Discovery of Mitochondrial Complex I Inhibitors as Anticancer and Radiosensitizer Drugs Based on Compensatory Stimulation of Lactate Release
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Cell Culture
2.3. Metabolic Profiling
2.4. 3D Cultures
2.5. Statistical Analysis
3. Results
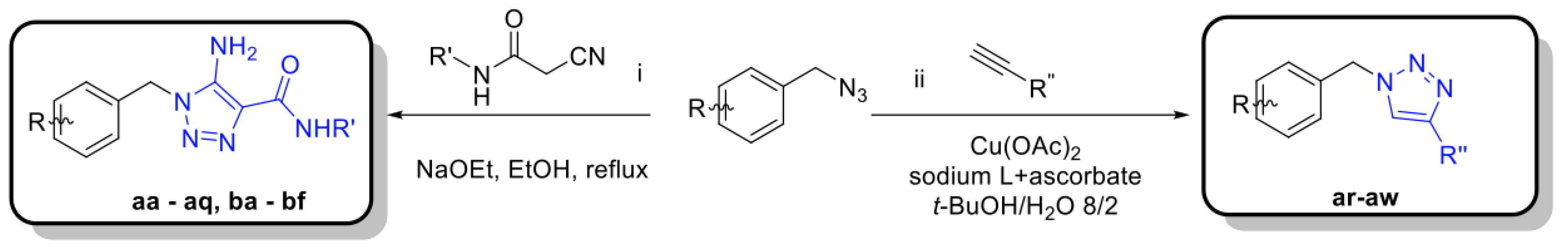
3.1. Synthesis of Carboxyamidotriazole Derivatives
3.2. Three-Step Procedure to Select for Mitochondrial Complex I Inhibitors as Anticancer Drugs
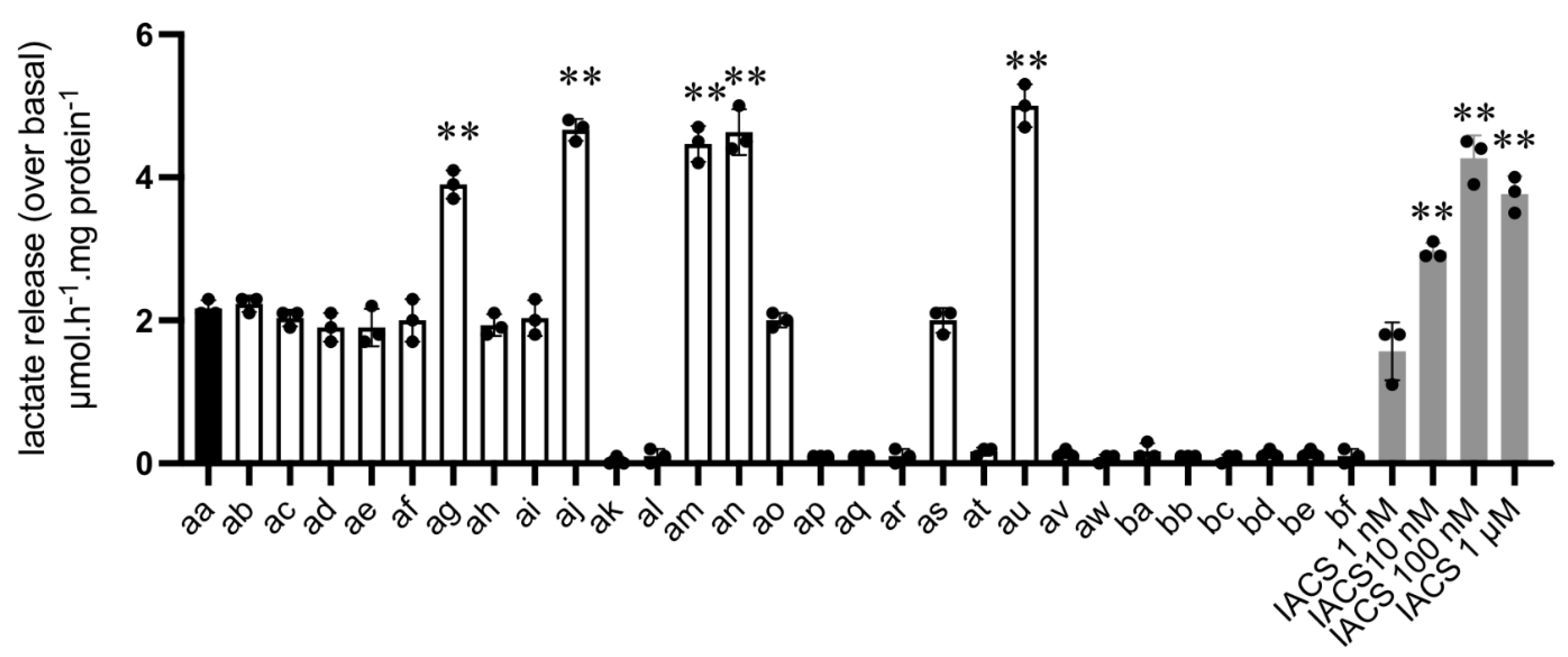
3.3. L-Lactate Release Measurement as a Primary Assay to Identify Potential OXPHOS Inhibitors
3.4. Validation of Complex I Inhibitors Using Seahorse-Based OCR Measurements
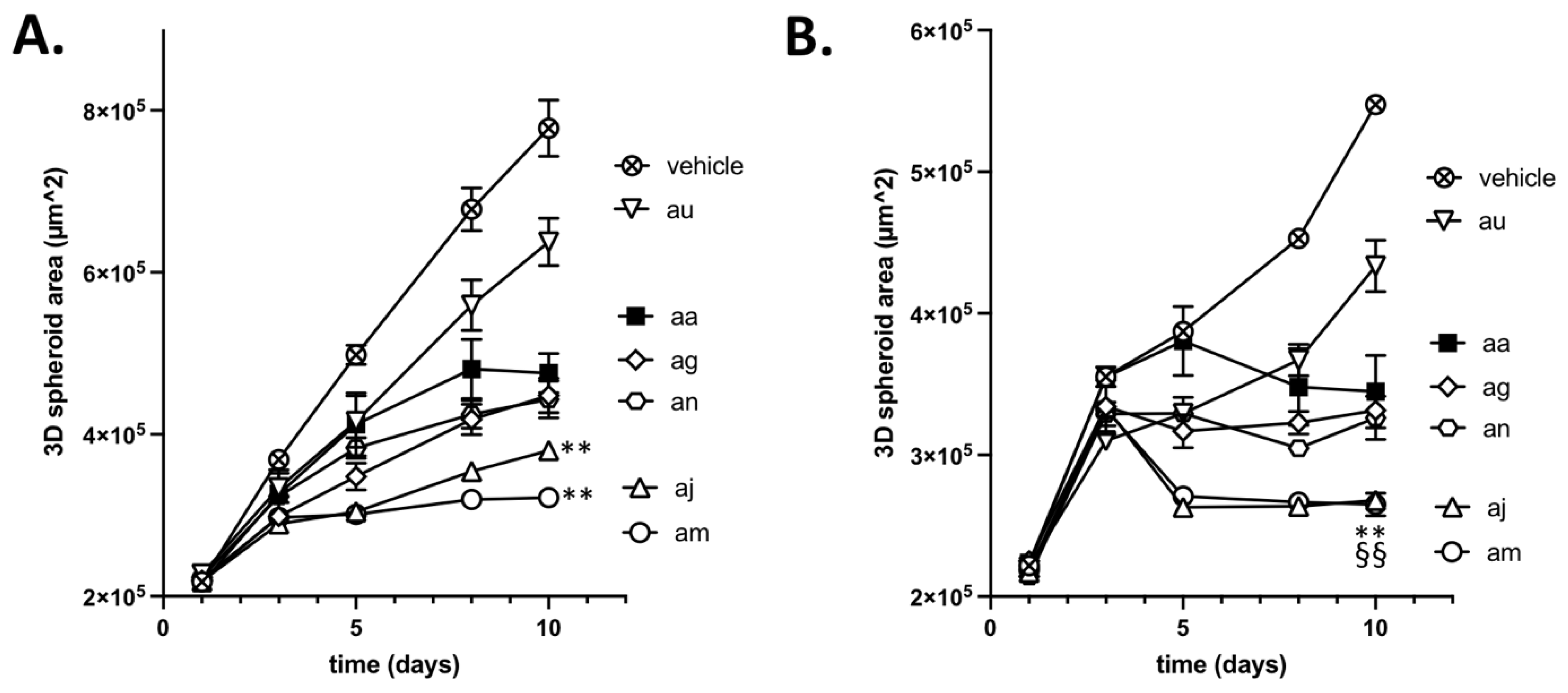
3.5. Validation of the Cytotoxic and Radiosensitizing Potentials of aa Derivatives
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sica, V.; Bravo-San Pedro, J.M.; Stoll, G.; Kroemer, G. Oxidative phosphorylation as a potential therapeutic target for cancer therapy. Int. J. Cancer 2020, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Boreel, D.F.; Span, P.N.; Heskamp, S.; Adema, G.J.; Bussink, J. Targeting oxidative phosphorylation to increase the efficacy of radio- and immune-combination therapy. Clin. Cancer Res. 2021, 27, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.L.; Coelho, A.R.; Marques, R.; Oliveira, P.J. Cancer cell metabolism: Rewiring the mitochondrial hub. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166016. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Cancer cell metabolism and mitochondria: Nutrient plasticity for tca cycle fueling. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 7–15. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Hardy, M.; Topchyan, P.; Zander, R.; Volberding, P.; Cui, W.; Kalyanaraman, B. Potent inhibition of tumour cell proliferation and immunoregulatory function by mitochondria-targeted atovaquone. Sci. Rep. 2020, 10, 17872. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. Therapeutic targeting of tumor cells and tumor immune microenvironment vulnerabilities. Front. Oncol. 2022, 12, 816504. [Google Scholar] [CrossRef]
- Ellinghaus, P.; Heisler, I.; Unterschemmann, K.; Haerter, M.; Beck, H.; Greschat, S.; Ehrmann, A.; Summer, H.; Flamme, I.; Oehme, F.; et al. Bay 87-2243, a highly potent and selective inhibitor of hypoxia-induced gene activation has antitumor activities by inhibition of mitochondrial complex i. Cancer Med. 2013, 2, 611–624. [Google Scholar] [CrossRef]
- Molina, J.R.; Sun, Y.; Protopopova, M.; Gera, S.; Bandi, M.; Bristow, C.; McAfoos, T.; Morlacchi, P.; Ackroyd, J.; Agip, A.A.; et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 2018, 24, 1036–1046. [Google Scholar] [CrossRef]
- Saito, K.; Zhang, Q.; Yang, H.; Yamatani, K.; Ai, T.; Ruvolo, V.; Baran, N.; Cai, T.; Ma, H.; Jacamo, R.; et al. Exogenous mitochondrial transfer and endogenous mitochondrial fission facilitate aml resistance to oxphos inhibition. Blood Adv. 2021, 5, 4233–4255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, Y.; Zhang, S.; Liu, Y.; Guo, H.; Ahmed, M.; Bell, T.; Zhang, H.; Han, G.; Lorence, E.; et al. Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci. Transl. Med. 2019, 11, eaau1167. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hu, C.; Kluza, J.; Ke, W.; Tian, G.; Giurgiu, M.; Bleilevens, A.; Campos, A.R.; Charbono, A.; Stickeler, E.; et al. Metabolic targeting of cancer by a ubiquinone uncompetitive inhibitor of mitochondrial complex i. Cell Chem. Biol. 2022, 29, 436–450.e15. [Google Scholar] [CrossRef] [PubMed]
- Lissanu Deribe, Y.; Sun, Y.; Terranova, C.; Khan, F.; Martinez-Ledesma, J.; Gay, J.; Gao, G.; Mullinax, R.A.; Khor, T.; Feng, N.; et al. Mutations in the swi/snf complex induce a targetable dependence on oxidative phosphorylation in lung cancer. Nat. Med. 2018, 24, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Bastien, E.; Draoui, N.; Doix, B.; Mignion, L.; Jordan, B.F.; Marchand, A.; Vanherck, J.C.; Chaltin, P.; Schakman, O.; et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat. Commun. 2018, 9, 1208. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Atlante, A. Mitochondria and l-lactate metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef] [Green Version]
- de Bari, L.; Atlante, A. Including the mitochondrial metabolism of l-lactate in cancer metabolic reprogramming. Cell Mol. Life Sci. 2018, 75, 2763–2776. [Google Scholar] [CrossRef]
- Lemire, J.; Mailloux, R.J.; Appanna, V.D. Mitochondrial lactate dehydrogenase is involved in oxidative-energy metabolism in human astrocytoma cells (ccf-sttg1). PLoS ONE 2008, 3, e1550. [Google Scholar] [CrossRef] [Green Version]
- Passarella, S.; Paventi, G.; Pizzuto, R. The mitochondrial l-lactate dehydrogenase affair. Front. Neurosci. 2014, 8, 407. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Mahieu, N.G.; Huang, X.; Singh, M.; Crawford, P.A.; Johnson, S.L.; Gross, R.W.; Schaefer, J.; Patti, G.J. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 2016, 12, 937–943. [Google Scholar] [CrossRef]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef] [PubMed]
- Feron, O. Pyruvate into lactate and back: From the warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 2009, 92, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Sonveaux, P.; Vegran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [Green Version]
- Boidot, R.; Vegran, F.; Meulle, A.; Le Breton, A.; Dessy, C.; Sonveaux, P.; Lizard-Nacol, S.; Feron, O. Regulation of monocarboxylate transporter mct1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012, 72, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Mignen, O.; Brink, C.; Enfissi, A.; Nadkarni, A.; Shuttleworth, T.J.; Giovannucci, D.R.; Capiod, T. Carboxyamidotriazole-induced inhibition of mitochondrial calcium import blocks capacitative calcium entry and cell proliferation in hek-293 cells. J. Cell Sci. 2005, 118, 5615–5623. [Google Scholar] [CrossRef] [Green Version]
- Ju, R.; Guo, L.; Li, J.; Zhu, L.; Yu, X.; Chen, C.; Chen, W.; Ye, C.; Zhang, D. Carboxyamidotriazole inhibits oxidative phosphorylation in cancer cells and exerts synergistic anti-cancer effect with glycolysis inhibition. Cancer Lett. 2016, 370, 232–241. [Google Scholar] [CrossRef]
- Corbet, C.; Draoui, N.; Polet, F.; Pinto, A.; Drozak, X.; Riant, O.; Feron, O. The sirt1/hif2alpha axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Res. 2014, 74, 5507–5519. [Google Scholar] [CrossRef] [Green Version]
- Draoui, N.; Schicke, O.; Seront, E.; Bouzin, C.; Sonveaux, P.; Riant, O.; Feron, O. Antitumor activity of 7-aminocarboxycoumarin derivatives, a new class of potent inhibitors of lactate influx but not efflux. Mol. Cancer Ther. 2014, 13, 1410–1418. [Google Scholar] [CrossRef] [Green Version]
- Bol, V.; Bol, A.; Bouzin, C.; Labar, D.; Lee, J.A.; Janssens, G.; Porporato, P.E.; Sonveaux, P.; Feron, O.; Gregoire, V. Reprogramming of tumor metabolism by targeting mitochondria improves tumor response to irradiation. Acta Oncol. 2015, 54, 266–274. [Google Scholar] [CrossRef]
- Brand, S.; Ko, E.J.; Viayna, E.; Thompson, S.; Spinks, D.; Thomas, M.; Sandberg, L.; Francisco, A.F.; Jayawardhana, S.; Smith, V.C.; et al. Discovery and optimization of 5-amino-1,2,3-triazole-4-carboxamide series against trypanosoma cruzi. J. Med. Chem. 2017, 60, 7284–7299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochis, R.J.; Chabala, J.C.; Harris, E.; Peterson, L.H.; Barash, L.; Beattie, T.; Brown, J.E.; Graham, D.W.; Waksmunski, F.S.; Tischler, M.; et al. Benzylated 1,2,3-triazoles as anticoccidiostats. J. Med. Chem. 1991, 34, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.G.; Le Grand, D.; Dowling, M.; Brocklehurst, C.E.; Chinn, C.; Elphick, L.; Faller, M.; Freeman, M.; Furminger, V.; Gasser, C.; et al. Development of autotaxin inhibitors: A series of zinc binding triazoles. Bioorg. Med. Chem. Lett. 2018, 28, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Pinto, A.; Martherus, R.; Santiago de Jesus, J.P.; Polet, F.; Feron, O. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 2016, 24, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: Oxphos protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]
- Doerrier, C.; Garcia-Souza, L.F.; Krumschnabel, G.; Wohlfarter, Y.; Meszaros, A.T.; Gnaiger, E. High-resolution fluorespirometry and oxphos protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol. Biol. 2018, 1782, 31–70. [Google Scholar]
- Iuso, A.; Repp, B.; Biagosch, C.; Terrile, C.; Prokisch, H. Assessing mitochondrial bioenergetics in isolated mitochondria from various mouse tissues using seahorse xf96 analyzer. Methods Mol. Biol. 2017, 1567, 217–230. [Google Scholar]
- Hashimoto, T.; Hussien, R.; Brooks, G.A. Colocalization of mct1, cd147, and ldh in mitochondrial inner membrane of l6 muscle cells: Evidence of a mitochondrial lactate oxidation complex. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1237–E1244. [Google Scholar] [CrossRef]
- De Bari, L.; Chieppa, G.; Marra, E.; Passarella, S. L-lactate metabolism can occur in normal and cancer prostate cells via the novel mitochondrial l-lactate dehydrogenase. Int. J. Oncol. 2010, 37, 1607–1620. [Google Scholar]
- Hussien, R.; Brooks, G.A. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol. Genom. 2011, 43, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acin-Perez, R.; Latorre-Pellicer, A.; Colas, C.; Balsa, E.; Perales-Clemente, E.; Quiros, P.M.; Calvo, E.; Rodriguez-Hernandez, M.A.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Protasoni, M.; Perez-Perez, R.; Lobo-Jarne, T.; Harbour, M.E.; Ding, S.; Penas, A.; Diaz, F.; Moraes, C.T.; Fearnley, I.M.; Zeviani, M.; et al. Respiratory supercomplexes act as a platform for complex iii-mediated maturation of human mitochondrial complexes i and iv. EMBO J. 2020, 39, e102817. [Google Scholar] [CrossRef] [PubMed]
- Letts, J.A.; Fiedorczuk, K.; Degliesposti, G.; Skehel, M.; Sazanov, L.A. Structures of respiratory supercomplex i+iii2 reveal functional and conformational crosstalk. Mol. Cell 2019, 75, 1131–1146.e6. [Google Scholar] [CrossRef] [PubMed]



| Compound No. |  i (aa–aw) | 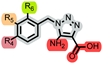 ii (ba–bf) | |||||
|---|---|---|---|---|---|---|---|
| X | R1 | R2 | R3 | R4 | R5 | R6 | |
| aa | -Cl | 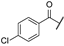 | -NH2 | -CONH2 | - | - | - |
| ab | -Cl |  | -NH2 | -CONH2 | - | - | - |
| ac | -Cl | 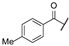 | -NH2 | -CONH2 | - | - | - |
| ad | -Cl | 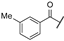 | -NH2 | -CONH2 | - | - | - |
| ae | -Cl | 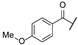 | -NH2 | -CONH2 | - | - | - |
| af | -Cl | 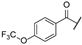 | -NH2 | -CONH2 | - | - | - |
| ag | -Cl |  | -NH2 | -CONH2 | - | - | - |
| ah | -Cl | 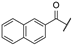 | -NH2 | -CONH2 | - | - | - |
| ai | -Cl | 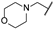 | -NH2 | -CONH2 | - | - | - |
| aj | -Cl | 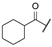 | -NH2 | -CONH2 | - | - | - |
| ak | -Cl |  | -NH2 | 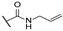 | - | - | - |
| al | -Cl |  | -NH2 | -CONH2 | - | - | - |
| am | -Cl | 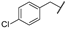 | -NH2 | -CONH2 | - | - | - |
| an | -F |  | -NH2 | -CONH2 | - | - | - |
| ao | -Cl | 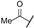 | -NH2 | -CONH2 | - | - | - |
| ap | -Me | -H | -NH2 | -CONH2 | - | - | - |
| aq | -Cl | -H | -NH2 | -CONH2 | - | - | - |
| ar | -Cl | 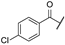 | -H | -COOH | - | - | - |
| as | -Cl |  | -H | -CONH2 | - | - | - |
| at | -Cl | 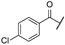 | -H | -CH2NH2 | - | - | - |
| au | -Cl |  | -H | -COOCH3 | - | - | - |
| av | -Cl |  | -H | -CH2OH | - | - | - |
| aw | -F | 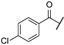 | -H | -CONH2 | - | - | - |
| ba | - | - | - | - | 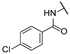 | -H | -H |
| bb | - | - | - | - | -H | 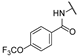 | -H |
| bc | - | - | - | - | -H | 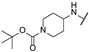 | -H |
| bd | - | - | - | - | -H | 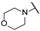 | -H |
| be | - | - | - | - | -H | -H |  |
| bf | - | - | - | - | -H | -H | 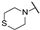 |
| Assay #1 | Assay #2 | Assay #3 | |
|---|---|---|---|
| compounds | [LAC] increase (µmol·h−1·mg−1) | OCR inhib. (IC50, µM) | 3D growth inhib. (%) |
| aa | ~2 | 1 < x < 10 | 25 < x < 50 |
| ab | ~2 | ~10 | n.d. |
| ac | ~2 | ~10 | n.d. |
| ad | ~2 | ~10 | n.d. |
| ae | ~2 | ~10 | n.d. |
| af | ~2 | ~10 | n.d. |
| ag | ~4 | 1 < x < 10 | 25 < x < 50 |
| ah | ~2 | ~10 | n.d. |
| ai | ~2 | >10 | n.d. |
| aj | ~4 | 1 < x < 10 | >50 |
| ak | ~0 | >10 | n.d. |
| al | ~0 | >10 | n.d. |
| am | ~4 | ~10 | >50 |
| an | ~4 | ~10 | 25 < x < 50 |
| ao | ~2 | >10 | n.d. |
| ap | ~0 | >10 | n.d. |
| aq | ~0 | >10 | n.d. |
| ar | ~0 | >10 | n.d. |
| as | ~2 | >10 | n.d. |
| at | ~0 | >10 | n.d. |
| au | ~4 | 1 < x < 10 | <25 |
| av | ~0 | >10 | n.d. |
| aw | ~0 | >10 | n.d. |
| ba | ~0 | >10 | n.d. |
| bb | ~0 | >10 | n.d. |
| bc | ~0 | >10 | n.d. |
| bd | ~0 | >10 | n.d. |
| be | ~0 | >10 | n.d. |
| bf | ~0 | >10 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, J.; Cadassou, O.; Corbet, C.; Riant, O.; Feron, O. Discovery of Mitochondrial Complex I Inhibitors as Anticancer and Radiosensitizer Drugs Based on Compensatory Stimulation of Lactate Release. Cancers 2022, 14, 5454. https://doi.org/10.3390/cancers14215454
Lan J, Cadassou O, Corbet C, Riant O, Feron O. Discovery of Mitochondrial Complex I Inhibitors as Anticancer and Radiosensitizer Drugs Based on Compensatory Stimulation of Lactate Release. Cancers. 2022; 14(21):5454. https://doi.org/10.3390/cancers14215454
Chicago/Turabian StyleLan, Junjie, Octavia Cadassou, Cyril Corbet, Olivier Riant, and Olivier Feron. 2022. "Discovery of Mitochondrial Complex I Inhibitors as Anticancer and Radiosensitizer Drugs Based on Compensatory Stimulation of Lactate Release" Cancers 14, no. 21: 5454. https://doi.org/10.3390/cancers14215454
APA StyleLan, J., Cadassou, O., Corbet, C., Riant, O., & Feron, O. (2022). Discovery of Mitochondrial Complex I Inhibitors as Anticancer and Radiosensitizer Drugs Based on Compensatory Stimulation of Lactate Release. Cancers, 14(21), 5454. https://doi.org/10.3390/cancers14215454








