 2013 [55] | WT | ✓ | ✓ | ✓ | |
| VEGF | Garcia 2011 [56] | MT | ✓ | ✓ | ✓ |
| WT | ✓ | ✓ | ✓ | ||
| Li 2015 [57] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
| Li 2018 [58] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
| Tabernero 2012 [59] | WT | ✓ | X | X | |
| MT | ✓ | X | X | ||
| VEGF + Chemo | Bennouna 2013 [60] | MT | ✓ | ✓ | ✓ |
| WT | ✓ | ✓ | ✓ | ||
| Bennouna 2018 [61] | WT | ✓ | ✓ | ✓ | |
| Goey 2016 [62] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
| Hurwitz 2009 [63] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| Nakayama 2018 [64] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| Price 2011 [65] | WT | ✓ | X | X | |
| MT | ✓ | X | X | ||
| Smith 2013 [66] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
| Tabernero 2013 [67] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
| Tabernero 2015 [68] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
| Van Cutsem 2012 [69] | WT | ✓ | X | X | |
| MT | ✓ | X | X | ||
| VEGF + EGFR | Hagman 2016 [19] | WT | ✓ | X | X |
| Siu 2013 [70] | WT | ✓ | ✓ | ✓ | |
| Tournigand 2015 [71] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| VEGF + EGFR + Chemo | Liu 2015 [72] | MT | ✓ | ✓ | ✓ |
| WT | ✓ | ✓ | ✓ | ||
| PACCE (Iri-CT) [73] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| PACCE (Ox-CT) [73] | MT | ✓ | ✓ | ✓ | |
| WT | ✓ | ✓ | ✓ | ||
| Not assigned | Bendell 2017 [74] | WT | ✓ | X | X |
| MT | ✓ | X | X | ||
| Cohn 2013 [75] | MT | ✓ | ✓ | ✓ | |
| Elez 2015 [76] | WT | ✓ | X | X | |
| Hecht 2017 [77] | MT | ✓ | ✓ | ✓ | |
| Hill 2018 [78] | WT | ✓ | ✓ | ✓ | |
| Peeters 2013 [79] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
| Peeters 2013 (2) [80] | MT | X | ✓ | X | |
| WT | X | ✓ | X | ||
| Sclafani 2015 [81] | WT | ✓ | ✓ | ✓ | |
| VanCutsem 2014 [82] | WT | ✓ | ✓ | ✓ | |
| Watkins 2012 [83] | WT | ✓ | X | X | |
| Xu 2018 [84] | MT | ✓ | X | X | |
| WT | ✓ | X | X | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poad, H.; Khan, S.; Wheaton, L.; Thomas, A.; Sweeting, M.; Bujkiewicz, S. The Validity of Surrogate Endpoints in Sub Groups of Metastatic Colorectal Cancer Patients Defined by Treatment Class and KRAS Status. Cancers 2022, 14, 5391. https://doi.org/10.3390/cancers14215391
Poad H, Khan S, Wheaton L, Thomas A, Sweeting M, Bujkiewicz S. The Validity of Surrogate Endpoints in Sub Groups of Metastatic Colorectal Cancer Patients Defined by Treatment Class and KRAS Status. Cancers. 2022; 14(21):5391. https://doi.org/10.3390/cancers14215391
Chicago/Turabian StylePoad, Heather, Sam Khan, Lorna Wheaton, Anne Thomas, Michael Sweeting, and Sylwia Bujkiewicz. 2022. "The Validity of Surrogate Endpoints in Sub Groups of Metastatic Colorectal Cancer Patients Defined by Treatment Class and KRAS Status" Cancers 14, no. 21: 5391. https://doi.org/10.3390/cancers14215391
APA StylePoad, H., Khan, S., Wheaton, L., Thomas, A., Sweeting, M., & Bujkiewicz, S. (2022). The Validity of Surrogate Endpoints in Sub Groups of Metastatic Colorectal Cancer Patients Defined by Treatment Class and KRAS Status. Cancers, 14(21), 5391. https://doi.org/10.3390/cancers14215391






