BRCA1/2 Mutation Testing in Patients with HER2-Negative Advanced Breast Cancer: Real-World Data from the United States, Europe, and Israel
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
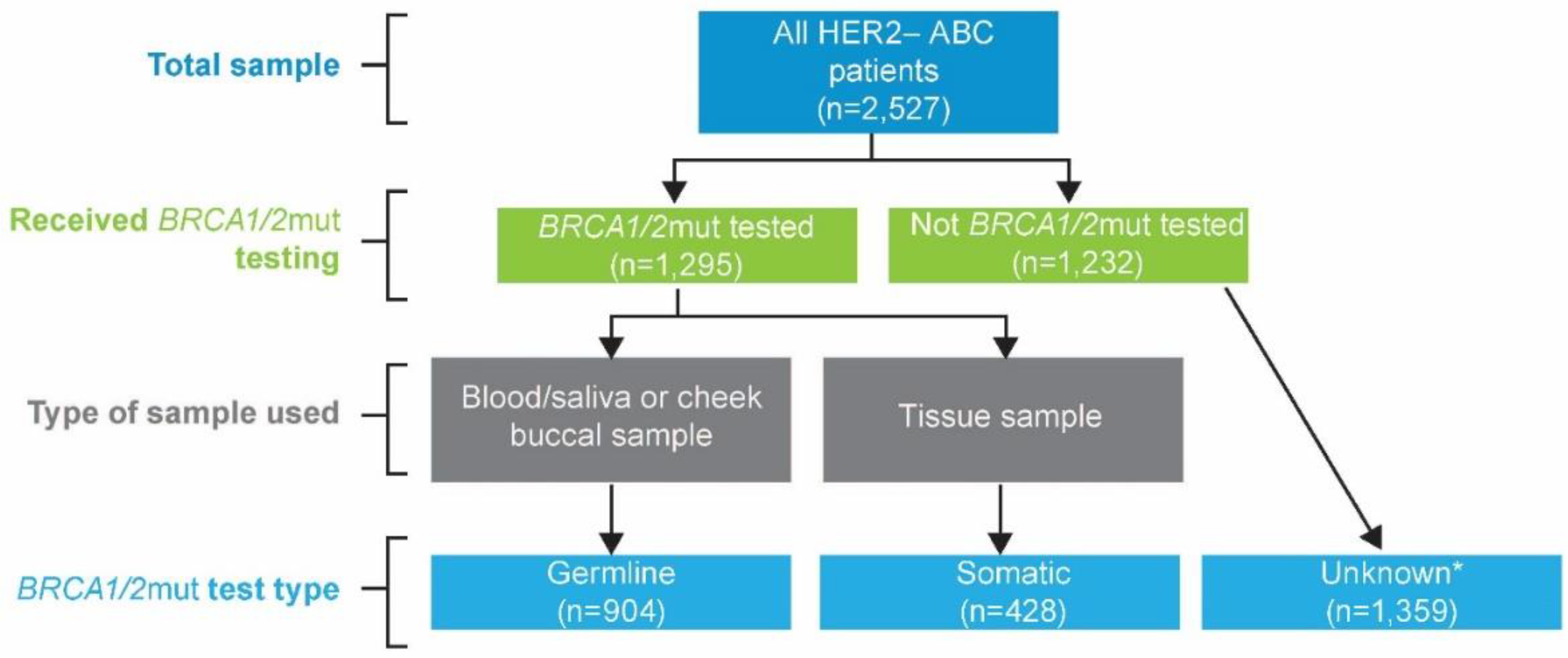
2.1. Data Source and Study Design
2.2. Outcomes and Measures
2.3. Statistical Analysis
3. Results
3.1. BRCA1/2 Mutation Testing in the United States
3.2. BRCA1/2 Mutation Testing in the European Union 4
3.3. BRCA1/2 Mutation Testing in Israel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadzicki, D.; Evans, D.G.; Harris, H.; Julian-Reynier, C.; Nippert, I.; Schmidtke, J.; Tibben, A.; van Asperen, C.J.; Schlegelberger, B. Genetic testing for familial/hereditary breast cancer—Comparison of guidelines and recommendations from the UK, France, the Netherlands and Germany. J. Community Genet. 2011, 2, 53–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujol, P.; Barberis, M.; Beer, P.; Friedman, E.; Piulats, J.M.; Capoluongo, E.D.; Garcia Foncillas, J.; Ray-Coquard, I.; Penault-Llorca, F.; Foulkes, W.D.; et al. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur. J. Cancer 2021, 146, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Yadav, S.; Hu, C.; Wunderle, M.; Haberle, L.; Hart, S.N.; Rubner, M.; Polley, E.C.; Lee, K.Y.; Gnanaolivu, R.D.; et al. Mutations in BRCA1/2 and other panel genes in patients with metastatic breast cancer—Association with patient and disease characteristics and effect on prognosis. J. Clin. Oncol. 2021, 39, 1619–1630. [Google Scholar] [CrossRef]
- Tung, N.; Lin, N.U.; Kidd, J.; Allen, B.A.; Singh, N.; Wenstrup, R.J.; Hartman, A.R.; Winer, E.P.; Garber, J.E. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J. Clin. Oncol. 2016, 34, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Meynard, G.; Villanueva, C.; Thiery-Vuillemin, A.; Mansi, L.; Montcuquet, P.; Meneveau, N.; Chaigneau, L.; Bazan, F.; Almotlak, H.; Dobi, E.; et al. Real-life study of BRCA genetic screening in metastatic breast cancer. Ann. Oncol. 2017, 28, V94. [Google Scholar] [CrossRef]
- Neviere, Z.; De La Motte Rouge, T.; Floquet, A.; Johnson, A.; Berthet, P.; Joly, F. How and when to refer patients for oncogenetic counseling in the era of PARP inhibitors. Ther. Adv. Med. Oncol. 2020, 12, 1758835919897530. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration. FDA Approves Olaparib for Germline BRCA-Mutated Metastatic Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-germline-brca-mutated-metastatic-breast-cancer (accessed on 31 January 2022).
- US Food and Drug Administration. FDA Approves Talazoparib for gBRCAm HER2-Negative Locally Advanced or Metastatic Breast Cancer. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer (accessed on 31 January 2022).
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Robson, M.; Ruddy, K.J.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Li, W.; Tung, N.; Armstrong, A.; et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur. J. Cancer 2019, 120, 20–30. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Goncalves, A.; Rugo, H.S.; Lee, K.H.; Fehrenbacher, L.; Mina, L.A.; Diab, S.; Blum, J.L.; Chakrabarti, J.; Elmeliegy, M.; et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: Detailed safety analyses from the phase III EMBRACA trial. Oncologist 2020, 25, e439–e450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, S.A.; Xu, B.; Li, W.; Robson, M.; Ouyang, Q.; Yeh, D.C.; Iwata, H.; Park, Y.H.; Sohn, J.H.; Tseng, L.M.; et al. Olaparib monotherapy for Asian patients with a germline BRCA mutation and HER2-negative metastatic breast cancer: OlympiAD randomized trial subgroup analysis. Sci. Rep. 2020, 10, 8753. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Gonzalez-Santiago, S.; Ramon, Y.C.T.; Aguirre, E.; Ales-Martinez, J.E.; Andres, R.; Balmana, J.; Grana, B.; Herrero, A.; Llort, G.; Gonzalez-Del-Alba, A.; et al. SEOM clinical guidelines in hereditary breast and ovarian cancer (2019). Clin. Transl. Oncol. 2020, 22, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, P.; Benford, M.; Harris, N.; Karavali, M.; Piercy, J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes—A means to understand. Curr. Med. Res. Opin. 2008, 24, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- European Pharmaceutical Market Research Association. EphMRA Code of Conduct. 2020. Available online: https://www.ephmra.org/sites/default/files/2022-08/EPHMRA%202022%20Code%20of%20Conduct.pdf (accessed on 1 September 2021).
- US Department of Health & Human Services. Summary of the HIPAA Privacy Rule. Available online: https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html (accessed on 26 May 2021).
- Referenced with Permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2022. © National Comprehensive Cancer Network, Inc. 2022. All Rights Reserved. Accessed July 5, 2022. To View the Most Recent and Complete Version of the Guideline, NCCN Makes No Warranties of Any Kind Whatsoever Regarding Their Content, Use or Application and Disclaims any Responsibility for Their Application or Use in any Way. Available online: NCCN.org (accessed on 1 September 2021).
- Lux, M.P.; Lewis, K.; Rider, A.; Niyazov, A. Real-world multi-country study of BRCA1/2 mutation testing among adult women with HER2-negative advanced breast cancer. Future Oncol. 2022, 18, 1089–1101. [Google Scholar] [CrossRef]
- Bayraktar, S.; Gutierrez-Barrera, A.M.; Liu, D.; Tasbas, T.; Akar, U.; Litton, J.K.; Lin, E.; Albarracin, C.T.; Meric-Bernstam, F.; Gonzalez-Angulo, A.M.; et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res. Treat. 2011, 130, 145–153. [Google Scholar] [CrossRef]
- Walsh, E.M.; Mangini, N.; Fetting, J.; Armstrong, D.; Chan, I.S.; Connolly, R.M.; Fiallos, K.; Lehman, J.; Nunes, R.; Petry, D.; et al. Olaparib use in patients with metastatic breast cancer harboring somatic BRCA1/2 mutations or mutations in non-BRCA1/2, DNA damage repair genes. Clin. Breast Cancer 2022, 22, 319–325. [Google Scholar] [CrossRef]
- Russo, A.; Incorvaia, L.; Capoluongo, E.; Tagliaferri, P.; Gori, S.; Cortesi, L.; Genuardi, M.; Turchetti, D.; De Giorgi, U.; Di Maio, M.; et al. Implementation of preventive and predictive BRCA testing in patients with breast, ovarian, pancreatic, and prostate cancer: A position paper of Italian Scientific Societies. ESMO Open 2022, 7, 100459. [Google Scholar] [CrossRef]
- US National Institutes of Health. BRCA Gene Mutations: Cancer Risk and Genetic Testing. Available online: https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet#r18 (accessed on 27 April 2022).
- The Health Policy Partnership. Genetic Testing for BRCA Mutations: A Policy Paper. Available online: https://www.healthpolicypartnership.com/app/uploads/Genetic-testing-for-BRCA-mutations-a-policy-paper.pdf (accessed on 23 May 2022).
- Abacan, M.; Alsubaie, L.; Barlow-Stewart, K.; Caanen, B.; Cordier, C.; Courtney, E.; Davoine, E.; Edwards, J.; Elackatt, N.J.; Gardiner, K.; et al. The global state of the genetic counseling profession. Eur. J. Hum. Genet. 2019, 27, 183–197. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef] [PubMed]


| p Value (vs. Not Tested) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Any BRCA1/2mut Testing (n = 298) | g +/− s BRCA1/2mut Testing (n = 190) | sBRCA1/2mut-Only Testing (n = 75) | Unknown BRCA1/2mut Testing (n = 33) | No BRCA1/2mut Testing (n = 109) | All Tested | g +/− s | s Only | Unknown | |
| Mean patient age, y | 62.5 | 62.9 | 60.7 | 64.5 | 68.9 | <0.001 | <0.001 | <0.001 | <0.001 |
| Race | |||||||||
| White/Caucasian | 200 (67) | 126 (66) | 50 (67) | 24 (73) | 66 (61) | 0.240 | 0.320 | 0.439 | 0.223 |
| African American | 71 (24) | 40 (21) | 22 (29) | 9 (27) | 30 (28) | 0.440 | 0.206 | 0.868 | 1.00 |
| Employed | 99 (33) | 62 (33) | 27 (36) | 10 (30) | 20 (18) | 0.003 | 0.010 | 0.010 | 0.151 |
| Premenopausal | 24 (8) | 13 (7) | 10 (14) | 1 (3) | 2 (2) | 0.022 | 0.095 | 0.004 | 0.560 |
| Family history of BRCA1/2-related cancer * | 56 (19) | 36 (19) | 15 (20) | 5 (15) | 7 (6) | 0.002 | 0.003 | 0.010 | 0.150 |
| HR status | |||||||||
| HR+/HER2− | 222 (74) | 140 (74) | 59 (79) | 23 (70) | 103 (94) | <0.001 | <0.001 | 0.002 | <0.001 |
| TNBC | 76 (26) | 50 (26) | 16 (21) | 10 (30) | 6 (6) | ||||
| Academic medical center | 122 (41) | 89 (47) | 19 (25) | 14 (42) | 31 (28) | 0.021 | 0.002 | 0.737 | 0.140 |
| Community-based center | 176 (59) | 101 (53) | 56 (75) | 19 (58) | 78 (72) | ||||
| United States | EU4 | Israel | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR+/ HER2− (n = 325) | TNBC (n = 82) | p Value | HR+/ HER2− (n = 1703) | TNBC (n = 223) | p Value | HR+/ HER2− (n = 141) | TNBC (n = 53) | p Value | |
| Any BRCA1/2mut testing | 222 (68) | 76 (93) | <0.001 | 631 (37) | 174 (78) | <0.001 | 139 (99) | 53 (100) | >0.99 |
| g +/− sBRCA1/2mut testing | 140 (43) | 50 (61) | 0.004 | 401 (24) | 127 (57) | <0.001 | 135 (96) | 51 (96) | >0.99 |
| sBRCA1/2mut-only testing | 59 (18) | 16 (20) | 0.752 | 155 (9) | 31 (14) | 0.029 | 1 (1) | 2 (4) | 0.182 |
| Unknown BRCA1/2mut testing | 23 (7) | 10 (12) | 0.171 | 75 (4) | 16 (7) | 0.090 | 3 (2) | 0 (0) | 0.563 |
| No BRCA1/2mut testing | 103 (32) | 6 (7) | 1072 (63) | 49 (22) | 2 (1) | 0 (0) | |||
| United States | EU4 | |||||
|---|---|---|---|---|---|---|
| Academic | Community | p Value | Academic | Community | p Value | |
| HR+/HER2− | (n = 121) | (n = 204) | (n = 951) | (n = 752) | ||
| Any BRCA1/2mut testing | 91 (75) | 131 (64) | 0.048 | 386 (41) | 245 (33) | 0.001 |
| g +/− sBRCA1/2mut testing | 65 (54) | 75 (37) | 0.004 | 236 (25) | 165 (22) | 0.168 |
| sBRCA1/2mut-only testing | 15 (12) | 44 (22) | 0.039 | 109 (11) | 46 (6) | <0.001 |
| Unknown BRCA1/2mut testing | 11 (9) | 12 (6) | 0.274 | 41 (4) | 34 (5) | 0.905 |
| No BRCA1/2mut testing | 30 (25) | 73 (36) | 565 (59) | 507 (67) | ||
| TNBC | (n = 32) | (n = 50) | (n = 123) | (n = 100) | ||
| Any BRCA1/2mut testing | 31 (97) | 45 (90) | 0.396 | 109 (89) | 65 (65) | <0.001 |
| g +/− sBRCA1/2mut testing | 24 (75) | 26 (52) | 0.063 | 77 (63) | 50 (50) | 0.077 |
| sBRCA1/2mut-only testing | 4 (13) | 12 (24) | 0.259 | 25 (20) | 6 (6) | 0.003 |
| Unknown BRCA1/2mut testing | 3 (9) | 7 (14) | 0.733 | 7 (6) | 9 (9) | 0.436 |
| No BRCA1/2mut testing | 1 (3) | 5 (10) | 14 (11) | 35 (35) | ||
| United States | EU4 | Israel | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Family History | No History | p Value | Family History | No History | p Value | Family History | No History | p Value | |
| HR+/HER2− | (n = 43) | (n = 234) | (n = 280) | (n = 1356) | (n = 101) | (n = 39) | |||
| Any BRCA1/2mut testing | 36 (84) | 156 (67) | 0.030 | 173 (62) | 437 (32) | <0.001 | 100 (99) | 38 (97) | 0.481 |
| g +/− sBRCA1/2mut testing | 22 (51) | 99 (42) | 0.317 | 120 (43) | 274 (20) | <0.001 | 98 (97) | 37 (95) | 0.618 |
| sBRCA1/2mut-only testing | 11 (26) | 45 (19) | 0.408 | 33 (12) | 111 (8) | 0.063 | 1 (1) | 0 (0) | 1.00 |
| Unknown BRCA1/2mut testing | 3 (7) | 12 (5) | 0.711 | 20 (7) | 52 (4) | 0.024 | 1 (1) | 1 (3) | 0.481 |
| No BRCA1/2mut testing | 7 (16) | 78 (33) | 107 (38) | 919 (68) | 1 (1) | 1 (3) | |||
| TNBC | (n = 20) | (n = 57) | (n = 57) | (n = 157) | (n = 30) | (n = 20) | |||
| Any BRCA1/2mut testing | 20 (100) | 53 (93) | 0.568 | 51 (89) | 118 (75) | 0.023 | 30 (100) | 20 (100) | 1.00 |
| g +/− sBRCA1/2mut testing | 14 (70) | 35 (61) | 0.594 | 38 (67) | 86 (55) | 0.158 | 30 (100) | 20 (100) | 1.00 |
| sBRCA1/2mut-only testing | 4 (20) | 12 (21) | 1.00 | 6 (11) | 24 (15) | 0.505 | 0 (0) | 0 (0) | 1.00 |
| Unknown BRCA1/2mut testing | 2 (10) | 6 (11) | 1.00 | 7 (12) | 8 (5) | 0.125 | 0 (0) | 0 (0) | 1.00 |
| No BRCA1/2mut testing | 0 (0) | 4 (7) | 6 (11) | 39 (25) | 0 (0) | 0 (0) | |||
| p Value (vs. Not Tested) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Any BRCA1/2mut Testing (n = 805) | g +/− sBRCA1/2mut Testing (n = 528) | sBRCA1/2mut-Only Testing (n = 186) | Unknown BRCA1/2mut Testing (n = 91) | No BRCA1/2mut Testing (n = 1121) | All Tested | g +/− s | s Only | Unknown | |
| Mean patient age, y | 58.0 | 57.9 | 59.7 | 55.3 | 66.7 | <0.001 | <0.001 | <0.001 | <0.001 |
| Race | |||||||||
| White/Caucasian | 752 (93) | 489 (93) | 180 (97) | 83 (91) | 1063 (95) | 0.199 | 0.092 | 0.357 | 0.148 |
| Employed | 206 (26) | 124 (23) | 51 (27) | 31 (34) | 124 (11) | <0.001 | <0.001 | <0.001 | <0.001 |
| Premenopausal | 118 (15) | 75 (14) | 25 (14) | 18 (20) | 33 (3) | <0.001 | <0.001 | <0.001 | <0.001 |
| Family history of BRCA1/2-related cancer * | 224 (28) | 158 (30) | 39 (21) | 27 (30) | 113 (10) | <0.001 | <0.001 | <0.001 | <0.001 |
| HR status | |||||||||
| HR+/HER2− | 631 (78) | 401 (76) | 155 (83) | 75 (82) | 1072 (96) | <0.001 | <0.001 | <0.001 | <0.001 |
| TNBC | 174 (22) | 127 (24) | 31 (17) | 16 (18) | 49 (4) | ||||
| Academic medical center | 495 (61) | 313 (59) | 134 (72) | 48 (53) | 579 (52) | <0.001 | 0.004 | <0.001 | 0.913 |
| Community-based center | 310 (39) | 215 (41) | 52 (28) | 43 (47) | 542 (48) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahtani, R.; Niyazov, A.; Arondekar, B.; Lewis, K.; Rider, A.; Massey, L.; Lux, M.P. BRCA1/2 Mutation Testing in Patients with HER2-Negative Advanced Breast Cancer: Real-World Data from the United States, Europe, and Israel. Cancers 2022, 14, 5399. https://doi.org/10.3390/cancers14215399
Mahtani R, Niyazov A, Arondekar B, Lewis K, Rider A, Massey L, Lux MP. BRCA1/2 Mutation Testing in Patients with HER2-Negative Advanced Breast Cancer: Real-World Data from the United States, Europe, and Israel. Cancers. 2022; 14(21):5399. https://doi.org/10.3390/cancers14215399
Chicago/Turabian StyleMahtani, Reshma, Alexander Niyazov, Bhakti Arondekar, Katie Lewis, Alex Rider, Lucy Massey, and Michael Patrick Lux. 2022. "BRCA1/2 Mutation Testing in Patients with HER2-Negative Advanced Breast Cancer: Real-World Data from the United States, Europe, and Israel" Cancers 14, no. 21: 5399. https://doi.org/10.3390/cancers14215399






