Reinduction of Hedgehog Inhibitors after Checkpoint Inhibition in Advanced Basal Cell Carcinoma: A Series of 12 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
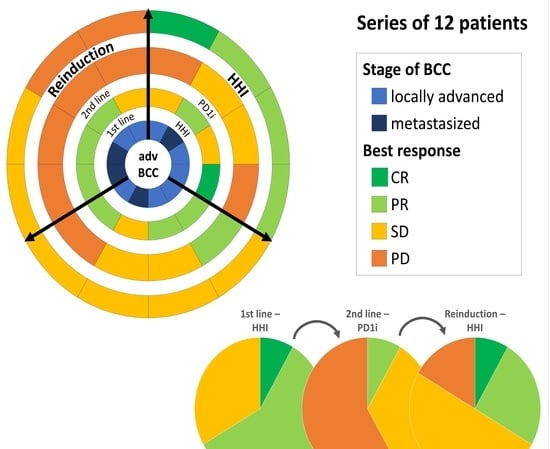
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dreier, J.; Cheng, P.F. Basal cell carcinomas in a tertiary referral centre: A systematic analysis. Br. J. Dermatol. 2014, 171, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Grob, J.J.; Guminski, A. Position statement on classification of basal cell carcinomas. Part 1: Unsupervised clustering of experts as a way to build an operational classification of advanced basal cell carcinoma based on pattern recognition. Eur. Acad. Dermatol. Venereol. 2021, 35, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Grob, J.J.; Gaudy-Marqueste, C. Position statement on classification of basal cell carcinomas. Part 2: EADO proposal for new operational staging system adapted to basal cell carcinomas. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: A joint expert opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ruiz, E.S. Current Perspectives in the Treatment of Locally Advanced Basal Cell Carcinoma. Drug. Des. Devel. Ther. 2022, 16, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Sekulic, A. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 848–857. [Google Scholar] [CrossRef]
- In, G.K.; Nallagangula, A. Clinical activity of PD-1 inhibition in the treatment of locally advanced or metastatic basal cell carcinoma. J. Immunother. Cancer 2022, 10, e004839. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.D. Abstract CT165. In Proceedings of the AACR Annual Meeting, New Orleans, LA, USA, 8–13 April 2022. [Google Scholar]
- Davis, C.M.; Lewis, K.D. Brief overview: Cemiplimab for the treatment of advanced basal cell carcinoma: PD-1 strikes again. Ther. Adv. Med. Oncol. 2022, 14, 17588359211066147. [Google Scholar] [CrossRef] [PubMed]
- Bassompierre, A.; Dalac, S. Efficacy of sonic hedgehog inhibitors rechallenge, after initial complete response in recurrent advanced basal cell carcinoma: A retrospective study from the CARADERM database. ESMO. Open 2021, 6, 100284. [Google Scholar] [CrossRef] [PubMed]
- Herms, F.; Lambert, J. Follow-Up of Patients With Complete Remission of Locally Advanced Basal Cell Carcinoma After Vismodegib Discontinuation: A Multicenter French Study of 116 Patients. J. Clin. Oncol. 2019, 37, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Kunstfeld, R. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): A randomised, regimen-controlled, double-blind, phase 2 trial. Lancet. Oncol. 2017, 18, 404–412. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hauschild, A. Quality-of-life analysis with intermittent vismodegib regimens in patients with multiple basal cell carcinomas: Patient-reported outcomes from the MIKIE study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e526–e529. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Danés, A.; Larsimont, J.C. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature 2018, 562, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Plaka, M. A Practical Guide for the Follow-Up of Patients with Advanced Basal Cell Carcinoma During Treatment with Hedgehog Pathway Inhibitors. Oncologist 2019, 24, e755–e764. [Google Scholar] [CrossRef] [PubMed]
- Habashy, S.; Jafri, A. Hedgehog Pathway Inhibitors: Clinical Implications and Resistance in the Treatment of Basal Cell Carcinoma. Cureus 2021, 13, e13859. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuizen, T.; Reinders, M.G. Acquired resistance to the Hedgehog pathway inhibitor vismodegib due to smoothened mutations in treatment of locally advanced basal cell carcinoma. J. Am. Acad. Dermatol. 2014, 71, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, H.J.; Pau, G. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell 2015, 27, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Trieu, K.G.; Tsai, S.Y. Basal cell carcinomas acquire secondary mutations to overcome dormancy and progress from microscopic to macroscopic disease. Cell Rep. 2022, 39, 110779. [Google Scholar] [CrossRef] [PubMed]
- Ridky, T.W.; Cotsarelis, G. Vismodegib resistance in basal cell carcinoma: Not a smooth fit. Cancer Cell 2015, 27, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.Q.; Chen, L. Switching Hedgehog inhibitors and other strategies to address resistance when treating advanced basal cell carcinoma. Oncotarget 2021, 12, 2089–2100. [Google Scholar] [CrossRef] [PubMed]


| Pat. ID | Sex | Age at HHI Initiation (Years) | Stage at Diagnosis | Site of Primary BCC or laBCC | Site of Metastasis at Diagnosis | Predisposing Disease |
|---|---|---|---|---|---|---|
| 1 | f | 59 | mBCC | Left flank | Lung, retroperitoneal | None |
| 2 | f | 41 | laBCC | Upper lip, inner corner of the left and right eye | Gorlin–Goltz syndrome | |
| 3 | m | 31 | laBCC | Head | None | |
| 4 | f | 70 | mBCC | Left mamma | Lung, bone, pleural, lymph node | None |
| 5 | m | 77 | mBCC | Right shoulder | lung, bone | None |
| 6 | m | 87 | laBCC | left ear | None | |
| 7 | m | 82 | mBCC | Left flank | Subcutaneous, bone, lung | None |
| 8 | f | 84 | laBCC | Right ear conch | None | |
| 9 | m | 38 | laBCC | Right shoulder | None | |
| 10 | f | 64 | laBCC | Scalp | - | None |
| 11 | m | 81 | laBCC | Retroauricular right | None | |
| 12 | f | 66 | laBCC | Corner of the left eye | None |
| Pat. ID | Type of 1st HHI | Duration 1st HHI (Months) | Best Response 1st HHI | Reason EOT 1st HHI | Type of PD1i | Duration PD1i (Months) | Best Response PD1i | Reason EOT PD1i | Time to HHI Reinduction (Months) | Type of 2nd HHI | Duration 2nd HHI (Months) | Best Response 2nd HHI | Reason EOT 2nd HHI | Further Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | Vismodegib | 13.2 | SD | Progress | Pembrolizumab | 4 | PD | Progress | 18.2 | Sonidegib | 9 | CR | Ongoing | - |
| 1 | Vismodegib | 22.4 | PR | Progress | Pembrolizumab | 2.1 | SD | No drug approval | 27.6 | Vismodegib | 20.1 | PR | Progress | Sonidegib/pembrolizumab |
| 2 | Vismodegib | 64.5 | SD | Progress | Pembrolizumab | 6.5 | SD | Pat’s request | 6.5 | Sonidegib | 29 | PR | Ongoing | - |
| 8 | Sonidegib | 5.1 | CR | AE | Cemiplimab | 4.1 | PD | Progress | 27.4 | Sonidegib | 2 | PR | Ongoing | - |
| 6 | Vismodegib/Sonidegib | 22.3 | PR | Progress | Cemiplimab | 16.3 | PR | Progress | 18.3 | Sonidegib | 10.2 | SD | Progress | - |
| 9 | Vismodegib | 19.2 | PR | Progress | Cemiplimab | 8.1 | SD | Progress | 25.4 | Sonidegib | 13.2 | SD | Progress | Cemiplimab |
| 5 | Vismodegib | 26 | SD | Progress | Pembrolizumab | 5.9 | SD | Progress | 5.9 | Vismodegib | 4.1 | SD | AE | - |
| 3 | Vismodegib | 27 | PR | Pat’s request | Cemiplimab | 0.8 | PD | AE | 3.8 | Sonidegib | 3 | SD | Pat’s request | Cemiplimab |
| 4 | Vismodegib | 6.8 | PR | Progress | Cemiplimab | 2.1 | PD | Progress | 4 | Sonidegib | 3.1 | SD | Progress | Carboplatin/paclitaxel/vismodegib/radiation |
| 7 | Vismodegib | 25.4 | PR | Progress | Cemiplimab | 3.1 | PD | Progress | 4.1 | Sonidegib | 3 | SD | Ongoing | - |
| 11 | Sonidegib | 12.7 | PR | AE | Cemiplimab | 2.1 | PD | Progress | 20.4 | Vismodegib | 1 | PD | Progress | - |
| 12 | Vismodegib | 2 | SD | AE | Pembrolizumab | 5 | PD | Progress | 69 | Sonidegib | 3 | PD | Ongoing | - |
| Pat. ID | AEs to 1st HHI | AEs to PD1i | AEs to 2nd HHI |
|---|---|---|---|
| 1 | Alopecia, weight loss, loss of appetite, muscle pain, arthralgia | None | Loss of appetite, weight loss |
| 2 | Alopecia, muscle spasm, depression | None | Muscle spasms, depression, weight gain, amenorrhea, brittle nails, fatigue |
| 3 | Alopecia, muscle spasms, weight loss | Vasovagal presyncope, myocarditis | None |
| 4 | none | None | None |
| 5 | Dysgeusia (I), alopecia (I), weight loss (III), muscle spasm (I) | None | Loss of appetite, weight loss (III) |
| 6 | Muscle spasms (I), dysgeusia (I) | None | None |
| 7 | dysgeusia, alopecia | None | None |
| 8 | Muscle spasm, arthralgia, dysgeusia, nausea, weight loss | Fatigue (I), weakness (I), pruritus (I) | None |
| 9 | Muscle spasms (II), dysgeusia (II), alopecia (III) | Exacerbation of psoriasis | None |
| 10 | Nausea, alopecia | None | Nausea |
| 11 | Muscle spasms (II), dysgeusia (III), weight loss (III) | None | Dysgeusia (II), weight loss (II) |
| 12 | Unknown | None | Myalgia, diarrhea, nausea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeTemple, V.K.; Hassel, J.C.; Sachse, M.M.; Grimmelmann, I.; Leiter, U.; Gebhardt, C.; Eckardt, J.; Pföhler, C.; Angela, Y.; Hübbe, H.; et al. Reinduction of Hedgehog Inhibitors after Checkpoint Inhibition in Advanced Basal Cell Carcinoma: A Series of 12 Patients. Cancers 2022, 14, 5469. https://doi.org/10.3390/cancers14215469
DeTemple VK, Hassel JC, Sachse MM, Grimmelmann I, Leiter U, Gebhardt C, Eckardt J, Pföhler C, Angela Y, Hübbe H, et al. Reinduction of Hedgehog Inhibitors after Checkpoint Inhibition in Advanced Basal Cell Carcinoma: A Series of 12 Patients. Cancers. 2022; 14(21):5469. https://doi.org/10.3390/cancers14215469
Chicago/Turabian StyleDeTemple, Viola K., Jessica C. Hassel, Michael M. Sachse, Imke Grimmelmann, Ulrike Leiter, Christoffer Gebhardt, Julia Eckardt, Claudia Pföhler, Yenny Angela, Hanna Hübbe, and et al. 2022. "Reinduction of Hedgehog Inhibitors after Checkpoint Inhibition in Advanced Basal Cell Carcinoma: A Series of 12 Patients" Cancers 14, no. 21: 5469. https://doi.org/10.3390/cancers14215469
APA StyleDeTemple, V. K., Hassel, J. C., Sachse, M. M., Grimmelmann, I., Leiter, U., Gebhardt, C., Eckardt, J., Pföhler, C., Angela, Y., Hübbe, H., & Gutzmer, R. (2022). Reinduction of Hedgehog Inhibitors after Checkpoint Inhibition in Advanced Basal Cell Carcinoma: A Series of 12 Patients. Cancers, 14(21), 5469. https://doi.org/10.3390/cancers14215469