Expression of Intermediate Filaments in the Developing Testis and Testicular Germ Cell Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Immunohistochemistry
2.3. Immunofluorescence
2.4. Image Capture
2.5. Quantification of Cells
2.6. Statistical Analysis
3. Results
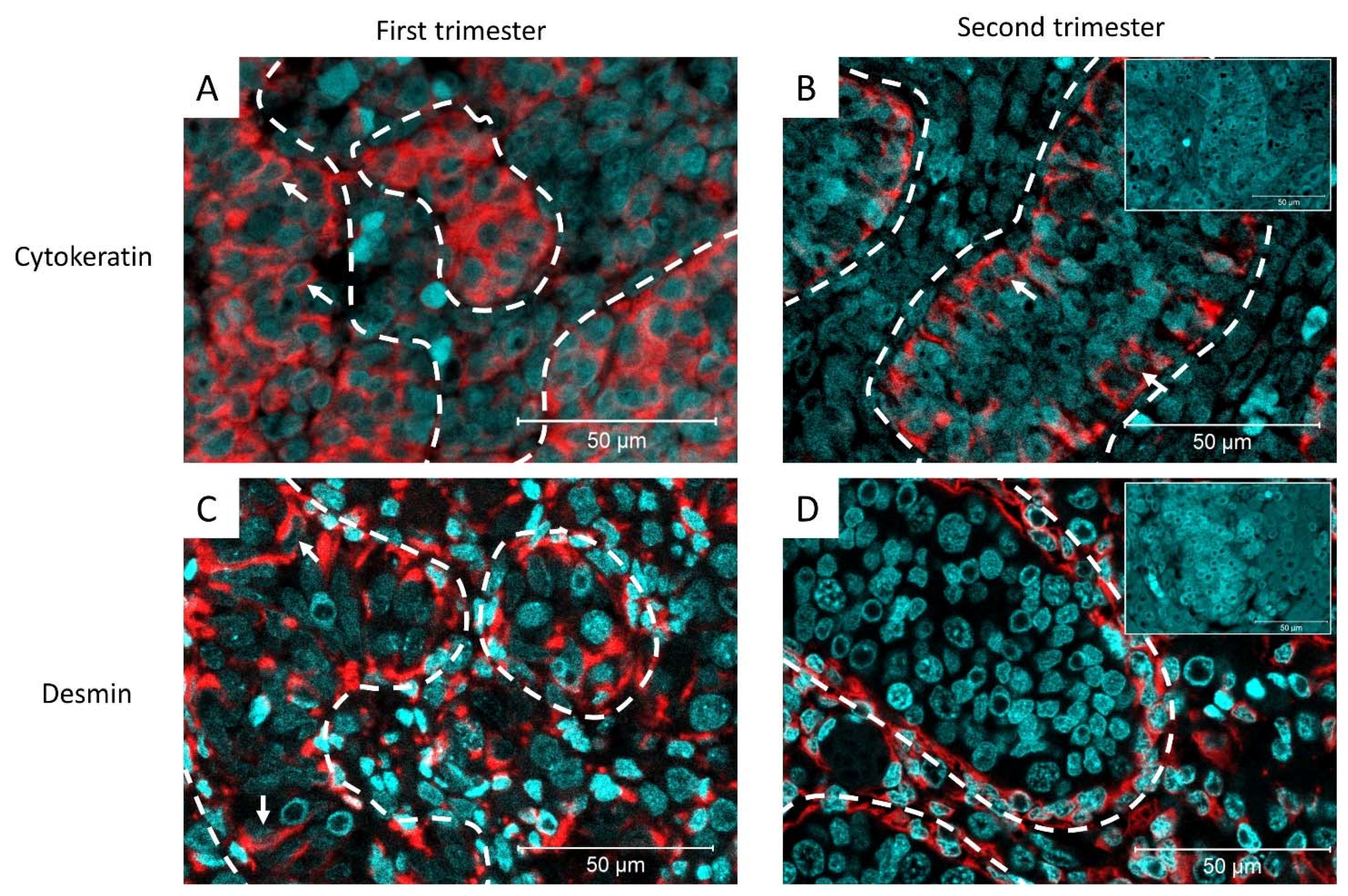
3.1. Expression of Cytokeratin and Desmin during Human Fetal Testis Development
3.2. Cytokeratin and Desmin Expression in Normal Adult Human Testis
3.3. Cytokeratin and Desmin Expression in Testicular Germ Cell Cancer (TGCC) Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Human Fetal Testis Tissue Samples | ||||||
|---|---|---|---|---|---|---|
| ICH or IF Performed | ||||||
| Sample ID | GW | Desmin/ SOX9 | Desmin/ POU5F1 | Desmin/ SOX9 | Cytokeratin/ AR/POU5F1 | Cytokeratin |
| HFT1 | 8 | X | ||||
| HFT2 | 10 | X | ||||
| HFT3 | 11 | X | X | |||
| HFT4 | 12 | X | ||||
| HFT5 | 12+6 | X | X | X | ||
| HFT6 | 13 | X | X | |||
| HFT7 | 14 | X | ||||
| HFT8 | 14 | X | ||||
| HFT9 | 14 | X | ||||
| HFT10 | 15 | X | ||||
| HFT11 | 15 | X | ||||
| HFT12 | 15 | X | ||||
| HFT13 | 15 | X | ||||
| HFT14 | 15+2 | X | ||||
| HFT15 | 16 | X | ||||
| HFT16 | 16 | X | ||||
| HFT17 | 16 | X | ||||
| HFT18 | 16 | X | ||||
| HFT19 | 16 | X | ||||
| HFT20 | 16+4 | X | ||||
| HFT21 | 17 | X | ||||
| HFT22 | 17 | X | ||||
| HFT23 | 17 | X | ||||
| HFT24 | 17 | X | ||||
| HFT25 | 17 | X | ||||
| HFT26 | 17 | X | ||||
| HFT27 | 18 | X | ||||
| HFT28 | 18 | X | ||||
| HFT29 | 18 | X | ||||
| HFT30 | 18 | X | ||||
| HFT31 | 18 | X | ||||
| HFT32 | 19 | X | ||||
| HFT33 | 19 | X | ||||
| HFT34 | 19 | X | ||||
| HFT35 | 20 | X | ||||
| HFT36 | 20 | X | ||||
| Normal Adult Testis Samples | ||||
|---|---|---|---|---|
| IHC or IF Performed | ||||
| Sample ID | Desmin | Desmin/ SOX9 | Cytokeratin/ AR/POU5F1 | Cytokeratin |
| NAT1 | X | |||
| NAT2 | X | |||
| NAT3 | X | X | X | |
| NAT4 | X | |||
| NAT5 | X | |||
| Childhood and Adult Pre-Invasive TGCC Samples | ||||
|---|---|---|---|---|
| IHC of IF Performed | ||||
| Sample ID | Age | Desmin | Cytokeratin/ AR/POU5F1 | Cytokeratin |
| PR1 | 5 | X | ||
| PR2 | 7 | X | ||
| PR3 | 12 | X | X | |
| PR4 | 17 | X | ||
| PR5 | 26 | X | ||
| PR6 | 28 | X | ||
| PR7 | 36 | X | ||
| Seminoma Samples | |||||
|---|---|---|---|---|---|
| IHC or IF Performed | |||||
| Sample ID | Desmin/ SOX9 | Desmin/ POU5F1 | Desmin | Cytokeratin/ AR/POU5F1 | Cytokeratin |
| S1 | X | X | |||
| S2 | X | ||||
| S3 | X | ||||
| S4 | X | ||||
| S5 | X | ||||
| S6 | X | ||||
| S7 | X | ||||
| S8 | X | X | |||
| S9 | X | X | X | ||
| S10 | X | X | |||
| S11 | X | ||||
| S12 | X | X | |||
| S13 | X | ||||
| S14 | X | ||||
| S15 | X | ||||
| Non-Seminoma Samples | |||||
|---|---|---|---|---|---|
| IHC or IF Performed | |||||
| Sample ID | Desmin/ SOX9 | Desmin/ POU5F1 | Desmin | Cytokeratin/ AR/POU5F1 | Cytokeratin |
| NS1 | X | ||||
| NS2 | X | ||||
| NS3 | X | ||||
| NS4 | X | X | |||
| NS5 | X | X | X | ||
| NS6 | |||||
| NS7 | X | ||||
| NS8 | X | ||||
| NS9 | X | X | X | ||
| NS10 | X | X | X | ||
| NS11 | X | X | |||
| NS12 | X | ||||
| NS13 | X | ||||
| NS14 | X | ||||
| Biopsies ‘Distant’ from the Tumour | ||
|---|---|---|
| IHC or IF Performed | ||
| Sample ID | Desmin | Cytokeratin/AR/POU5F1 |
| BD1 | X | |
| BD2 | X | |
| BD3 | X | |
| BD4 | X | |
| BD5 | X | |
| BD6 | X | |
| BD7 | X | |
| BD8 | X | |
| BD9 | X | |
| BD10 | X | |
| BD11 | X | |
| BD12 | X | |
| BD13 | X | |
| BD14 | X | |
| Biopsies ‘Adjacent’ from the Tumour | |
|---|---|
| IHC or IF Performed | |
| Cytokeratin/AR/POU5F1 | |
| BA15 | X |
| BA5 | X |
| BA6 | X |
| BA7 | X |
| BA8 | X |
| BA11 | X |
| BA14 | X |
| BA4 | X |
References
- Trabert, B.; Chen, J.; Devesa, S.S.; Bray, F.; Mcglynn, K.A. International Patterns and Trends in Testicular Cancer Incidence, Overall and by Histologic Subtype, 1973–2007. Andrology 2015, 3, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajpert-De Meyts, E. Developmental Model for the Pathogenesis of Testicular Carcinoma in Situ: Genetic and Environmental Aspects. Hum. Reprod. Update 2006, 12, 303–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lie, P.P.Y.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Cytoskeletal Dynamics and Spermatogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Wayne Vogl, A.; Vaid, K.S.; Guttman, J.A. The Sertoli Cell Cytoskeleton. Adv. Exp. Med. Biol. 2008, 636, 186–211. [Google Scholar] [CrossRef]
- Skakkebaek, N.E. Trends in Male Reproductive Health. Environmental Aspects. Adv. Exp. Med. Biol. 1998, 444, 1–2. [Google Scholar]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and Functional Maturation of Sertoli Cells, and Their Relevance to Disorders of Testis Function in Adulthood. Reproduction 2003, 125, 769–784. [Google Scholar]
- Sharpe, R.M.; Skakkebaek, N.E. Testicular Dysgenesis Syndrome: Mechanistic Insights and Potential New Downstream Effects. Fertil. Steril. 2008, 89, e33–e38. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.-M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef] [Green Version]
- Rogatsch, H.; Hittmair, A.; Mikuz, G.; Feichtinger, H.; Jezek, D. Expression of Vimentin, Cytokeratin, and Desmin in Sertoli Cells of Human Fetal, Cryptorchid, and Tumour-Adjacent Testicular Tissue. Virchows Arch. 1996, 427, 497–502. [Google Scholar] [CrossRef]
- Bergmann, M.; Kliesch, S. The Distribution Pattern of Cytokeratin and Vimentin Immunoreactivity in Testicular Biopsies of Infertile Men. Anat. Embryol. 1994, 190, 515–520. [Google Scholar] [CrossRef]
- Nistal, M.; Gonzalez-Peramato, P.; Regadera, J.; Serrano, A.; Tarin, V.; De Miguel, M.P. Primary Testicular Lesions Are Associated with Testicular Germ Cell Tumors of Adult Men. Am. J. Surg. Pathol. 2006, 30, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Stosiek, P.; Kasper, M.; Karsten, U. Expression of Cytokeratins 8 and 18 in Human Sertoli Cells of Immature and Atrophic Seminiferous Tubules. Differentiation 1990, 43, 66–70. [Google Scholar] [CrossRef]
- Friel, A.; Houghton, J.A.; Glennon, M.; Lavery, R.; Smith, T.; Nolan, A.; Maher, M. A Preliminary Report on the Implication of RT-PCR Detection of DAZ, RBMY1, USP9Y and Protamine-2 MRNA in Testicular Biopsy Samples from Azoospermic Men. Int. J. Androl. 2002, 25, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Norton, A.J.; Jordan, S.; Yeomans, P. Brief, High-Temperature Heat Denaturation (Pressure Cooking): A Simple and Effective Method of Antigen Retrieval for Routinely Processed Tissues. J. Pathol. 1994, 173, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Franke, F.E.; Pauls, K.; Rey, R.; Marks, A.; Bergmann, M.; Steger, K. Differentiation Markers of Sertoli Cells and Germ Cells in Fetal and Early Postnatal Human Testis. Anat Embryol. 2004, 209, 169–177. [Google Scholar] [CrossRef]
- Maymon, B.B.-S. Maturation Phenotype of Sertoli Cells in Testicular Biopsies of Azoospermic Men. Hum. Reprod. 2000, 15, 1537–1542. [Google Scholar] [CrossRef] [Green Version]
- Welsh, M.; Saunders, P.T.K.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Smith, L.B.; Sharpe, R.M.; Koopman, P.; Gubbay, J.; Vivian, N.; et al. Identification in Rats of a Programming Window for Reproductive Tract Masculinization, Disruption of Which Leads to Hypospadias and Cryptorchidism. J. Clin. Invest. 2008, 118, 1479–1490. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, L.; Smith, L.B.; Rebourcet, D. Sertoli Cells as Key Drivers of Testis Function. Semin. Cell Dev. Biol. 2022, 121, 2–9. [Google Scholar] [CrossRef]
- Lucas-Herald, A.K.; Mitchell, R.T. Testicular Sertoli Cell Hormones in Differences in Sex Development. Front. Endocrinol. 2022, 13, 919670. [Google Scholar] [CrossRef]
- Chemes, H.E.; Rey, R.A.; Nistal, M.; Regadera, J.; Musse, M.; González-Peramato, P.; Serrano, A. Physiological Androgen Insensitivity of the Fetal, Neonatal, and Early Infantile Testis Is Explained by the Ontogeny of the Androgen Receptor Expression in Sertoli Cells. J. Clin. Endocrinol. Metab. 2008, 93, 4408–4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, E.; Huang, H.; Masch, R.J.; McFadden, D.E.; Wu, X.-R.; Ostrer, H. Immunolocalization of Androgen Receptor and Estrogen Receptors Alpha and Beta in Human Fetal Testis and Epididymis. J. Urol. 2005, 174, 1695–1698; discussion 1698. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Quian, C.A.; Martínez-García, F.; Nistal, M.; Regadera, J. Androgen Receptor Distribution in Adult Human Testis. J. Clin. Endocrinol. Metab. 1999, 84, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.; Baal, N.; Wilhelm, J.; Sarode, P.; Weigel, R.; Schumacher, V.; Nettersheim, D.; Schorle, H.; Schröck, C.; Bergmann, M.; et al. On the Origin of Germ Cell Neoplasia in Situ: Dedifferentiation of Human Adult Sertoli Cells in Cross Talk with Seminoma Cells in Vitro. Neoplasia 2021, 23, 731–742. [Google Scholar] [CrossRef]
- Steger, K.; Rey, R.; Kliesch, S.; Louis, F.; Schleicher, G.; Bergmann, M. Immunohistochemical Detection of Immature Sertoli Cell Markers in Testicular Tissue of Infertile Adult Men: A Preliminary Study. Int. J. Androl. 1996, 19, 122–128. [Google Scholar] [PubMed]
- Tran, D.; Picard, J.Y.; Campargue, J.; Josso, N. Immunocytochemical Detection of Anti-Mullerian Hormone in Sertoli Cells of Various Mammalian Species Including Human. J. Histochem. Cytochem. 1987, 35, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Cupp, A.S.; Skinner, M.K. Sertoli Cell Biology; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 9780126477511. [Google Scholar]








| Antibody | Species Raised | Dilution for IHC | Dilution for IF | Clonality | Cat. No. | Manufacturer |
|---|---|---|---|---|---|---|
| Androgen receptor | rabbit | N/A | 1:3000 | Poly-clonal | ab74272 | Abcam Ltd. (Cambridge, UK) |
| Pan-cytokeratin (cytokeratin 1, 4, 5, 6, 8, 10, 13, 18, and 19) | mouse | 1:3000 | 1:5000 | Mono-clonal | C2562 | Sigma-Aldrich (Poole, UK) |
| Desmin | rabbit | 1:3500 | 1:3500 | Poly-clonal | ab15200 | Abcam Ltd. (Cambridge, UK) |
| POU5F1 | goat | N/A | 1:150 | Poly-clonal | sc-8626 | Santa Cruz (Heidelberg, Germany) |
| SOX-9 | rabbit | N/A | 1:5000 | Poly-clonal | A5535 | Merk Millipore (Hertfordshire, UK) |
| Sample Number | Age | Number of Images | Total Counted Sertoli Cells | |
|---|---|---|---|---|
| HFT1 | 8 GW | 2 | 655 | First trimester |
| HFT2 | 10 GW | 3 | 1212 | |
| HFT3 | 11 GW | 2 | 718 | |
| HFT4 | 12 GW | 1 | 233 | |
| HFT11 | 15 GW | 2 | 772 | Second trimester |
| HFT12 | 15 GW | 1 | 456 | |
| HFT15 | 16 GW | 3 | 1318 | |
| HFT16 | 16 GW | 2 | 467 | |
| HFT21 | 17 GW | 2 | 628 | |
| HFT22 | 17 GW | 1 | 404 | |
| HFT23 | 17 GW | 1 | 335 | |
| HFT27 | 18 GW | 2 | 711 | |
| HFT28 | 18 GW | 2 | 998 | |
| HFT29 | 18 GW | 1 | 454 | |
| HFT30 | 18 GW | 3 | 1478 | |
| HFT32 | 19 GW | 2 | 602 | |
| HFT33 | 19 GW | 1 | 384 | |
| HFT35 | 20 GW | 4 | 1619 |
| Sample Number | Number of Images | Total Counted Sertoli Cells | TGCC Type |
|---|---|---|---|
| SEM4 | 3 | 182 | seminoma |
| SEM9 | 2 | 95 | |
| SEM15 | 2 | 124 | |
| NS1 | 2 | 98 | non-seminoma |
| NS9 | 3 | 244 | |
| NS6 | 2 | 182 |
| Adjacent to Tumour | Distant to Tumour | ||||
|---|---|---|---|---|---|
| Sample ID | Number of Images | Total Counted Sertoli Cells | Sample ID | Number of Images | Total Counted Sertoli Cells |
| BA4 | 1 | 20 | BD4 | 2 | 270 |
| BA5 | 3 | 299 | BD5 | 1 | 22 |
| BA6 | 2 | 142 | BD6 | 2 | 90 |
| BA8 | 3 | 169 | BD8 | 2 | 132 |
| BA11 | 2 | 63 | BD11 | 3 | 46 |
| BA14 | 2 | 55 | BD14 | 2 | 144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Moll, M.E.; Looijenga, L.H.J.; Donat, R.; Shukla, C.J.; Jørgensen, A.; Mitchell, R.T. Expression of Intermediate Filaments in the Developing Testis and Testicular Germ Cell Cancer. Cancers 2022, 14, 5479. https://doi.org/10.3390/cancers14225479
Camacho-Moll ME, Looijenga LHJ, Donat R, Shukla CJ, Jørgensen A, Mitchell RT. Expression of Intermediate Filaments in the Developing Testis and Testicular Germ Cell Cancer. Cancers. 2022; 14(22):5479. https://doi.org/10.3390/cancers14225479
Chicago/Turabian StyleCamacho-Moll, Maria E., Leendert H. J. Looijenga, Roland Donat, Chitranjan J. Shukla, Anne Jørgensen, and Rod T. Mitchell. 2022. "Expression of Intermediate Filaments in the Developing Testis and Testicular Germ Cell Cancer" Cancers 14, no. 22: 5479. https://doi.org/10.3390/cancers14225479
APA StyleCamacho-Moll, M. E., Looijenga, L. H. J., Donat, R., Shukla, C. J., Jørgensen, A., & Mitchell, R. T. (2022). Expression of Intermediate Filaments in the Developing Testis and Testicular Germ Cell Cancer. Cancers, 14(22), 5479. https://doi.org/10.3390/cancers14225479








