Merkel Cell Carcinoma of the External Ear: Population-Based Analysis and Survival Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Variable Selection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel Cell Carcinoma: Current United States Incidence and Projected Increases based on Changing Demographics. J. Am. Dermatol. 2017, 78, 457–463.e2. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.Q.; Waldeck, K.; Vergara, I.A.; Schröder, J.; Madore, J.; Wilmott, J.S.; Colebatch, A.J.; de Paoli-Iseppi, R.; Li, J.; Lupat, R.; et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015, 75, 5228–5234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Peñas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Lemos, B.D.; Storer, B.E.; Lyer, J.G.; Phillips, J.L.; Bichakjian, C.K.; Fang, L.C.; Johnson, T.M.; Liegois-Kwon, N.J.; Otley, C.C.; Paulson, K.G.; et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J. Am. Acad. Dermatol. 2010, 63, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Albores-Saavedra, J.; Batich, K.; Chable-Montero, F.; Sagy, N.; Schwartz, A.; Henson, D.E. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population based study. J. Cutan. Pathol. 2009, 37, 20–27. [Google Scholar] [CrossRef]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Clinical Factors Associated With Merkel Cell Polyomavirus Infection in Merkel Cell Carcinoma. J. Natl. Cancer Inst. 2009, 101, 938–945. [Google Scholar] [CrossRef]
- Scampa, M.; Merat, R.; Tzika, E.; Kalbermatten, D.; Oranges, C. Survival outcomes and epidemiology of Merkel cell carcinoma of the lower limb and hip: A Surveillance, Epidemiology, and End Results analysis 2000–2018. JAAD Int. 2022, 7, 13–21. [Google Scholar] [CrossRef]
- Huang, G.-S.; Chang, W.-C.; Lee, H.-S.; Taylor, J.; Cheng, T.-Y.; Chen, C.Y. Merkel Cell Carcinoma Arising from the Subcutaneous Fat of the Arm with Intact Skin. Dermatol. Surg. 2005, 31, 717–719. [Google Scholar] [CrossRef]
- Toker, C. Trabecular Carcinoma of the Skin. Am. J. Dermatopathol. 1983, 4, 497–500. [Google Scholar] [CrossRef]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 triet. Lancet Oncol. 2016, 17, 12. [Google Scholar] [CrossRef] [Green Version]
- McCarty, M.; Lentsch, E.; Cerrati, E.; Stadelmann, W. Melanoma of the ear: Results of a cartilage-sparing approach to resection. Eur. Arch. Otorhinolaryngol. 2013, 270, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Shinogi, T.; Nagase, K.; Inoue, T.; Sato, K.; Onita, A.; Takamori, A.; Narisawa, Y. Merkel cell carcinoma: A systematic review of the demographic and clinical characteristics of 847 cases in Japan. J. Dermatol. 2021, 48, 1027–1034. [Google Scholar] [CrossRef]
- Ezaldein, H.; Ventura, A.; DeRuyter, N.; Yin, E.; Giunta, A. Understanding the influence of patient demographics on disease severity, treatment strategy, and survival outcomes in merkel cell carcinoma: A surveillance, epidemiology, and end-results study. Oncoscience 2017, 4, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.; Camp, E.R.; Lentsch, E. Merkel cell carcinoma: Identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER data. Laryngoscope 2012, 122, 1283–1290. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, D.; Zhao, J.; Zhu, B.; Xie, J. Clinical Features and Prognosis of Merkel Cell Carcinoma in Elderly Patients. Med. Sci. Monit. 2020, 26, e924570-1–e924570-13. [Google Scholar] [CrossRef]
- Grabowski, J.; Saltzstein, S.; Sadler, G.; Tahir, Z.; Blair, S. A Comparison of Merkel Cell Carcinoma and Melanoma: Results from the California Cancer Registry. Clin. Med. Oncol. 2008, 2, 327–333. [Google Scholar] [CrossRef]
- Zaar, O.; Gillstedt, M.; Lindelöf, B.; Wennberg-Larkö, A.-M.; Paoli, J. Merkel cell carcinoma incidence is increasing in Sweden. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1708–1713. [Google Scholar] [CrossRef]
- Youlden, D.; Soyer, P.; Youl, P.; Fritschi, L.; Baade, P. Incidence and Survival for Merkel Cell Carcinoma in Queensland, Australia, 1993–2010. JAMA Dermatol. 2014, 150, 864–872. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V. The Protective Role of Melanin Against UV Damage in Human Skin†. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Brewer, J. Merkel Cell Carcinoma in Immunosuppressed Patients. Cancers 2014, 6, 1328–1350. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.; Luu, M.; Barker, C.A.; Gharavi, N.M.; Hamid, O.; Shiao, S.L.; Nguyen, A.T.; Lu, D.J.; Ho, A.S.; Zumsteg, Z.S. Improved survival in women versus men with merkel cell carcinoma. J. Am. Acad. Dermatol. 2020, 84, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Andrini, E.; Siepe, G.; Mosconi, C.; Ambrosini, V.; Ricci, C.; Marchese, P.V.; Ricco, G.; Casadei, R.; Campana, D. Lymph node ratio predicts efficacy of postoperative radiation therapy in nonmetastatic Merkel cell carcinoma: A population—Based analysis. Cancer Med. 2022. [Google Scholar] [CrossRef]
- Santamaria-Barria, J.; Boland, G.; Yeap, B.; Nardi, V.; Dias-Santagata, D.; Cusack, J. Merkel Cell Carcinoma: 30-Year Experience from a Single Institution. Ann. Surg. Oncol. 2012, 20, 1365–1373. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, D.; Zhao, J.; Zhu, B.; Xie, J. Does regional lymph node status have a predictive effect on the prognosis of Merkel cell carcinoma? J. Plast. Reconstr. Aesthet. Surg. 2020, 74, 845–856. [Google Scholar] [CrossRef]
- Maloney, N.; Nguyen, K.; So, N.; Aasi, S.; Zaba, L. Risk factors for and prognostic impact of positive surgical margins after excision of Merkel cell carcinoma. J. Am. Acad. Dermatol. 2021, 87, 444–446. [Google Scholar] [CrossRef]
- Tai, P. A Practical Update of Surgical Management of Merkel Cell Carcinoma of the Skin. ISRN Surg. 2013, 2013, 850797. [Google Scholar] [CrossRef] [Green Version]
- Jouary, T.; Leyral, C.; Dreno, B.; Doussau, A.; Sassolas, B.; Beylot-Barry, M.; Renaud-Vilmer, C.; Guillot, B.; Bernard, P.; Lok, C.; et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: A multicentric prospective randomized study. Ann. Oncol. 2011, 23, 1074–1080. [Google Scholar] [CrossRef]
- Agarwal, C.; Johns, D.; Tanner, P.; Andtbacka, R. Osseointegrated Prosthetic Ear Reconstruction in Cases of Skin Malignancy: Technique, Outcomes, and Patient Satisfaction. Ann. Plast. Surg. 2017, 80, 32–39. [Google Scholar] [CrossRef]
- Crisan, D.; Colosi, H.A.; Manea, A.; Kastler, S.; Lipke, A.; Crisan, M.; Scharffetter-Kochanek, K.; Schneider, L.A. Retrospective Analysis of Complication Rates Associated With Auricular Reconstruction After Skin Cancer Surgery. J. Cutan. Med. Surg. 2019, 24, 120347541989084. [Google Scholar] [CrossRef]
- Medina-Franco, H.; Urist, M.; Fiveash, J.; Heslin, M.; Bland, K.; Beenken, S. Multimodality Treatment of Merkel Cell Carcinoma: Case Series and Literature Review of 1024 Cases. Ann. Surg. Oncol. 2011, 8, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.; Bowne, W.; Jaques, D.; Brennan, M.; Busam, K.; Coit, D. Merkel Cell Carcinoma: Prognosis and Treatment of Patients from a Single Institution. J. Clin. Oncol. 2005, 23, 2300–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, C.; Kwan, W. Radiotherapy and Conservative Surgery in the Locoregional Management of Merkel Cell Carcinoma: The British Columbia Cancer Agency Experience. Ann. Surg. Oncol. 2015, 23, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Jouary, T.; Kubica, E.; Dalle, S.; Pages, C.; Duval-Modeste, A.B.; Guillot, B.; Mansard, S.; Saiag, P.; Aubin, F.; Bedane, C.; et al. Sentinel Node Status and Immunosuppression: Recurrence Factors in Localized Merkel Cell Carcinoma. Acta Derm. Venereol. 2015, 95, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Tarantola, T.I.; Vallow, L.A.; Halyard, M.Y.; Weenig, R.H.; Warschaw, K.E.; Grotz, T.E.; Jakub, J.W.; Roenigk, R.K.; Brewer, J.D.; Weaver, A.L.; et al. Prognostic factors in Merkel cell carcinoma: Analysis of 240 cases. J. Am. Acad. Dermatol. 2012, 68, 425–432. [Google Scholar] [CrossRef]
- Fields, R.C.; Busam, K.J.; Chou, J.F.; Panageas, K.S.; Pulitzer, M.P.; Allen, P.J.; Kraus, D.H.; Brady, M.S.; Coit, D.G. Recurrence after complete resection and selective use of adjuvant therapy for stage I through III Merkel cell carcinoma. Cancer 2012, 118, 3311–3320. [Google Scholar] [CrossRef]
- Hasan, S.; Liu, L.; Triplet, J.; Li, Z.; Mansur, D. The Role of Postoperative Radiation and Chemoradiation in Merkel Cell Carcinoma: A Systematic Review of the Literature. Front. Oncol. 2013, 3, 276. [Google Scholar] [CrossRef] [Green Version]
- Palencia, R.; Sandhu, A.; Webb, S.; Blaikie, T.; Bharmal, M. Systematic literature review of current treatments for stage I–III Merkel cell carcinoma. Future Oncol. 2021, 17, 4813–4822. [Google Scholar] [CrossRef]
- Kadakia, S.; Badhey, A.; Inman, J.; Mourad, M.; Ducic, Y. Surgical management of temporal bone osteoradionecrosis: Single surgeon experience of 47 cases. Am. J. Otolaryngol. 2017, 38, 688–691. [Google Scholar] [CrossRef]
- Clark, J.R.; McCluskey, S.A.; Hall, F.; Lipa, J.; Neligan, P.; Brown, D.; Irish, J.; Gullane, P.; Gilbert, R. Predictors of morbidity following free flap reconstruction for cancer of the head and neck. Head Neck 2007, 29, 1090–1101. [Google Scholar] [CrossRef] [PubMed]


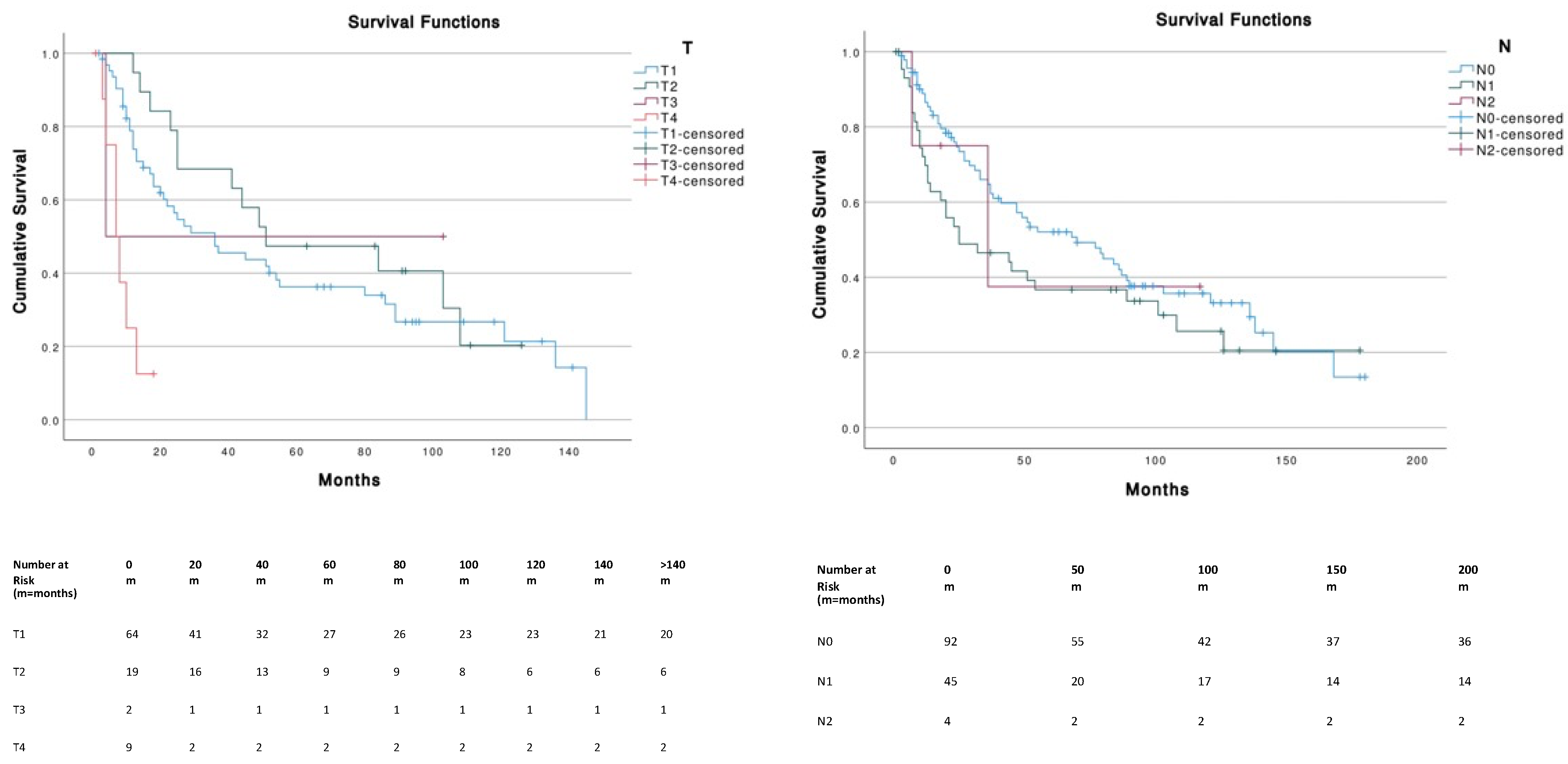
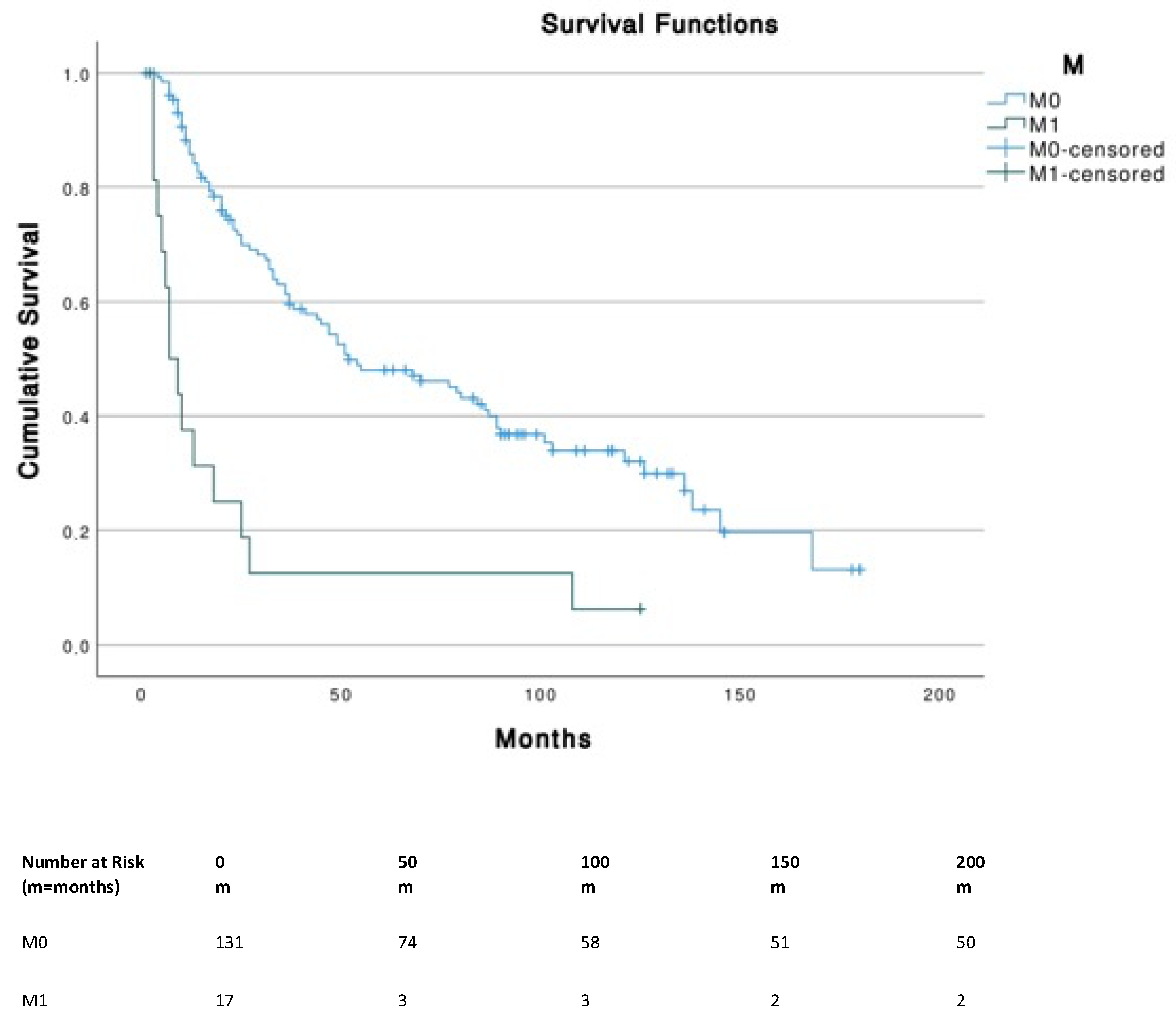
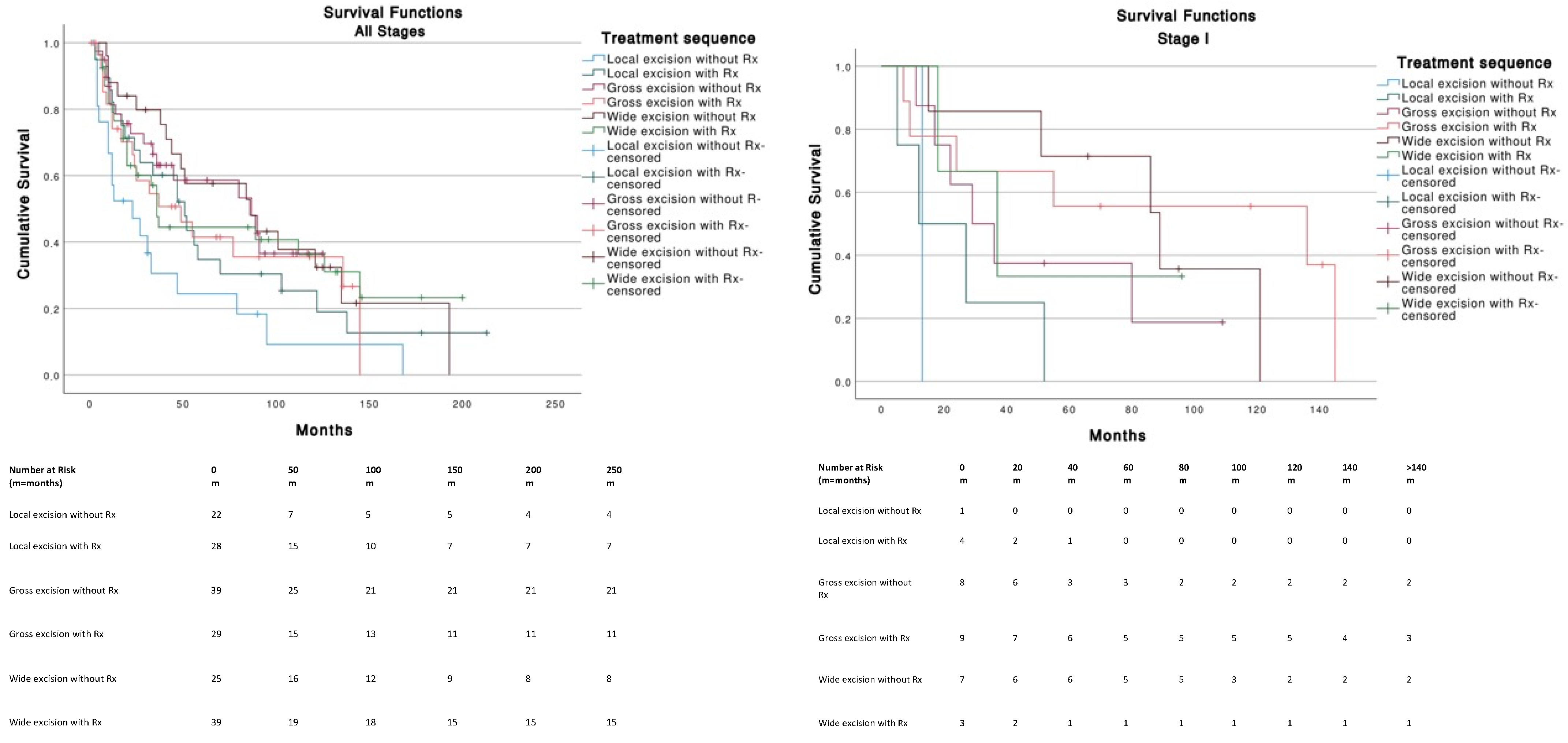

| Variable | MCC on External Ear (n = 210) |
|---|---|
| Sex | |
| Female | 50 (23.8%) |
| Male | 160 (76.2%) |
| Age at diagnosis | |
| Mean (SD) | 77.98 (10.785) |
| Median (min–max) | 80.00 (43–99) |
| Race | |
| White | 191 (91.8%) |
| Asian/Pacific Islander | 10 (4.8%) |
| Black | 6 (2.9%) |
| American Indian/Alaska Native | 1 (0.5%) |
| State | |
| California | 95 (45.2%) |
| Connecticut | 5 (2.4%) |
| Georgia | 25 (11.9%) |
| Hawaii | 4 (1.9%) |
| Iowa | 12 (5.7%) |
| Kentucky | 12 (5.7%) |
| Louisiana | 17 (8.1%) |
| New Jersey | 21 (10%) |
| New Mexico | 7 (3.3%) |
| Seattle | 8 (3.8%) |
| Utah | 4 (1.9%) |
| UV exposure | |
| ≥180 days | 148 (70.5%) |
| <180 days | 62 (29.5%) |
| TNM | |
| T | |
| T1 | 64 (68.1%) |
| T2 | 19 (20.2%) |
| T3 | 2 (2.1%) |
| T4 | 9 (9.6%) |
| N | |
| N0 | 92 (65.2%) |
| N1 | 45 (31.9%) |
| N2 | 4 (2.8%) |
| M | |
| M0 | 131 (88.5%) |
| M1 | 17 (11.5%) |
| Stage | |
| Stage I | 33 (36.3%) |
| Stage II | 11 (12.1%) |
| Stage III (nodal) | 32 (35.2%) |
| Stage IV (distant metastasis) | 15 (16.5%) |
| Variable | MCC on External Ear (n = 210) |
|---|---|
| Surgery | |
| No | 28 (13.3%) |
| Local excision (no margin) | 50 (23.8%) |
| Gross excision (<1 cm margin) | 68 (32.4%) |
| Wide excision (>1 cm) | 64 (30.5%) |
| Surgery/Radiotherapy (Rx) | |
| Local excision with Rx | 28 (25.0%) |
| Local excision without Rx | 22 (18.2%) |
| Gross excision with Rx | 29 (37.9%) |
| Gross excision without Rx | 39 (53.8%) |
| Wide excision with Rx | 39 (38.5%) |
| Wide excision without Rx | 25 (32.0%) |
| Multimodal treatment | |
| Surgery without Radiotherapy | 86 (41.0%) |
| Surgery with Radiotherapy | 96 (45.7%) |
| Radiotherapy without Chemotherapy | 93 (44.3%) |
| Radiotherapy with Chemotherapy | 16 (7.6%) |
| Radiotherapy | |
| Yes | 109 (51.9%) |
| No/unknown | 101 (48.1%) |
| Chemotherapy | |
| Yes | 19 (9.0%) |
| No/unknown | 191 (91.0%) |
| No Radiotherapy and No Chemotherapy (n = 84) | Radiotherapy and No Chemotherapy (n = 83) | Chemotherapy and No Radiotherapy (n = 3) | Radiotherapy and Chemotherapy (n = 16) | |
|---|---|---|---|---|
| Local Excision (n = 50) | 22 | 24 | 0 | 4 |
| Gross Excision (n = 68) | 38 | 27 | 1 | 2 |
| Wide Excision (n = 64) | 24 | 32 | 1 | 7 |
| No Surgery (n = 28) | 0 | 0 | 1 | 3 |
| Variable | B | p Value | Exp (B) | 95% CI for Exp (B) |
|---|---|---|---|---|
| Age a | ||||
| <65 | ||||
| 65–79 | −1.626 | <0.001 | 0.197 | 0.100/0.386 |
| ≥80 | −0.897 | <0.001 | 0.408 | 0.271/0.613 |
| Sex a | ||||
| Male | ||||
| Female | −0.624 | 0.003 | 0.536 | 0.353/0.813 |
| Tumor Size a | ||||
| T1 | 0.000 | |||
| T2 | −0.173 | 0.589 | 0.841 | 0.450/1.575 |
| T3 | −0.823 | 0.422 | 0.439 | 0.059/3.274 |
| T4 | 1.769 | 0.000 | 5.864 | 2.437/14.106 |
| Lymph node status a | ||||
| N0 | 0.449 | |||
| N1 | 0.227 | 0.217 | 1.323 | 0.848/2.064 |
| N2 | 0.723 | 0.686 | 1.339 | 0.324/5.529 |
| Metastasis a | ||||
| M0 | ||||
| M1 | 1.712 | 0.000 | 5.539 | 3.023/10.149 |
| Stage a | ||||
| I | 0.000 | |||
| II | 0.036 | 0.936 | 1.036 | 0.433/2.482 |
| III | −0.095 | 0.750 | 0.909 | 0.505/1.636 |
| IV | 1.447 | 0.000 | 4.248 | 2.082/8.667 |
| Treatment a | ||||
| Local excision without Rx | 0.201 | |||
| Local excision with Rx | −0.575 | 0.079 | 0.563 | 0.297/1.068 |
| Gross excision without Rx | −0.787 | 0.020 | 0.455 | 0.235/0.883 |
| Gross excision with Rx | −0.744 | 0.029 | 0.475 | 0.244/0.928 |
| Wide excision without Rx | −0.649 | 0.060 | 0.523 | 0.265/1.029 |
| Wide excision with Rx | −0.642 | 0.042 | 0.526 | 0.283/0.978 |
| Radiotherapy a | ||||
| No/unknown | ||||
| Yes | −0.112 | 0.525 | 0.894 | 0.633/1.263 |
| Chemotherapy a | ||||
| No/unknown | ||||
| Yes | 0.167 | 0.547 | 1.181 | 0.687/2.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.S.; Scampa, M.; Martineau, J.; Giordano, S.; Kalbermatten, D.F.; Oranges, C.M. Merkel Cell Carcinoma of the External Ear: Population-Based Analysis and Survival Outcomes. Cancers 2022, 14, 5653. https://doi.org/10.3390/cancers14225653
Alves AS, Scampa M, Martineau J, Giordano S, Kalbermatten DF, Oranges CM. Merkel Cell Carcinoma of the External Ear: Population-Based Analysis and Survival Outcomes. Cancers. 2022; 14(22):5653. https://doi.org/10.3390/cancers14225653
Chicago/Turabian StyleAlves, André S., Matteo Scampa, Jérôme Martineau, Salvatore Giordano, Daniel F. Kalbermatten, and Carlo M. Oranges. 2022. "Merkel Cell Carcinoma of the External Ear: Population-Based Analysis and Survival Outcomes" Cancers 14, no. 22: 5653. https://doi.org/10.3390/cancers14225653
APA StyleAlves, A. S., Scampa, M., Martineau, J., Giordano, S., Kalbermatten, D. F., & Oranges, C. M. (2022). Merkel Cell Carcinoma of the External Ear: Population-Based Analysis and Survival Outcomes. Cancers, 14(22), 5653. https://doi.org/10.3390/cancers14225653








