Recurrence of Oral Leukoplakia after CO2 Laser Resection: A Prospective Longitudinal Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
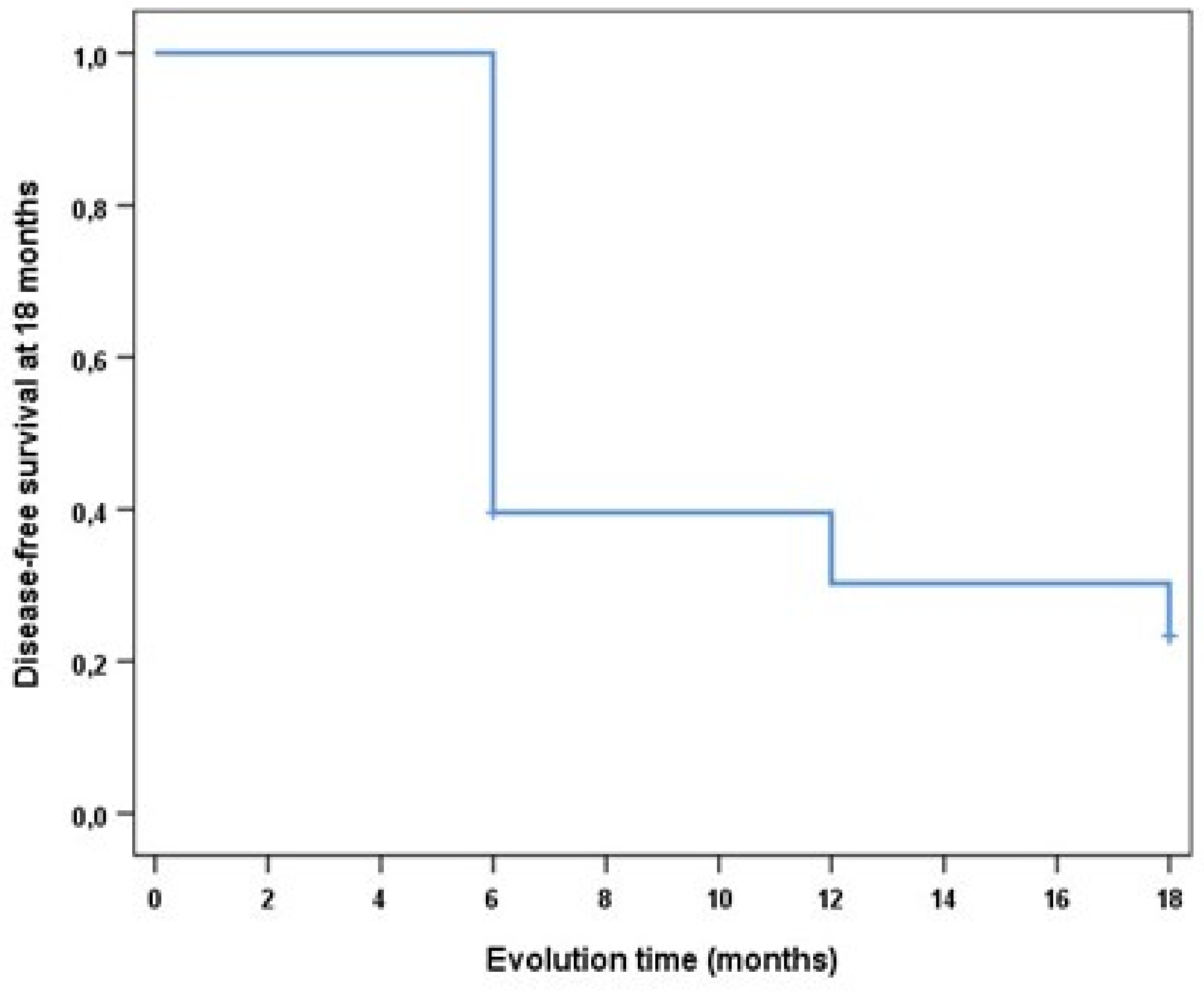
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pentenero, M.; Sutera, S.; Lodi, G.; Bagan, J.V.; Farah, C.S. Oral leukoplakia diagnosis and treatment in Europe and Australia: Oral Medicine Practitioners’ attitudes and practice. Oral Dis. 2022. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Petti, S.; Scully, C. Association between different alcoholic beverages and leukoplakia among non- to moderate-drinking adults: A matched case–control study. Eur. J. Cancer 2006, 42, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Petti, S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003, 39, 770–780. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classifcation of potentially malignant disorders of the oral mucosa. J. Oral. Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- González-Moles, M.; González Ruiz, L. Leucoplasia oral, una revisión de los aspectos esenciales de su diagnóstico y tratamiento. Actual Med. 2018, 803, 49–51. [Google Scholar] [CrossRef]
- Axell, T.; Pindborg, J.; Smith, C.; Van Der Waal, I. Oral white lesions with special reference to precancerous and tobacco. J. Oral Pathol. Med. 1996, 25, 49–54. [Google Scholar] [CrossRef]
- Martorell, A.; Botella, R.; Jv, B.; Sanmartín, O.; Guillén, C. Oral leukoplakia: Clinical, histopathologic, and molecular features and therapeutic approach. Actas Dermosifiliogr. 2009, 100, 669–684. [Google Scholar]
- Arduino, P.G.; Bagan, J.; El-Naggar, A.K.; Carrozzo, M. Urban legends series: Oral leukoplakia. Oral Dis. 2013, 19, 642–659. [Google Scholar] [CrossRef]
- Varela-Centelles, P.; Seoane, J.; Ulloa-Morales, Y.; Estany-Gestal, A.; Blanco-Hortas, A.; García-Pola, M.J.; Seoane-Romero, J.M. Oral cancer awareness in North-Western Spain: A population-based study. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e518–e525. [Google Scholar] [CrossRef]
- Tenore, G.; Nuvoli, A.; Mohsen, A.; Cassoni, A.; Battisti, A.; Terenzi, V.; Della Monaca, M.; Raponi, I.; Brauner, E.; De Felice, F.; et al. Tobacco, Alcohol and Family History of Cancer as Risk Factors of Oral Squamous Cell Carcinoma: Case-Control Retrospective Study. Appl. Sci. 2020, 10, 3896. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S. Oral leukoplakia remains a challenging condition. Oral Dis. 2018, 24, 179–183. [Google Scholar] [CrossRef]
- Brouns, E.; Baart, J.; Bloemena, E.; Karagozoglu, H.; Van Der Waal, I. The relevance of uniform reporting in oral leukoplakia: Definition, certainty factor and staging based on experience with 275 patients. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e19–e26. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Pathol. Med. 2016, 45, 155–166. [Google Scholar] [CrossRef]
- Holmstrup, P.; Vedtofte, P.; Reibel, J.; Stoltze, K. Long-term treatment outcome of oral premalignant lesions. Oral Oncol. 2006, 42, 461–474. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Tao, Y.; Hao, Y.; Jiang, L.; Dan, H.; Zeng, X.; Chen, Q.; Zhou, Y. Malignant transformation of oral leukoplakia treated with carbon dioxide laser: A meta-analysis. Lasers Med. Sci. 2019, 34, 209–221. [Google Scholar] [CrossRef]
- Gandara-Vila, P.; Sayáns, M.P.; Suarez-Penaranda, J.; Gallas-Torreira, M.; Martín, J.M.S.; Lopez, R.; Blanco-Carrion, A.; Garcia-Garcia, A. Survival study of leukoplakia malignant transformation in a region of northern Spain. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e413–e420. [Google Scholar] [CrossRef]
- Kindler, S.; Samietz, S.; Dickel, S.; Mksoud, M.; Kocher, T.; Lucas, C.; Seebauer, C.; Doberschütz, P.; Holtfreter, B.; Völzke, H.; et al. Prevalence and risk factors of potentially malignant disorders of the mucosa in the general population: Mucosa lesions a general health problem? Ann. Anat. 2021, 237, 151724. [Google Scholar] [CrossRef]
- Palaia, G.; Bellisario, A.; Pampena, R.; Pippi, R.; Romeo, U. Oral Proliferative Verrucous Leukoplakia: Progression to Malignancy and Clinical Implications. Systematic Review and Meta-Analysis. Cancers 2021, 13, 4085. [Google Scholar] [CrossRef]
- Proaño-Haro, A.; Bagan, L.; Bagan, J.V. Recurrences following treatment of proliferative verrucous leukoplakia: A systematic review and meta-analysis. J. Oral Pathol. Med. 2021, 50, 820–828. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M.; Mello, F.W.; Bagan, J.V.; Warnakulasuriya, S. Malignant transformation of oral proliferative verrucous leukoplakia: A systematic review and meta-analysis. Oral Dis. 2021, 27, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R.; Fitzpatrick, S.G.; Müller, S.; Eisenberg, E.; Upadhyaya, J.D.; Lingen, M.W.; Vigneswaran, N.; Woo, S.-B.; Bhattacharyya, I.; Bilodeau, E.A.; et al. Proliferative Verrucous Leukoplakia: An Expert Consensus Guideline for Standardized Assessment and Reporting. Head Neck Pathol. 2021, 15, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; López, S.P.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Ishii, J.; Fujita, K.; Komori, T. Laser surgery as a treatment for oral leukoplakia. Oral Oncol. 2003, 39, 759–769. [Google Scholar] [CrossRef]
- van der Hem, P.; Nauta, J.; van der Wal, J.; Roodenburg, J. The results of CO2 laser surgery in patients with oral leukoplakia: A 25 year follow up. Oral Oncol. 2005, 41, 31–37. [Google Scholar] [CrossRef]
- Lodi, G.; Franchini, R.; Warnakulasuriya, S.; Varoni, E.M.; Sardella, A.; Kerr, A.R.; Carrassi, A.; MacDonald, L.C.; Worthington, H.V. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst. Rev. 2016, 2016, CD001829. [Google Scholar] [CrossRef]
- Mogedas-Vegara, A.; Hueto-Madrid, J.-A.; Chimenos-Küstner, E.; Bescós-Atín, C. Oral leukoplakia treatment with the carbon dioxide laser: A systematic review of the literature. J. Cranio-Maxillofacial Surg. 2016, 44, 331–336. [Google Scholar] [CrossRef]
- Romeo, U.; Mohsen, M.; Palaia, G.; Bellisario, A.; Del Vecchio, A.; Tenore, G. CO2 laser ablation of oral leukoplakia: With or without extension of margins? Clin. Ter. 2020, 171, e209–e215. [Google Scholar] [CrossRef]
- Yang, S.-W.; Lee, Y.-S.; Chang, L.-C.; Yang, C.-H.; Luo, C.-M. An anatomical perspective on clinicopathological characteristics and treatment outcomes of dorsal and ventrolateral tongue leukoplakia after carbon dioxide laser surgery. BMC Oral Health 2021, 21, 45. [Google Scholar] [CrossRef]
- Monteiro, L.; Barbieri, C.; Warnakulasuriya, S.; Martins, M.; Salazar, F.; Pacheco, J.J.; Vescovi, P.; Meleti, M. Type of surgical treatment and recurrence of oral leukoplakia, A retrospective clinical study. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e520–e526. [Google Scholar] [CrossRef]
- Paglioni, M.D.P.; Migliorati, C.A.; Faustino, I.S.P.; Mariz, B.A.L.A.; Roza, A.L.O.C.; Vargas, P.A.; Leme, A.F.P.; Brandão, T.B.; Ribeiro, A.C.P.; Lopes, M.A.; et al. Laser excision of oral leukoplakia: Does it affect recurrence and malignant transformation? A systematic review and meta-analysis. Oral Oncol. 2020, 109, 104850. [Google Scholar] [CrossRef]
- Bagan, J.; Martorell, M.; Cebrián, J.L.; Rubert, A.; Bagán, L.; Mezquida, C.; Hervás, D. Effect of clinical and histologic features on time to malignancy in 224 cases of oral leukoplakia treated by surgery. Clin. Oral Investig. 2022, 26, 5181–5188. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Li, C.; Wu, L.; Tang, G. Management of oral leukoplakia by ablative fractional laser-assisted photodynamic therapy: A 3-year retrospective study of 48 patients. Lasers Surg. Med. 2022, 54, 682–687. [Google Scholar] [CrossRef]
- Kuribayashi, Y.; Tsushima, F.; Sato, M.; Morita, K.-I.; Omura, K. Recurrence patterns of oral leukoplakia after curative surgical resection: Important factors that predict the risk of recurrence and malignancy. J. Oral Pathol. Med. 2012, 41, 682–688. [Google Scholar] [CrossRef]
- Tiwari, L.; Kujan, O.; Farah, C.S. Optical fluorescence imaging in oral cancer and potentially malignant disorders: A systematic review. Oral Dis. 2020, 26, 491–510. [Google Scholar] [CrossRef]
- Brouns, E.; Baart, J.; Karagozoglu, K.; Aartman, I.; Bloemena, E.; Van Der Waal, I. Malignant transformation of oral leukoplakia in a well-defined cohort of 144 patients. Oral Dis. 2014, 20, e19–e24. [Google Scholar] [CrossRef]
- Evren, I.; Brouns, E.R.; Poell, J.B.; Wils, L.J.; Brakenhoff, R.H.; Bloemena, E.; de Visscher, J.G. Associations between clinical and histopathological characteristics in oral leukoplakia. Oral Dis. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Holmstrup, P.; Dabelsteen, E. Oral leukoplakia-to treat or not to treat. Oral Dis. 2016, 22, 494–497. [Google Scholar] [CrossRef]
- Angadi, P.V.; Savitha, J.K.; Rao, S.; Sivaranjini, Y. Oral field cancerization: Current evidence and future perspectives. Oral Maxillofac. Surg. 2012, 16, 171–180. [Google Scholar] [CrossRef]
- Holmstrup, P.; Vedtofte, P.; Reibel, J. Stoltze K Oral premalignant lesions: Is a biopsy reliable? J. Oral Pathol. Med. 2007, 36, 262–266. [Google Scholar] [CrossRef]
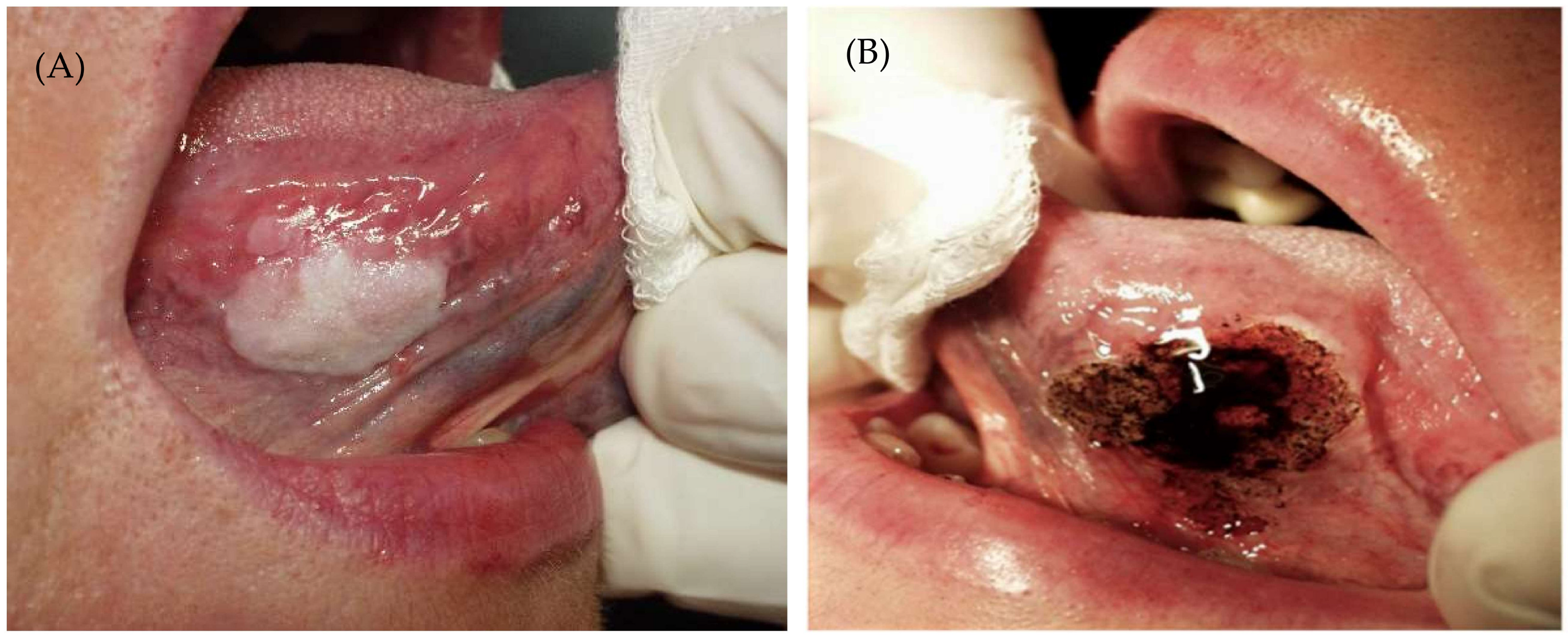
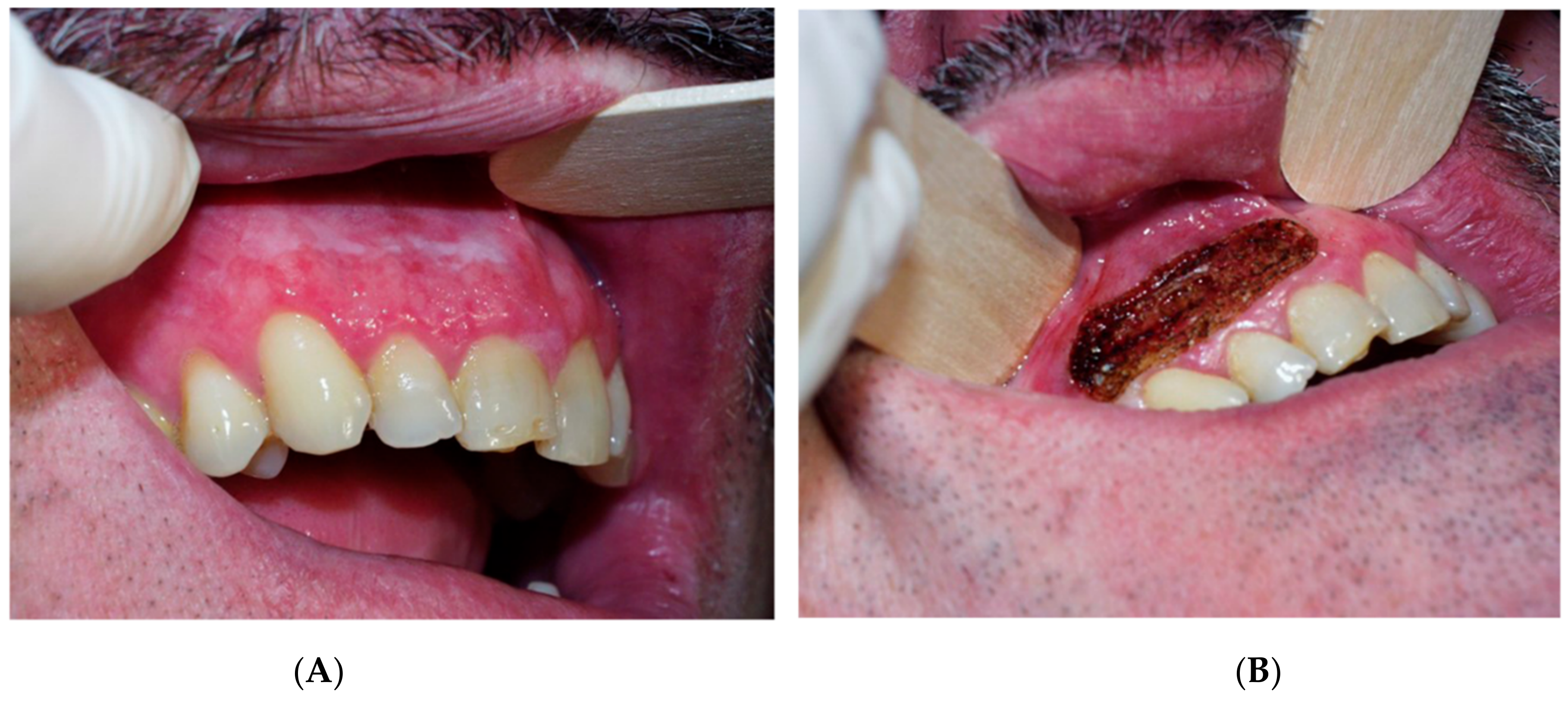
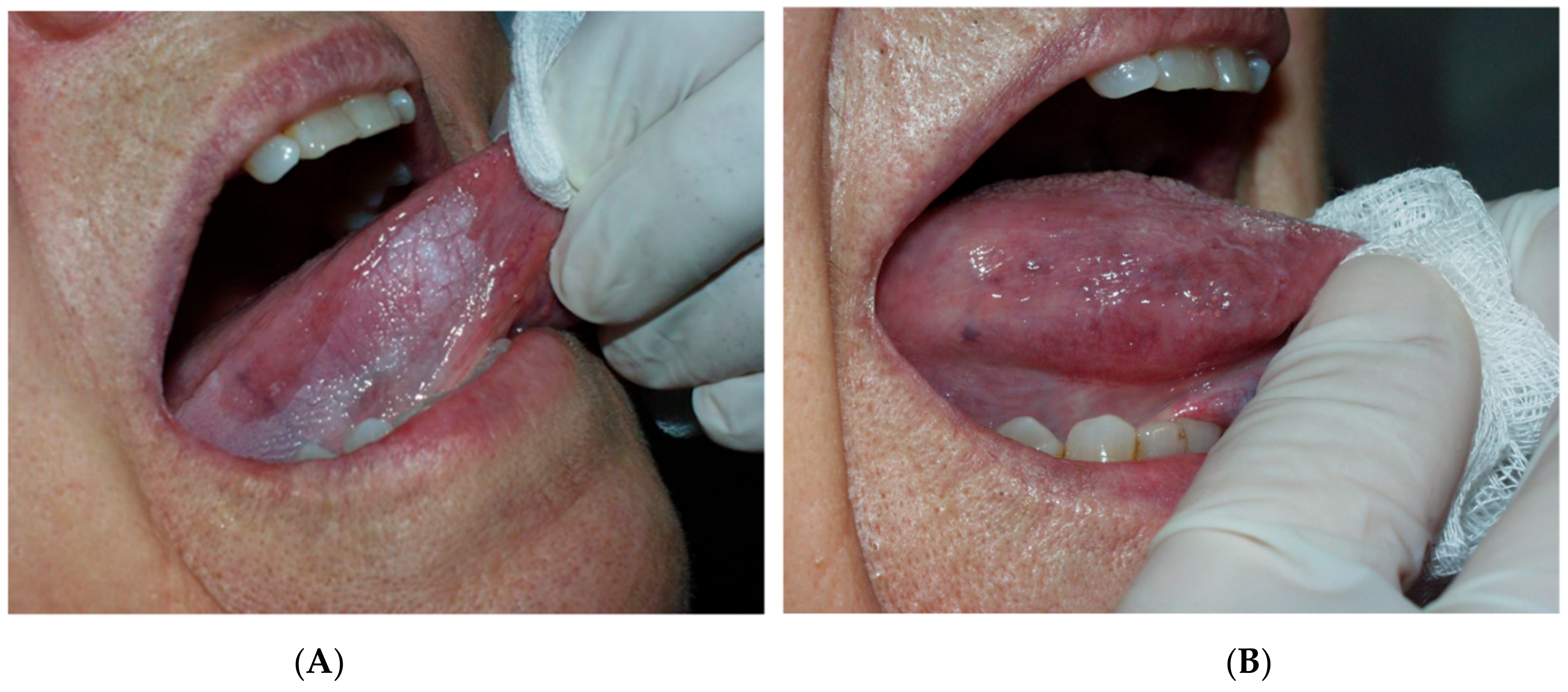
| Recurrence, n (%) | Univariate Logistic Regression | ||||
|---|---|---|---|---|---|
| No | Yes | OR | IC 95% | p-Value | |
| Clinical type | |||||
| Leucoplakia Homogeneous | 6 (33.3) | 12 (66.7) | 1 | ||
| Leucoplakia non-homogeneous | 15 (50) | 15 (50) | 2 | 0.59–6.73 | 0.263 |
| Location | |||||
| Lining Mucosa | 6 (46.2) | 7 (53.8) | 1 | ||
| Masticatory Mucosa | 13 (39.4) | 20 (60,6) | 1.319 | 0.36–4.81 | 0.675 |
| Size | |||||
| <2 cm | 11 (42.3) | 15 (57.7) | 1 | ||
| >2 cm | 10 (45.5) | 12 (54.5) | 0.88 | 0.28–2.76 | 0.827 |
| Dysplasia | |||||
| No | 14 (37.8) | 23 (62.2) | 1 | ||
| Yes | 7 (63.6) | 4 (36.4) | 0.348 | 0.09–1.41 | 0.138 |
| Recurrence, n (%) | Univariate Logistic Regression | ||||
|---|---|---|---|---|---|
| No | Yes | OR | IC 95% | p-Value | |
| Tobacco | |||||
| No | 10 (34.5) | 19 (65.5) | |||
| Yes | 9 (81.8) | 2 (18.2) | 0.163 | 0.02–1.40 | 0.098 |
| former smoker | 2 (25) | 6 (75) | 1.579 | 0.27–9.31 | 0.614 |
| Oral hygiene | |||||
| Good | 14 (45.2) | 17 (54.8) | |||
| Bad | 7 (41.2) | 10 (58.8) | 1.176 | 0.36–3.90 | 0.790 |
| Diabetes | |||||
| No | 19 (50) | 19 (50) | |||
| Yes | 2 (20) | 8 (80) | 4 | 0.75–21.35 | 0.105 |
| Body mass index | |||||
| Normal weight | 4 (28.6) | 10 (71.4) | |||
| overweight | 17 (50) | 17 (50) | 0.400 | 0.11–1.53 | 0.180 |
| Bruxism | |||||
| No | 17 (47.2) | 19 (52.8) | |||
| Yes | 4 (33.3) | 8 (66.7) | 1.789 | 0.46–7.02 | 0.404 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Lujan, A.; López-Jornet, P.; Pons-Fuster López, E. Recurrence of Oral Leukoplakia after CO2 Laser Resection: A Prospective Longitudinal Study. Cancers 2022, 14, 5455. https://doi.org/10.3390/cancers14215455
Rodriguez-Lujan A, López-Jornet P, Pons-Fuster López E. Recurrence of Oral Leukoplakia after CO2 Laser Resection: A Prospective Longitudinal Study. Cancers. 2022; 14(21):5455. https://doi.org/10.3390/cancers14215455
Chicago/Turabian StyleRodriguez-Lujan, Adela, Pia López-Jornet, and Eduardo Pons-Fuster López. 2022. "Recurrence of Oral Leukoplakia after CO2 Laser Resection: A Prospective Longitudinal Study" Cancers 14, no. 21: 5455. https://doi.org/10.3390/cancers14215455
APA StyleRodriguez-Lujan, A., López-Jornet, P., & Pons-Fuster López, E. (2022). Recurrence of Oral Leukoplakia after CO2 Laser Resection: A Prospective Longitudinal Study. Cancers, 14(21), 5455. https://doi.org/10.3390/cancers14215455







