Identification of MYEOV-Associated Gene Network as a Potential Therapeutic Target in Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Analysis
2.2. Gene Differential Expression Validation
2.3. Relative mRNA Levels Measurements
2.4. The Construction, Transfection, and Infection of Small Interfering RNA (siRNA)
2.5. CCK-8 Assay and Cell Colony Formation Assay
2.6. Western Blotting
2.7. Gene-Related Survival Validation
2.8. Gene Co-Expression Analysis
2.9. Alteration Prediction
2.10. PPI Analysis
2.11. Gene-Related Immune Infiltration Analysis
2.12. miRNA Analysis
2.13. Dual-Luciferase Reporter Assay
2.14. Statistical Analyses
3. Results
3.1. MYEOV as a Key Upregulated Gene Closely Related to Prognosis in Pancreatic Cancer
3.2. Gene Co-Expression Analysis of MYEOV in Pancreatic Cancer
3.3. PPI Analysis and Molecular Regulatory Network of MYEOV
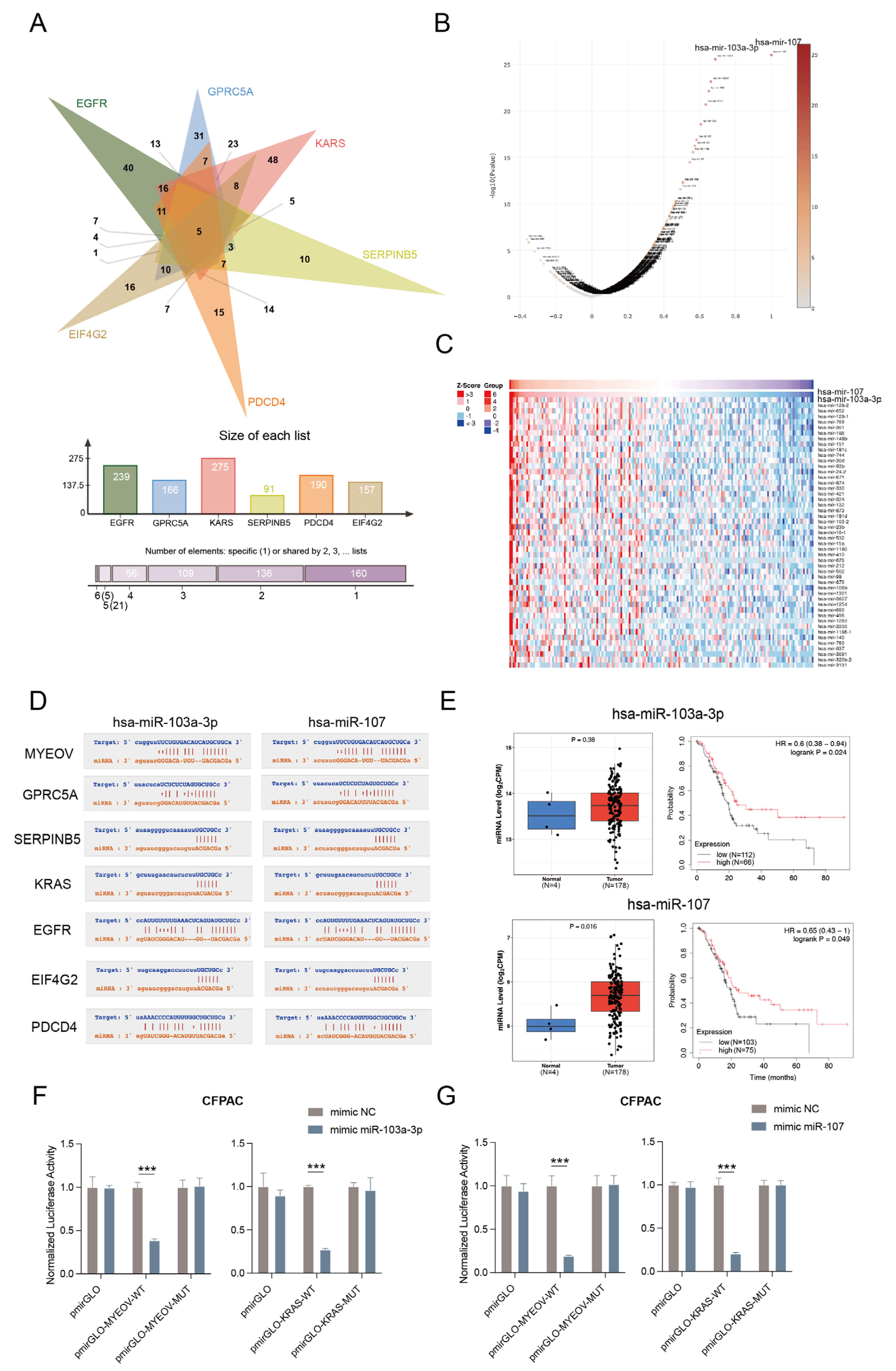
3.4. MYEOV as ceRNA for the Regulation of Multiple Genes
3.5. Analysis of the Role of Genes in MYEOV Regulatory Network on Pancreatic Cancer
3.6. Immune Infiltration Analysis of Genes Associated with the MYEOV Regulatory Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Nigri, J.; Lac, S.; Leca, J.; Bressy, C.; Berthezene, P.; Bartholin, L.; Chan, P.; Calvo, E.; Iovanna, J.L.; et al. TAp73 Loss Favors Smad-Independent TGF-β Signaling That Drives EMT in Pancreatic Ductal Adenocarcinoma. Cell Death Differ. 2016, 23, 1358–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saif, M.W. Advancements in the Management of Pancreatic Cancer: 2013. JOP J. Pancreas 2013, 14, 112–118. [Google Scholar] [CrossRef]
- Donahue, T.R.; Dawson, D.W. Leveraging Mechanisms Governing Pancreatic Tumorigenesis To Reduce Pancreatic Cancer Mortality. Trends Endocrinol. Metab. 2016, 27, 770–781. [Google Scholar] [CrossRef] [Green Version]
- Mayerle, J.; Kalthoff, H.; Reszka, R.; Kamlage, B.; Peter, E.; Schniewind, B.; Maldonado, S.G.; Pilarsky, C.; Heidecke, C.-D.; Schatz, P.; et al. Metabolic Biomarker Signature to Differentiate Pancreatic Ductal Adenocarcinoma from Chronic Pancreatitis. Gut 2018, 67, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Zhang, C.-Y. Diagnostic Biomarkers for Pancreatic Cancer: An Update. World J. Gastroenterol. 2021, 27, 7862–7865. [Google Scholar] [CrossRef]
- de Almeida, R.A.; Heuser, T.; Blaschke, R.; Bartram, C.R.; Janssen, J.W.G. Control of MYEOV Protein Synthesis by Upstream Open Reading Frames. J. Biol. Chem. 2006, 281, 695–704. [Google Scholar] [CrossRef]
- Fang, L.; Wu, S.; Zhu, X.; Cai, J.; Wu, J.; He, Z.; Liu, L.; Zeng, M.; Song, E.; Li, J.; et al. MYEOV Functions as an Amplified Competing Endogenous RNA in Promoting Metastasis by Activating TGF-β Pathway in NSCLC. Oncogene 2019, 38, 896–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Xu, X.; Feng, X.; Zhu, E.; Zhou, J.; Wang, G.; Tian, L.; Wang, B. A Novel Long Noncoding RNA PGC1β-OT1 Regulates Adipocyte and Osteoblast Differentiation through Antagonizing MiR-148a-3p. Cell Death Differ. 2019, 26, 2029–2045. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-Y.; Zhu, M.-X.; Lu, N.-H.; Liu, J.-Q.; Yang, Y.-W.; Zhang, Y.; Shi, Y.-D.; Feng, Z.-H.; Li, J.-X.; Qi, F.-Z.; et al. Circular RNA Circ_0020710 Drives Tumor Progression and Immune Evasion by Regulating the MiR-370-3p/CXCL12 Axis in Melanoma. Mol. Cancer 2020, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Bannoura, S.F.; Uddin, M.H.; Nagasaka, M.; Fazili, F.; Al-Hallak, M.N.; Philip, P.A.; El-Rayes, B.; Azmi, A.S. Targeting KRAS in Pancreatic Cancer: New Drugs on the Horizon. Cancer Metastasis Rev. 2021, 40, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Momeni, S.A.; Zarandi, P.K.; Chalbatani, G.M.; Dana, H.; Mirzaei, H.R.; Akbari, M.E.; Miri, S.R. The Role and Function of Ras-Association Domain Family in Cancer: A Review. Genes Dis. 2019, 6, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs Activate Gene Transcription Epigenetically as an Enhancer Trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yang, H.-S. The Role of Pdcd4 in Tumour Suppression and Protein Translation. Biol. Cell 2018, 110, 169–177. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.; Wang, Q.; Yang, H.-S. AKT Activation by Pdcd4 Knockdown Up-Regulates Cyclin D1 Expression and Promotes Cell Proliferation. Genes Cancer 2011, 2, 818–828. [Google Scholar] [CrossRef]
- Bednar, K.J.; Lee, J.H.; Ort, T. Tregs in Autoimmunity: Insights Into Intrinsic Brake Mechanism Driving Pathogenesis and Immune Homeostasis. Front. Immunol. 2022, 13, 932485. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, W.; Mathew, E.; Bednar, F.; Wan, S.; Collins, M.A.; Evans, R.A.; Welling, T.H.; Vonderheide, R.H.; di Magliano, M.P. CD4 + T Lymphocyte Ablation Prevents Pancreatic Carcinogenesis in Mice. Cancer Immunol. Res. 2014, 2, 423–435. [Google Scholar] [CrossRef]
- Deniger, D.C.; Maiti, S.N.; Mi, T.; Switzer, K.C.; Ramachandran, V.; Hurton, L.V.; Ang, S.; Olivares, S.; Rabinovich, B.A.; Huls, M.H.; et al. Activating and Propagating Polyclonal Gamma Delta T Cells with Broad Specificity for Malignancies. Clin. Cancer Res. 2014, 20, 5708–5719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMichael, E.L.; Jaime-Ramirez, A.C.; Guenterberg, K.D.; Luedke, E.; Atwal, L.S.; Campbell, A.R.; Hu, Z.; Tatum, A.S.; Kondadasula, S.V.; Mo, X.; et al. IL-21 Enhances Natural Killer Cell Response to Cetuximab-Coated Pancreatic Tumor Cells. Clin. Cancer Res. 2017, 23, 489–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Chen, C.; Shen, H.; He, B.Z.; Yu, D.; Jiang, S.; Zhao, S.; Gao, Z.; Zhu, Z.; Chen, X.; et al. GenTree, an Integrated Resource for Analyzing the Evolution and Function of Primate-Specific Coding Genes. Genome Res. 2019, 29, 682–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Lai, F.; Wang, X.; Shang, D.; Zou, J.; Luo, M.; Xia, X.; Cheng, H.; Zhou, R. Srag Regulates Autophagy via Integrating into a Preexisting Autophagy Pathway in Testis. Mol. Biol. Evol. 2021, 38, 128–141. [Google Scholar] [CrossRef]
- Puente, X.S.; Velasco, G.; Gutiérrez-Fernández, A.; Bertranpetit, J.; King, M.-C.; López-Otín, C. Comparative Analysis of Cancer Genes in the Human and Chimpanzee Genomes. BMC Genomics 2006, 7, 15. [Google Scholar] [CrossRef]
- Liang, E.; Lu, Y.; Shi, Y.; Zhou, Q.; Zhi, F. MYEOV Increases HES1 Expression and Promotes Pancreatic Cancer Progression by Enhancing SOX9 Transactivity. Oncogene 2020, 39, 6437–6450. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Fan, Z.; Liu, H.; Zhang, X.; Cai, Z.; Xu, L.; Luo, J.; Huang, Y.; He, L.; et al. Single-Cell Sequencing Reveals Variants in ARID1A, GPRC5A and MLL2 Driving Self-Renewal of Human Bladder Cancer Stem Cells. Eur. Urol. 2017, 71, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Greenhough, A.; Bagley, C.; Heesom, K.J.; Gurevich, D.B.; Gay, D.; Bond, M.; Collard, T.J.; Paraskeva, C.; Martin, P.; Sansom, O.J.; et al. Cancer Cell Adaptation to Hypoxia Involves a HIF-GPRC5A-YAP Axis. EMBO Mol. Med. 2018, 10, e8699. [Google Scholar] [CrossRef]
- Moyano-Galceran, L.; Pietilä, E.A.; Turunen, S.P.; Corvigno, S.; Hjerpe, E.; Bulanova, D.; Joneborg, U.; Alkasalias, T.; Miki, Y.; Yashiro, M.; et al. Adaptive RSK-EphA2-GPRC5A Signaling Switch Triggers Chemotherapy Resistance in Ovarian Cancer. EMBO Mol. Med. 2020, 12, e11177. [Google Scholar] [CrossRef]
- Zhou, H.; Telonis, A.G.; Jing, Y.; Xia, N.L.; Biederman, L.; Jimbo, M.; Blanco, F.; Londin, E.; Brody, J.R.; Rigoutsos, I. GPRC5A Is a Potential Oncogene in Pancreatic Ductal Adenocarcinoma Cells That Is Upregulated by Gemcitabine with Help from HuR. Cell Death Dis. 2016, 7, e2294. [Google Scholar] [CrossRef]
- Jahny, E.; Yang, H.; Liu, B.; Jahnke, B.; Lademann, F.; Knösel, T.; Rümmele, P.; Grützmann, R.; Aust, D.E.; Pilarsky, C.; et al. The G Protein-Coupled Receptor RAI3 Is an Independent Prognostic Factor for Pancreatic Cancer Survival and Regulates Proliferation via STAT3 Phosphorylation. PLoS ONE 2017, 12, e0170390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, M.A.; Bednar, F.; Zhang, Y.; Brisset, J.-C.; Galbán, S.; Galbán, C.J.; Rakshit, S.; Flannagan, K.S.; Adsay, N.V.; di Magliano, M.P. Oncogenic Kras Is Required for Both the Initiation and Maintenance of Pancreatic Cancer in Mice. J. Clin. Investig. 2012, 122, 639–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buscail, L. Exosomes for Targeting KRAS in the Treatment of Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 636–638. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; Quinn, M.C.; et al. Genomic Analyses Identify Molecular Subtypes of Pancreatic Cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, J.H.; Cheng, Y.; Wu, W.; Bhagat, T.; Yu, Y.; Abraham, J.M.; Ibrahim, S.; Ravich, W.; Roland, B.C.; et al. Long Non-Coding RNA HNF1A-AS1 Regulates Proliferation and Migration in Oesophageal Adenocarcinoma Cells. Gut 2014, 63, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Öhlund, D.; Rickelt, S.; Lidström, T.; Huang, Y.; Hao, L.; Zhao, R.T.; Franklin, O.; Bhatia, S.N.; Tuveson, D.A.; et al. Cancer Cell–Derived Matrisome Proteins Promote Metastasis in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2020, 80, 1461–1474. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Chen, X.; Wu, X.; Wang, X.; Wang, Y.; Lin, T.-Y.; Kurata, J.; Wu, J.; Vonderfecht, S.; Sun, G.; et al. Disruption of MicroRNA-21 by TALEN Leads to Diminished Cell Transformation and Increased Expression of Cell-Environment Interaction Genes. Cancer Lett. 2015, 356, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, S.S.; Blackhall, F.; Krzakowski, M.; Barrios, C.H.; Park, K.; Bover, I.; Heo, D.S.; Rosell, R.; Talbot, D.C.; Frank, R.; et al. Randomized Phase II Study of Dacomitinib (PF-00299804), an Irreversible Pan–Human Epidermal Growth Factor Receptor Inhibitor, Versus Erlotinib in Patients With Advanced Non–Small-Cell Lung Cancer. JCO 2012, 30, 3337–3344. [Google Scholar] [CrossRef] [Green Version]
- Longhi, M.T.; Magalhães, M.; Reina, J.; Freitas, V.M.; Cella, N. EGFR Signaling Regulates Maspin/SerpinB5 Phosphorylation and Nuclear Localization in Mammary Epithelial Cells. PLoS ONE 2016, 11, e0159856. [Google Scholar] [CrossRef] [Green Version]
- De Benedetti, A.; Harris, A.L. EIF4E Expression in Tumors: Its Possible Role in Progression of Malignancies. Int. J. Biochem. Cell Biol. 1999, 31, 59–72. [Google Scholar] [CrossRef]
- Beatty, G.L.; Gladney, W.L. Immune Escape Mechanisms as a Guide for Cancer Immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, E.R.; Wu, A.A.; Bigelow, E.; Sharma, R.; Mo, G.; Soares, K.; Solt, S.; Dorman, A.; Wamwea, A.; Yager, A.; et al. Immunotherapy Converts Nonimmunogenic Pancreatic Tumors into Immunogenic Foci of Immune Regulation. Cancer Immunol. Res. 2014, 2, 616–631. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, J.; Guo, Q.; Li, X.; Zou, X. Identification of MYEOV-Associated Gene Network as a Potential Therapeutic Target in Pancreatic Cancer. Cancers 2022, 14, 5439. https://doi.org/10.3390/cancers14215439
Chen Y, Wang J, Guo Q, Li X, Zou X. Identification of MYEOV-Associated Gene Network as a Potential Therapeutic Target in Pancreatic Cancer. Cancers. 2022; 14(21):5439. https://doi.org/10.3390/cancers14215439
Chicago/Turabian StyleChen, Yu, Jialun Wang, Qiyuan Guo, Xihan Li, and Xiaoping Zou. 2022. "Identification of MYEOV-Associated Gene Network as a Potential Therapeutic Target in Pancreatic Cancer" Cancers 14, no. 21: 5439. https://doi.org/10.3390/cancers14215439




