Low-Grade Ovarian Stromal Tumors with Genetic Alterations of the Wnt/β-Catenin Pathway That Is Crucial in Ovarian Follicle Development and Regulation
Abstract
:Simple Summary
Abstract
1. Wnt Pathway in the Development and Regulation of Ovarian Follicle Cycle
1.1. Wnt Pathway during the Embryonic Development of the Ovary
1.2. Synchronized Effort between the Wnt Pathway and Hormones in the Adult Ovary
1.3. Role of β-Catenin in Steroid Production and Regulation
2. Ovarian Stroma and Stromal Cell Types
3. Activation of Wnt/β-Catenin Signaling Pathway in Gynecological Neoplasms
4. The Family of Low-Grade Ovarian Stromal Tumors with β-Catenin Alterations
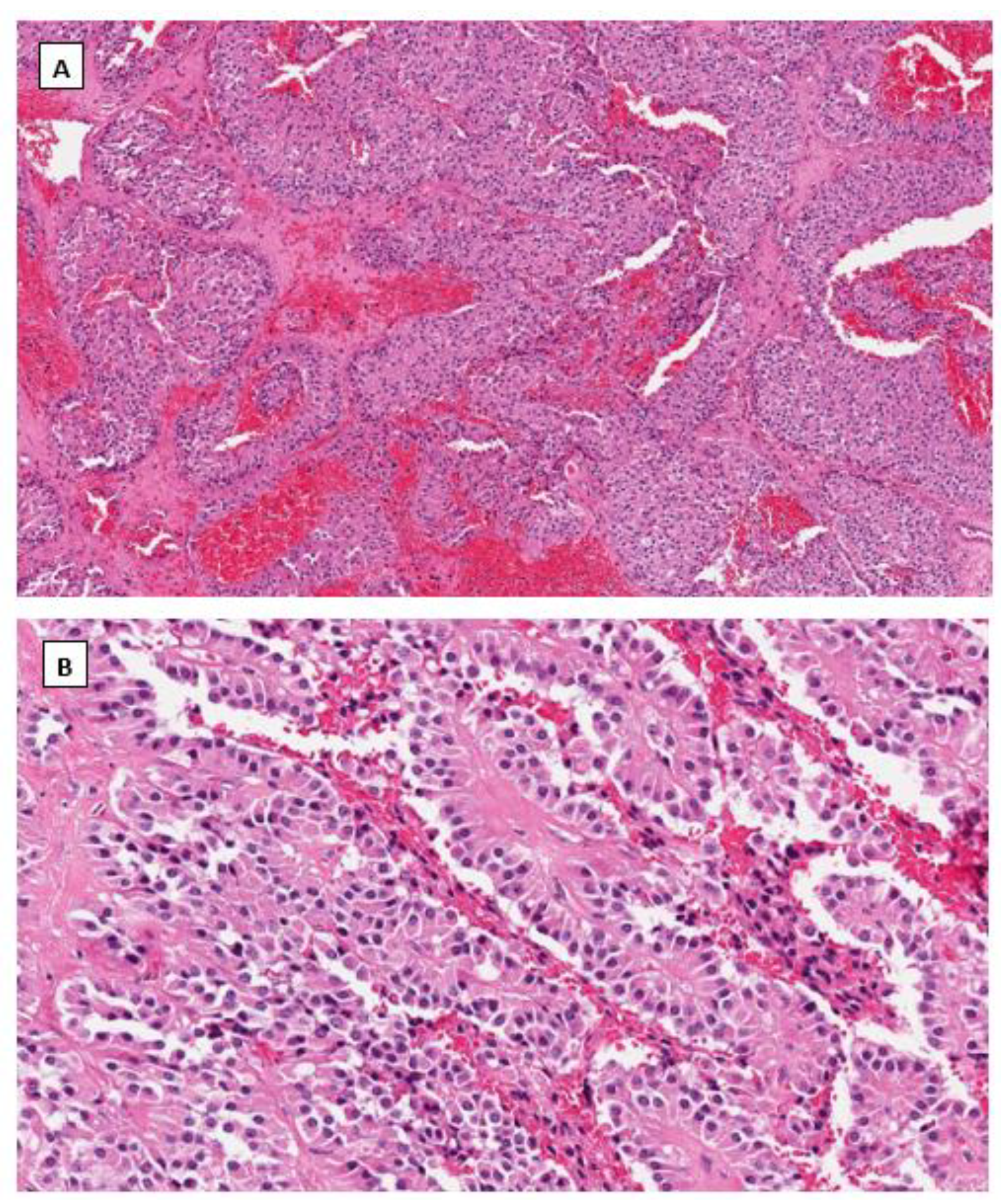
4.1. Solid Pseudopapillary Neoplasm (SPPN)
4.2. Microcystic Stromal Tumor (MCST)
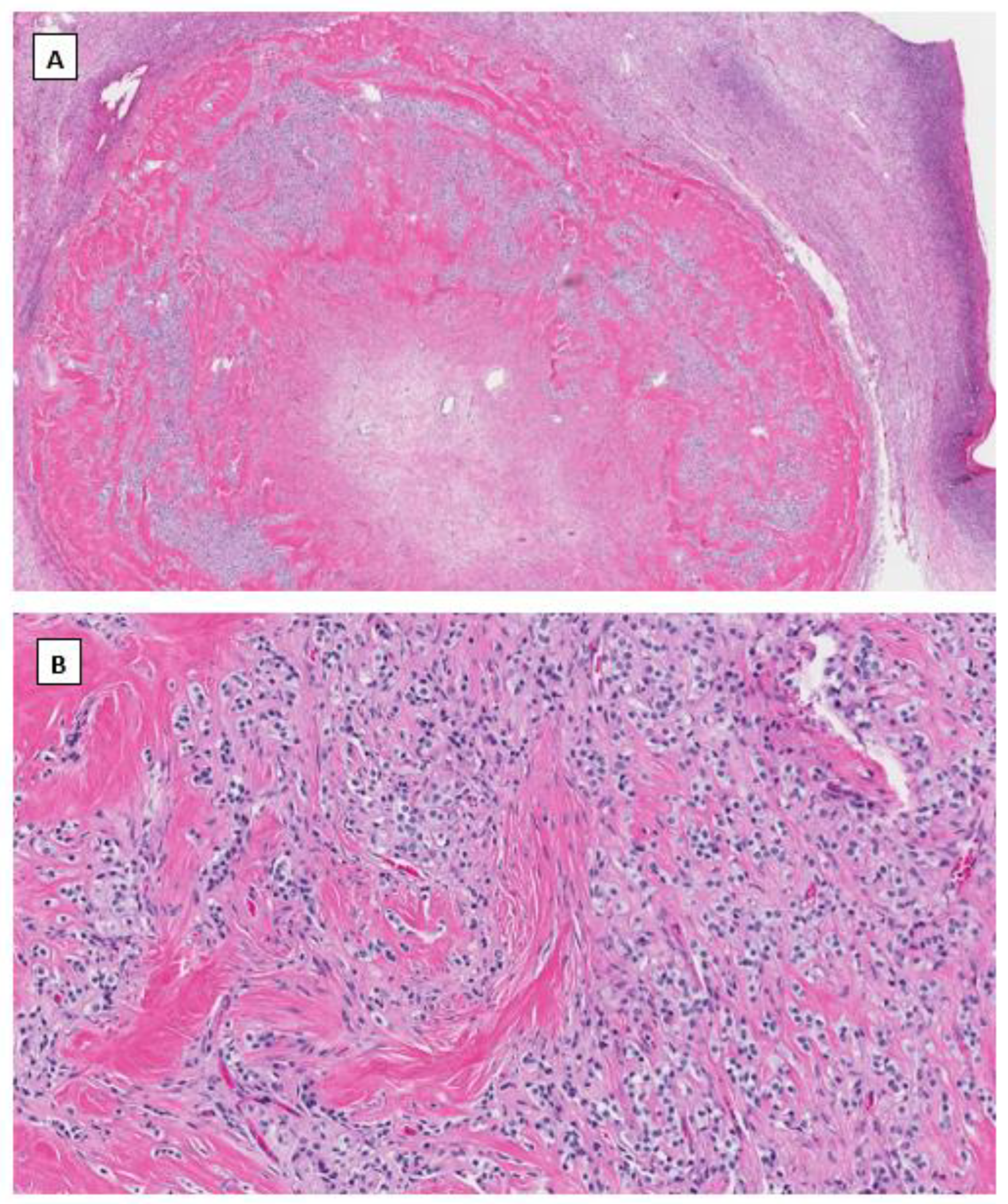
4.3. Signet Ring Stromal Cell Tumor (SRSCT)
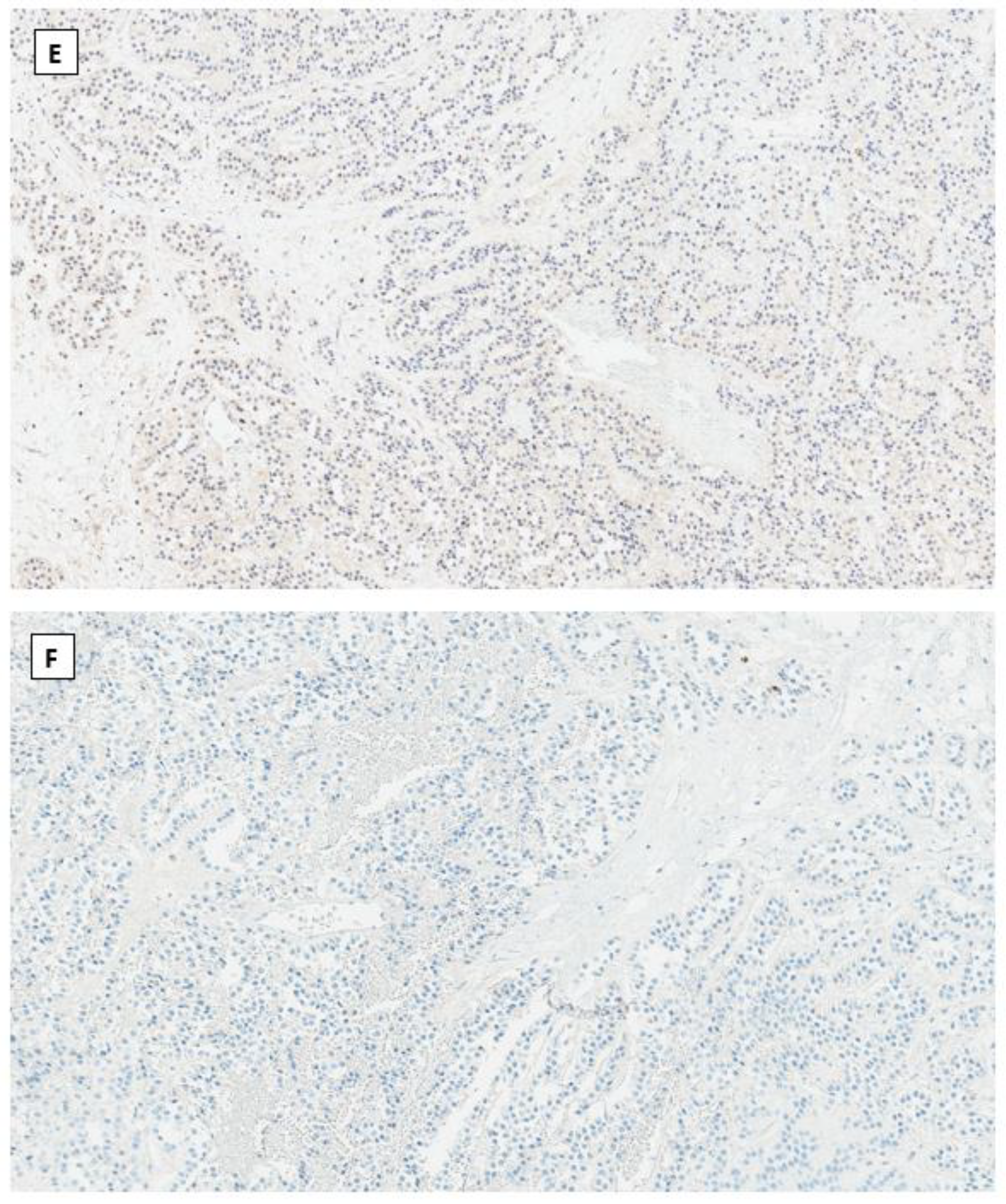
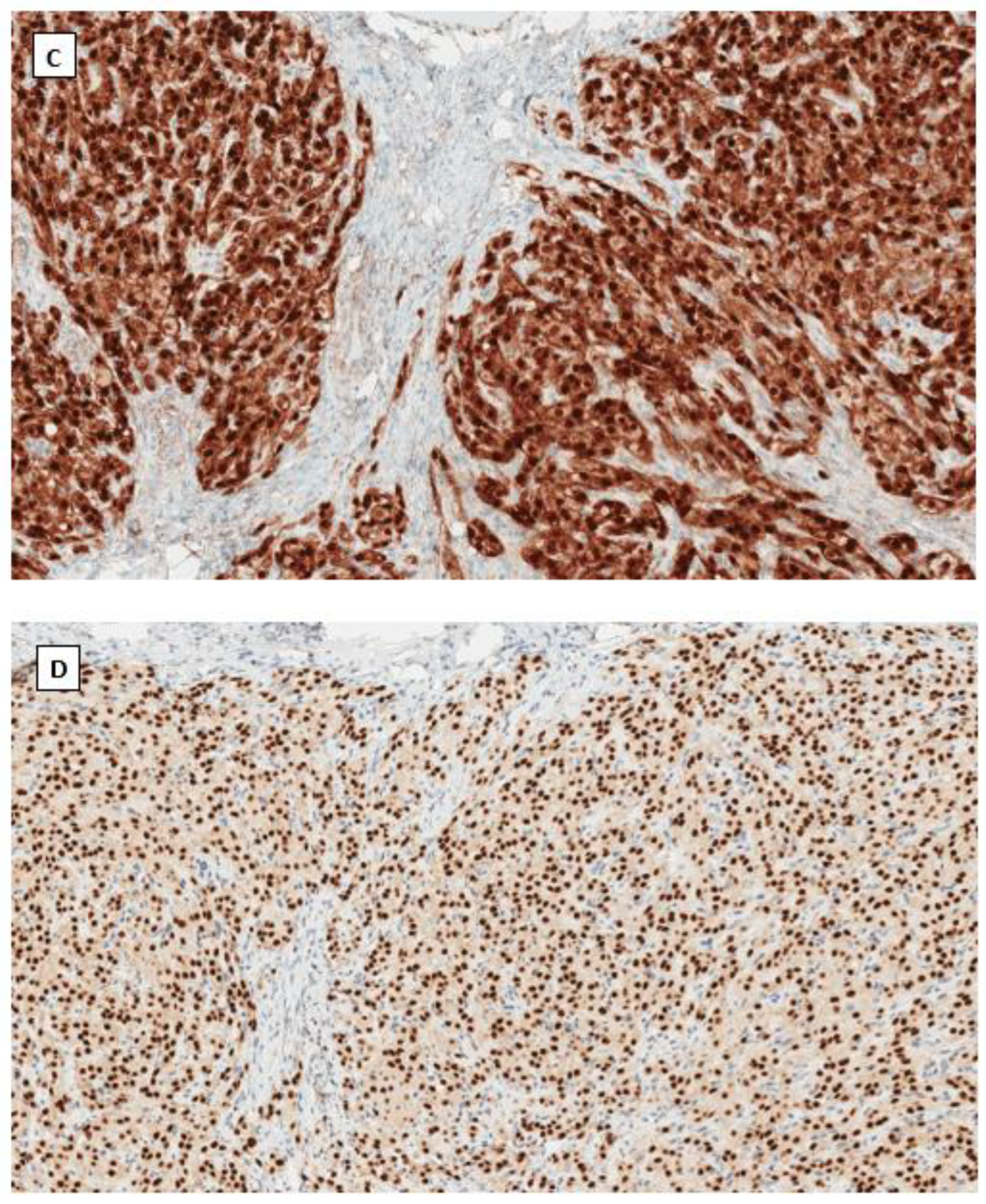
4.4. Immunohistochemical Phenotype Shared in Ovarian Low-Grade Stromal Tumors
Author Contributions
Funding
Conflicts of Interest
References
- Vainio, S.; Heikkila, M.; Kispert, A.; Chin, N.; McMahon, A.P. Female development in mammals is regulated by Wnt-4 signalling. Nature 1999, 397, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S. Maturation of ovarian follicles: Actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol. Rev. 1980, 60, 51–89. [Google Scholar] [CrossRef] [PubMed]
- Namwanje, M.; Brown, C.W. Activins and Inhibins: Roles in Development, Physiology, and Disease. Cold Spring Harb. Perspect. Biol. 2016, 8, 1–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, S.; Stark, J.; Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update 2008, 14, 367–378. [Google Scholar] [CrossRef]
- Gupta, P.S.; Folger, J.K.; Rajput, S.K.; Lv, L.; Yao, J.; Ireland, J.J.; Smith, G.W. Regulation and regulatory role of WNT signaling in potentiating FSH action during bovine dominant follicle selection. PLoS ONE 2014, 9, e100201. [Google Scholar] [CrossRef] [Green Version]
- Hernandez Gifford, J.A. The role of WNT signaling in adult ovarian folliculogenesis. Reproduction 2015, 150, R137–R148. [Google Scholar] [CrossRef] [Green Version]
- Boyer, A.; Lapointe, E.; Zheng, X.; Cowan, R.G.; Li, H.; Quirk, S.M.; DeMayo, F.J.; Richards, J.S.; Boerboom, D. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 3010–3025. [Google Scholar] [CrossRef] [Green Version]
- Castanon, B.I.; Stapp, A.D.; Gifford, C.A.; Spicer, L.J.; Hallford, D.M.; Hernandez Gifford, J.A. Follicle-stimulating hormone regulation of estradiol production: Possible involvement of WNT2 and beta-catenin in bovine granulosa cells. J. Anim. Sci. 2012, 90, 3789–3797. [Google Scholar] [CrossRef]
- Stapp, A.D.; Gomez, B.I.; Gifford, C.A.; Hallford, D.M.; Hernandez Gifford, J.A. Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells. PLoS ONE 2014, 9, e86432. [Google Scholar] [CrossRef] [Green Version]
- Law, N.C.; Weck, J.; Kyriss, B.; Nilson, J.H.; Hunzicker-Dunn, M. Lhcgr expression in granulosa cells: Roles for PKA-phosphorylated beta-catenin, TCF3, and FOXO1. Mol. Endocrinol. 2013, 27, 1295–1310. [Google Scholar] [CrossRef]
- Roy, L.; McDonald, C.A.; Jiang, C.; Maroni, D.; Zeleznik, A.J.; Wyatt, T.A.; Hou, X.; Davis, J.S. Convergence of 3’,5’-cyclic adenosine 5’-monophosphate/protein kinase A and glycogen synthase kinase-3beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology 2009, 150, 5036–5045. [Google Scholar] [CrossRef] [PubMed]
- Clement, P.B. Histology of the ovary. Am. J. Surg. Pathol. 1987, 11, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Briley, S.M.; Jasti, S.; McCracken, J.M.; Hornick, J.E.; Fegley, B.; Pritchard, M.T.; Duncan, F.E. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 2016, 152, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Bialecka, M.; Moustakas, I.; Lam, E.; Torrens-Juaneda, V.; Borggreven, N.V.; Trouw, L.; Louwe, L.A.; Pilgram, G.S.K.; Mei, H.; et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 2019, 10, 3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zheng, Y.; Li, J.; Yu, Y.; Zhang, W.; Song, M.; Liu, Z.; Min, Z.; Hu, H.; Jing, Y.; et al. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell 2020, 180, 585–600.e19. [Google Scholar] [CrossRef] [PubMed]
- Rotgers, E.; Jorgensen, A.; Yao, H.H. At the Crossroads of Fate-Somatic Cell Lineage Specification in the Fetal Gonad. Endocr. Rev. 2018, 39, 739–759. [Google Scholar] [CrossRef]
- Hummitzsch, K.; Anderson, R.A.; Wilhelm, D.; Wu, J.; Telfer, E.E.; Russell, D.L.; Robertson, S.A.; Rodgers, R.J. Stem cells, progenitor cells, and lineage decisions in the ovary. Endocr. Rev. 2015, 36, 65–91. [Google Scholar] [CrossRef] [Green Version]
- Kinnear, H.M.; Tomaszewski, C.E.; Chang, F.L.; Moravek, M.B.; Xu, M.; Padmanabhan, V.; Shikanov, A. The ovarian stroma as a new frontier. Reproduction 2020, 160, R25–R39. [Google Scholar] [CrossRef]
- Wagner, M.; Yoshihara, M.; Douagi, I.; Damdimopoulos, A.; Panula, S.; Petropoulos, S.; Lu, H.; Pettersson, K.; Palm, K.; Katayama, S.; et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat. Commun. 2020, 11, 1147. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.S.; Ren, Y.A.; Candelaria, N.; Adams, J.E.; Rajkovic, A. Ovarian Follicular Theca Cell Recruitment, Differentiation, and Impact on Fertility: 2017 Update. Endocr. Rev. 2018, 39, 1–20. [Google Scholar] [CrossRef]
- Young, J.M.; McNeilly, A.S. Theca: The forgotten cell of the ovarian follicle. Reproduction 2010, 140, 489–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Peng, J.; Matzuk, M.M.; Yao, H.H. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat. Commun. 2015, 6, 6934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, M.; Moh-Moh-Aung, A.; Zeng, Z.; Yoshimura, T.; Wani, Y.; Matsukawa, A. Ovarian stromal cells as a source of cancer-associated fibroblasts in human epithelial ovarian cancer: A histopathological study. PLoS ONE 2018, 13, e0205494. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Jeong, S. Mutation Hotspots in the beta-Catenin Gene: Lessons from the Human Cancer Genome Databases. Mol. Cells 2019, 42, 8–16. [Google Scholar]
- Bugter, J.M.; Fenderico, N.; Maurice, M.M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat. Rev. Cancer 2021, 21, 5–21. [Google Scholar] [CrossRef] [PubMed]
- McMellen, A.; Woodruff, E.R.; Corr, B.R.; Bitler, B.G.; Moroney, M.R. Wnt Signaling in Gynecologic Malignancies. Int. J. Mol. Sci. 2020, 21, 4272–4293. [Google Scholar] [CrossRef] [PubMed]
- Teeuwssen, M.; Fodde, R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8, 1658–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.H.L.; Hough, R.; Bernaudo, S.; Peng, C. Wnt/beta-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J. Ovarian Res. 2019, 12, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arend, R.C.; Londono-Joshi, A.I.; Samant, R.S.; Li, Y.; Conner, M.; Hidalgo, B.; Alvarez, R.D.; Landen, C.N.; Straughn, J.M.; Buchsbaum, D.J. Inhibition of Wnt/beta-catenin pathway by niclosamide: A therapeutic target for ovarian cancer. Gynecol. Oncol. 2014, 134, 112–120. [Google Scholar] [CrossRef]
- Rask, K.; Nilsson, A.; Brannstrom, M.; Carlsson, P.; Hellberg, P.; Janson, P.O.; Hedin, L.; Sundfeldt, K. Wnt-signalling pathway in ovarian epithelial tumours: Increased expression of beta-catenin and GSK3beta. Br. J. Cancer 2003, 89, 1298–1304. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Wang, Y.; Broaddus, R.; Sun, L.; Xue, F.; Zhang, W. Exon 3 mutations of CTNNB1 drive tumorigenesis: A review. Oncotarget 2018, 9, 5492–5508. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003, 94, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.; Oliva, E.; Young, R.H. Solid pseudopapillary neoplasm of the ovary: A report of 3 primary ovarian tumors resembling those of the pancreas. Am. J. Surg. Pathol. 2010, 34, 1514–1520. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Carter, M.; Zhao, Z.; Abidi, A.; Hodeib, M. Ovarian Solid Pseudopapillary Tumor Resembling Benign Hemorrhagic Cyst on Rapid Frozen Section. Case Rep. Obstet. Gynecol. 2020, 2020, 6473630. [Google Scholar] [CrossRef] [PubMed]
- Kominami, A.; Fujino, M.; Murakami, H.; Ito, M. Beta-catenin mutation in ovarian solid pseudopapillary neoplasm. Pathol. Int. 2014, 64, 460–464. [Google Scholar] [CrossRef]
- Irving, J.A.; Young, R.H. Microcystic stromal tumor of the ovary: Report of 16 cases of a hitherto uncharacterized distinctive ovarian neoplasm. Am. J. Surg. Pathol. 2009, 33, 367–375. [Google Scholar] [CrossRef]
- Maeda, D.; Shibahara, J.; Sakuma, T.; Isobe, M.; Teshima, S.; Mori, M.; Oda, K.; Nakagawa, S.; Taketani, Y.; Ishikawa, S.; et al. Beta-catenin (CTNNB1) S33C mutation in ovarian microcystic stromal tumors. Am. J. Surg. Pathol. 2011, 35, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Bai, Q.M.; Yang, F.; Wu, L.J.; Cheng, Y.F.; Shen, X.X.; Cai, X.; Zhou, X.Y.; Yang, W.T. Microcystic stromal tumour of the ovary: Frequent mutations of beta-catenin (CTNNB1) in six cases. Histopathology 2015, 67, 872–879. [Google Scholar] [CrossRef]
- Irving, J.A.; Lee, C.H.; Yip, S.; Oliva, E.; McCluggage, W.G.; Young, R.H. Microcystic Stromal Tumor: A Distinctive Ovarian Sex Cord-Stromal Neoplasm Characterized by FOXL2, SF-1, WT-1, Cyclin D1, and beta-catenin Nuclear Expression and CTNNB1 Mutations. Am. J. Surg. Pathol. 2015, 39, 1420–1426. [Google Scholar] [CrossRef]
- Kang, Y.N.; Cho, C.H.; Kwon, S.Y. Microcystic stromal tumor of the ovary with mutation in exon 3 of beta-catenin: A case report. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2015, 34, 121–125. [Google Scholar] [CrossRef]
- Na, K.; Kim, E.K.; Jang, W.; Kim, H.S. CTNNB1 Mutations in Ovarian Microcystic Stromal Tumors: Identification of a Novel Deletion Mutation and the Use of Pyrosequencing to Identify Reported Point Mutation. Anticancer Res. 2017, 37, 3249–3258. [Google Scholar] [PubMed]
- Lee, S.H.; Koh, Y.W.; Roh, H.J.; Cha, H.J.; Kwon, Y.S. Ovarian microcystic stromal tumor: A novel extracolonic tumor in familial adenomatous polyposis. Genes Chromosomes Cancer 2015, 54, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gallagher, R.L.; Price, G.R.; Bolton, E.; Joy, C.; Harraway, J.; Venter, D.J.; Armes, J.E. Ovarian Microcystic Stromal Tumor: A Rare Clinical Manifestation of Familial Adenomatous Polyposis. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2016, 35, 561–565. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Irving, J.A.; Chong, A.S.; Clarke, B.A.; Young, R.H.; Foulkes, W.D.; Rivera, B. Ovarian Microcystic Stromal Tumors Are Characterized by Alterations in the Beta-Catenin-APC Pathway and May be an Extracolonic Manifestation of Familial Adenomatous Polyposis. Am. J. Surg. Pathol. 2018, 42, 137–139. [Google Scholar] [CrossRef]
- Ramzy, I. Signet-ring stromal tumor of ovary. Histochemical, light, and electron microscopic study. Cancer 1976, 38, 166–172. [Google Scholar] [CrossRef]
- Dickersin, G.R.; Young, R.H.; Scully, R.E. Signet-ring stromal and related tumors of the ovary. Ultrastruct. Pathol. 1995, 19, 401–419. [Google Scholar] [CrossRef]
- Su, R.M.; Chang, K.C.; Chou, C.Y. Signet-ring stromal tumor of the ovary: A case report. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2003, 13, 90–93. [Google Scholar] [CrossRef]
- Suarez, A.; Palacios, J.; Burgos, E.; Gamallo, C. Signet-ring stromal tumor of the ovary: A histochemical, immunohistochemical and ultrastructural study. Virchows Arch. A Pathol. Anat. Histopathol. 1993, 422, 333–336. [Google Scholar] [CrossRef]
- Vang, R.; Bague, S.; Tavassoli, F.A.; Prat, J. Signet-ring stromal tumor of the ovary: Clinicopathologic analysis and comparison with Krukenberg tumor. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2004, 23, 45–51. [Google Scholar] [CrossRef]
- Hardisson, D.; Regojo, R.M.; Marino-Enriquez, A.; Martinez-Garcia, M. Signet-ring stromal tumor of the ovary: Report of a case and review of the literature. Pathol. Oncol. Res. POR 2008, 14, 333–336. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Nakazono, Y.; Hirotani, K.; Kawanaka, H. Signet ring cell-rich microcystic stromal tumor of the ovary: A poorly recognized variant. Hum. Pathol. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hayashi, Y.; Ohtsuki, Y.; Ikegami, N.; Toi, M.; Iguchi, M.; Hiroi, M. Signet-ring stromal tumor of the ovary: An immunohistochemical and ultrastructural study with a review of the literature. Med. Mol. Morphol. 2008, 41, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Shaco-Levy, R.; Kachko, L.; Mazor, M.; Piura, B. Ovarian signet-ring stromal tumor: A potential diagnostic pitfall. Int. J. Surg. Pathol. 2008, 16, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.M.; Ramzy, I. Perspectives on signet ring stromal cell tumor and related signet ring cell lesions of the gonads. Adv. Anat. Pathol. 2014, 21, 443–449. [Google Scholar] [CrossRef]
- Kopczynski, J.; Kowalik, A.; Chlopek, M.; Wang, Z.F.; Gozdz, S.; Lasota, J.; Miettinen, M. Oncogenic Activation of the Wnt/beta-Catenin Signaling Pathway in Signet Ring Stromal Cell Tumor of the Ovary. Appl. Immunohistochem. Mol. Morphol. AIMM 2016, 24, e28–e33. [Google Scholar] [CrossRef]
- Mishra, J.; Das, J.K.; Kumar, N. Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through beta-catenin. J. Biol. Chem. 2017, 292, 16406–16419. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Lu, W.; Lv, W. Overlap of microcystic stromal tumor and primary solid pseudopapillary neoplasm of the ovary. Int. J. Clin. Exp. Pathol. 2015, 8, 11792–11797. [Google Scholar]
- Santiago, L.; Daniels, G.; Wang, D.; Deng, F.M.; Lee, P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am. J. Cancer Res. 2017, 7, 1389–1406. [Google Scholar]
- Singhi, A.D.; Lilo, M.; Hruban, R.H.; Cressman, K.L.; Fuhrer, K.; Seethala, R.R. Overexpression of lymphoid enhancer-binding factor 1 (LEF1) in solid-pseudopapillary neoplasms of the pancreas. Mod. Pathol. 2014, 27, 1355–1363. [Google Scholar] [CrossRef]


| SPPN | MCST | SRSCT | |
|---|---|---|---|
| Clinicopathologic Features | Mean age 45 | Mean age 43 | Mean age 53 |
| Unilateral, solid and cystic | Unilateral, solid and well demarcated | Unilateral, solid and well demarcated | |
| Morphological features | |||
| Growth patterns | Solid, pseudopapillary and microcystic growth patterns | Solid and microcystic growth patterns | Solid, nested and trabecular growth patterns |
| Tumor cell Morphology | Uniform round nuclei, abundant eosinophilic cytoplasm with or without cytoplasmic vacuoles Lack of cytological atypia and mitosis | Uniform round nuclei, abundant eosinophilic cytoplasm with or without cytoplasmic vacuoles Lack of cytological atypia and mitosis | Signet ring-like cells with eccentric nuclei, and a single large cytoplasmic vacuole, admixed with spindle cells Lack of cytological atypia and mitosis |
| Immunophenotype | |||
| Diffuse positive | CD10, vimentin, CD56, Cyclin D1 | CD10, vimentin, CD56, Cyclin D1 | CD10, vimentin, CD56, Cyclin D1 |
| Variable positive | WT1, CD99, PR, CD117 | WT1, CD99, SF1, FOXL2 | WT1, CD99, SF1, FOXL2 |
| Totally negative | AE1/3, CAM5.2, EMA, inhibin, calretinin, SMA, synaptophysin, INSM1 | AE1/3, CAM5.2, EMA, inhibin, calretinin, SMA, synaptophysin, INSM1 | AE1/3, CAM5.2, EMA, inhibin, calretinin, SMA, synaptophysin, INSM1 |
| Mutations of Wnt signaling genes | CTNNB1 exon 3 | CTNNB1 exon 3 or APC gene | CTNNB1 exon 3 or APC gene |
| Immunohistochemical staining of β-catenin | Nuclear and cytoplasmic | Nuclear and cytoplasmic | Nuclear and cytoplasmic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Michener, C.M.; Yang, B. Low-Grade Ovarian Stromal Tumors with Genetic Alterations of the Wnt/β-Catenin Pathway That Is Crucial in Ovarian Follicle Development and Regulation. Cancers 2022, 14, 5622. https://doi.org/10.3390/cancers14225622
Zhang G, Michener CM, Yang B. Low-Grade Ovarian Stromal Tumors with Genetic Alterations of the Wnt/β-Catenin Pathway That Is Crucial in Ovarian Follicle Development and Regulation. Cancers. 2022; 14(22):5622. https://doi.org/10.3390/cancers14225622
Chicago/Turabian StyleZhang, Gloria, Chad M. Michener, and Bin Yang. 2022. "Low-Grade Ovarian Stromal Tumors with Genetic Alterations of the Wnt/β-Catenin Pathway That Is Crucial in Ovarian Follicle Development and Regulation" Cancers 14, no. 22: 5622. https://doi.org/10.3390/cancers14225622




