Impact of FLT3–ITD Mutation Status and Its Ratio in a Cohort of 2901 Patients Undergoing Upfront Intensive Chemotherapy: A PETHEMA Registry Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patients and Eligibility
2.3. Data Extraction
2.4. Treatment Schedules
2.5. Cytogenetic and Molecular Analysis
2.6. Analysis of FLT3–ITD and Ratio
2.7. Definitions and Study Endpoints
2.8. Statistical Methods
3. Results
3.1. Pretreatment Characteristics according to FLT3–ITD Allelic Ratio (FLT3–ITD AR)
3.2. Response to Induction Therapy according to FLT3–ITD Allelic Ratio
3.3. Survival Analyses according to FLT3–ITD Allelic Ratio
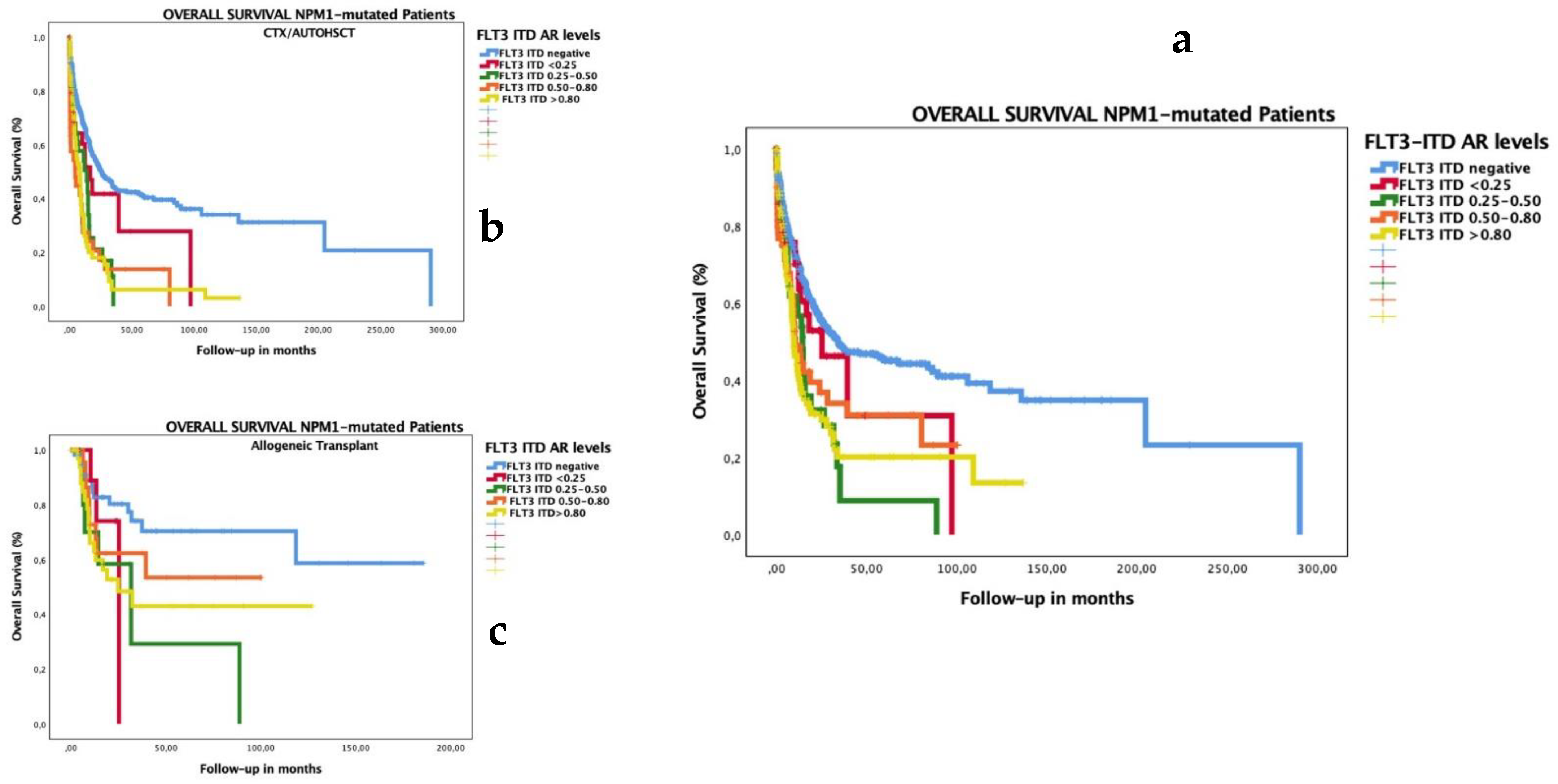
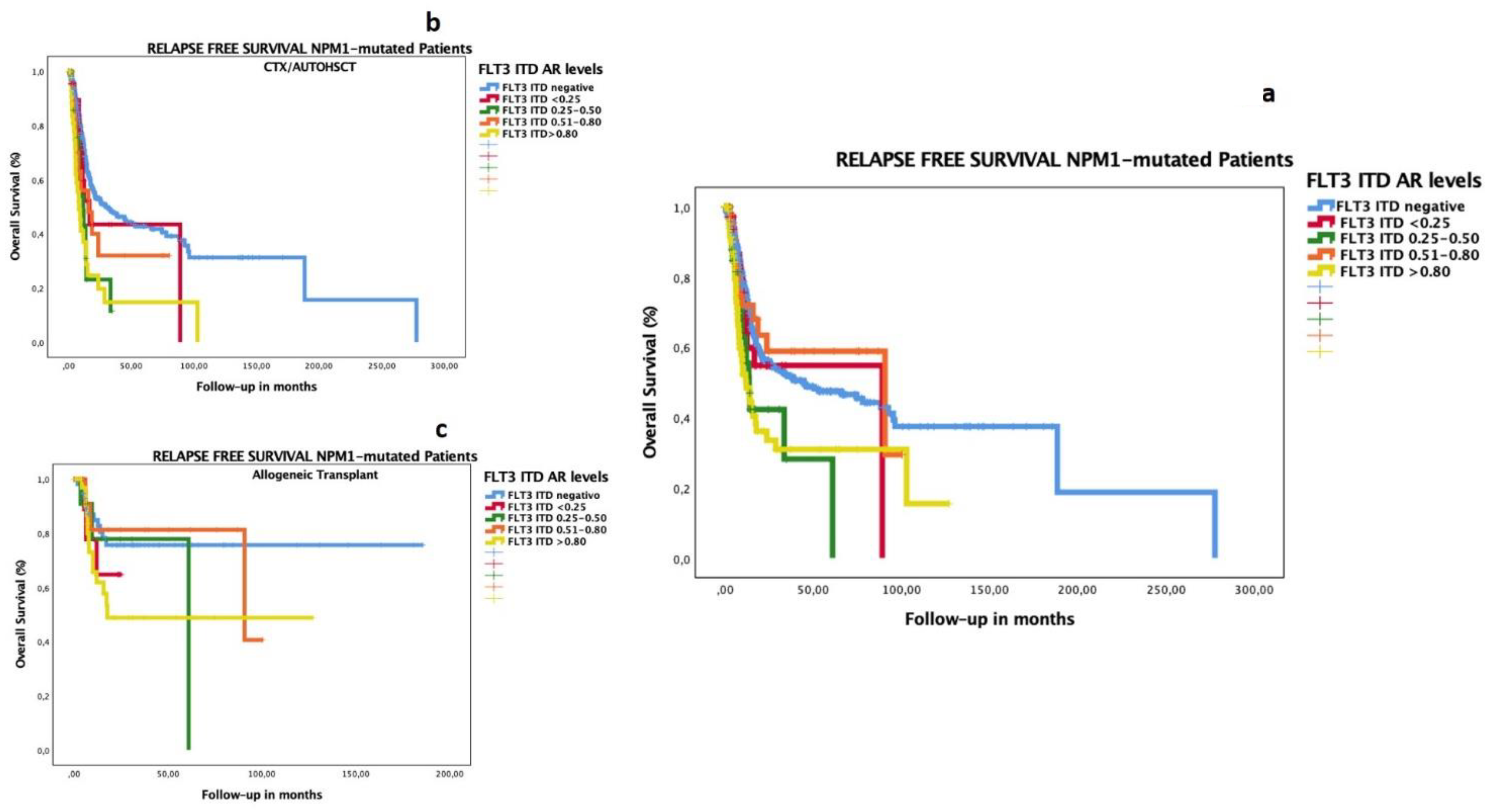
3.4. Impact of Low FLT3–ITD AR in Patients with Co-Occurrence of Mutated NPM1
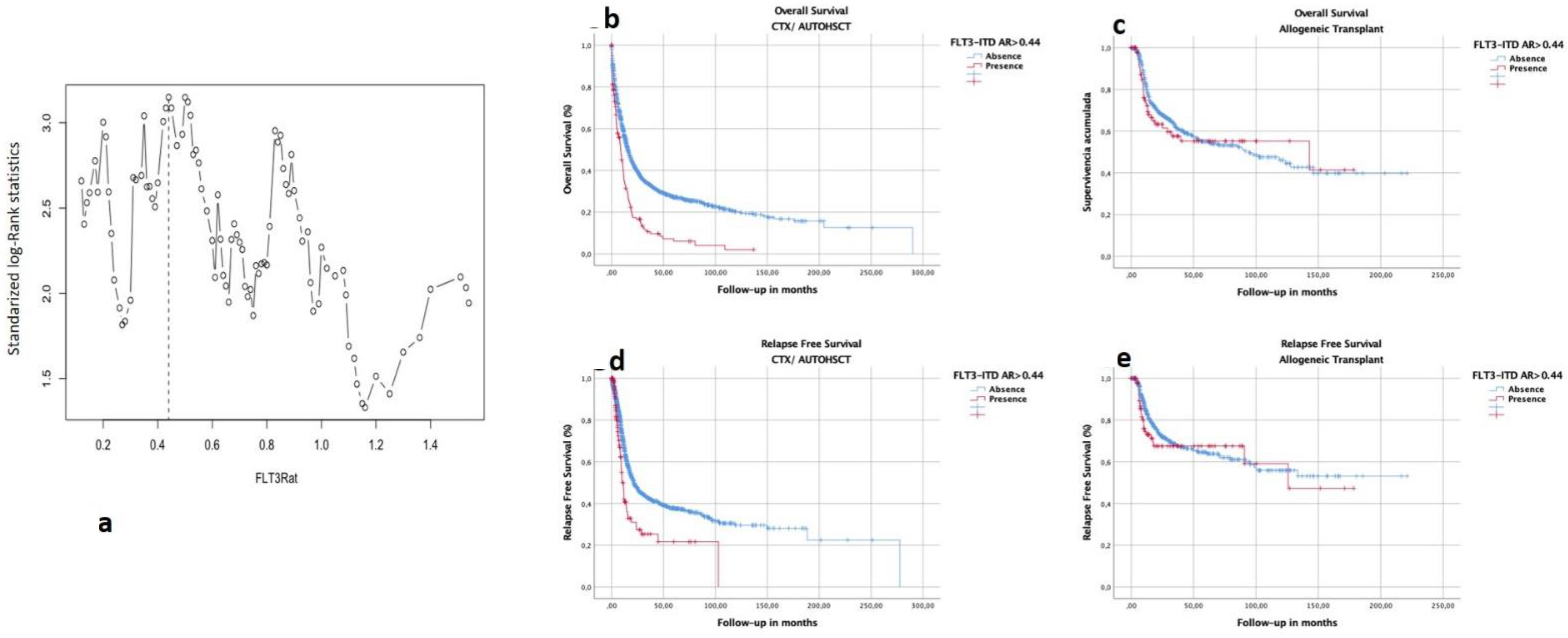
3.5. Selection of Optimal Cutoff of FLT3–ITD Allelic Ratio in This Cohort
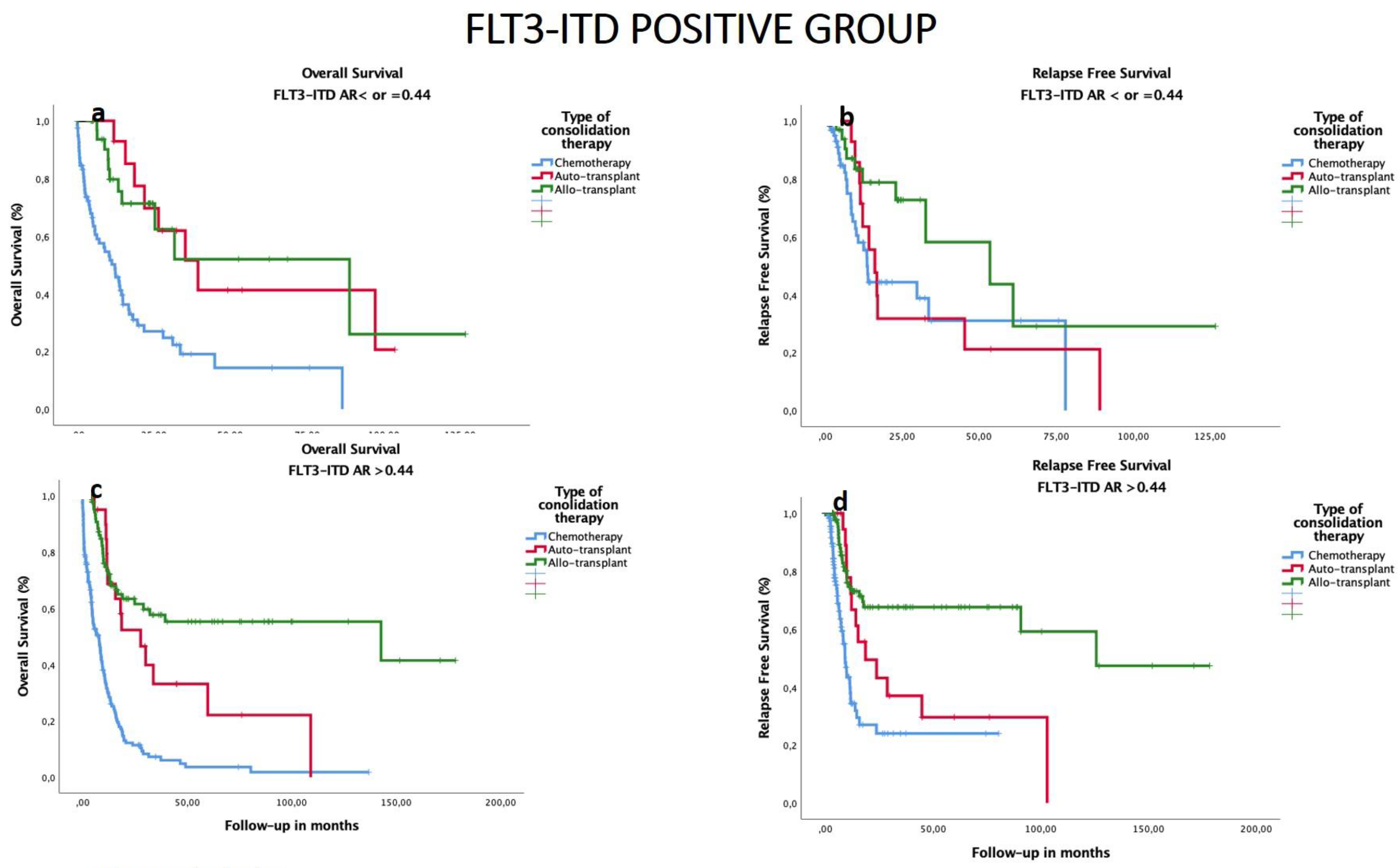
3.6. Impact of Consolidation Treatment according to FLT3–ITD AR in All Patients
3.7. Subgroup Analysis of OS and RFS by HSCT Type and Biological and Genomic Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Trial Registration
References
- Kiyoi, B.H.; Naoe, T.; Nakano, Y.; Yokota, S.; Minami, S.; Miyawaki, S.; Asou, N.; Kuriyama, K.; Jinnai, I.; Shimazaki, C.; et al. Prognostic Implication of FLT3 and N- RAS Gene Mutations in Acute Myeloid Leukemia. Blood 1999, 93, 3074–3080. [Google Scholar] [PubMed]
- Kottaridis, P.D.; Gale, R.E.; Frew, M.E.; Harrison, G.; Langabeer, S.E.; Belton, A.A.; Walker, H.; Wheatley, K.; Bowen, D.T.; Burnett, A.K.; et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001, 98, 1752–1759. [Google Scholar] [PubMed]
- Thiede, C.; Steudel, C.; Mohr, B.; Schaich, M.; Schäkel, U.; Platzbecker, U.; Wermke, M.; Bornhäuser, M.; Ritter, M.; Neubauer, A.; et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002, 99, 4326–4335. [Google Scholar] [CrossRef]
- Schnittger, S.; Schoch, C.; Dugas, M.; Kern, W.; Staib, P.; Wuchter, C.; Löffler, H.; Sauerland, C.M.; Serve, H.; Büchner, T.; et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002, 100, 59–66. [Google Scholar] [CrossRef]
- Fröhling, S.; Schlenk, R.F.; Breitruck, J.; Benner, A.; Kreitmeier, S.; Tobis, K.; Döhner, H.; Döhner, K. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: A study of the AML Study Group Ulm. Blood 2002, 100, 4372–4380. [Google Scholar] [CrossRef]
- Levis, M.; Small, D. FLT3: ITDoes matter in leukemia. Leukemia 2003, 17, 1738–1752. [Google Scholar] [CrossRef]
- Whitman, S.P.; Archer, K.J.; Feng, L.; Baldus, C.; Becknell, B.; Carlson, B.D.; Carroll, A.J.; Mrózek, K.; Vardiman, J.W.; George, S.L.; et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: A cancer and leukemia group B study. Cancer Res 2001, 61, 7233–7239. [Google Scholar]
- Schnittger, S.; Bacher, U.; Haferlach, C.; Alpermann, T.; Kern, W.; Haferlach, T. Diversity of the juxtamembrane and TKD1 mutations (exons 13-15) in the FLT3 gene with regards to mutant load, sequence, length, localization, and correlation with biological data. Genes Chromosomes Cancer 2012, 51, 910–924. [Google Scholar] [CrossRef]
- Stirewalt, D.L.; Pogosova-Agadjanyan, E.L.; Tsuchiya, K.; Joaquin, J.; Meshinchi, S. Copy-neutral loss of heterozygosity is prevalent and a late event in the pathogenesis of FLT3/ITD AML. Blood Cancer J. 2014, 4, e208. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Kayser, S.; Bullinger, L.; Kobbe, G.; Casper, J.; Ringhoffer, M.; Held, G.; Brossart, P.; Lübbert, M.; Salih, H.R.; et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 2014, 124, 3441–3449. [Google Scholar] [CrossRef]
- Boddu, P.C.; Kadia, T.M.; Garcia-Manero, G.; Cortes, J.; Alfayez, M.; Borthakur, G.; Konopleva, M.; Jabbour, E.J.; Daver, N.G.; DiNardo, C.D.; et al. Validation of the 2017 European LeukemiaNet classification for acute myeloid leukemia with NPM1 and FLT3-internal tandem duplication genotypes. Cancer 2019, 125, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Pratcorona, M.; Brunet, S.; Nomdedéu, J.; Ribera, J.M.; Tormo, M.; Duarte, R.; Escoda, L.; Guàrdia, R.; de Llano, M.P.Q.; Salamero, O.; et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: Relevance to post-remission therapy. Blood 2013, 121, 2734–2738. [Google Scholar] [CrossRef]
- Gale, R.E.; Green, C.; Allen, C.; Mead, A.J.; Burnett, A.K.; Hills, R.K.; Linch, D.C. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008, 111, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Pratz, K.W.; Levis, M. How I treat FLT3-mutated AML. Blood 2017, 129, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.D.; Schetelig, J.; Bochtler, T.; Schaich, M.; Schäfer-Eckart, K.; Hänel, M.; Rösler, W.; Einsele, H.; Kaufmann, M.; Serve, H.; et al. Allogeneic Stem Cell Transplantation Improves Survival in Patients with Acute Myeloid Leukemia Characterized by a High Allelic Ratio of Mutant FLT3-ITD. Biol. Blood Marrow Transplant. 2016, 22, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, G.; Nagafuji, K.; Miyamoto, T.; Kinukawa, N.; Takase, K.; Eto, T.; Kato, K.; Hayashi, S.; Kamimura, T.; Ohno, Y.; et al. FLT3 mutations in normal karyotype acute myeloid leukemia in first complete remission treated with autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2005, 36, 977–983. [Google Scholar] [CrossRef][Green Version]
- Bornhäuser, M.; Illmer, T.; Schaich, M.; Soucek, S.; Ehninger, G.; Thiede, C.; AML SHG 96 Study Group. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood 2007, 109, 2264–2265. [Google Scholar] [CrossRef]
- Rodríguez-Arbolí, E.; Martínez-Cuadrón, D.; Rodríguez-Veiga, R.; Carrillo-Cruz, E.; Gil-Cortés, C.; Serrano-López, J.; del Castillo, T.B.; Martínez-Sánchez, M.d.P.; Rodríguez-Medina, C.; Vidriales, B.; et al. Long-Term Outcomes After Autologous Versus Allogeneic Stem Cell Transplantation in Molecularly-Stratified Patients With Intermediate Cytogenetic Risk Acute Myeloid Leukemia: A PETHEMA Study. Transplant. Cell. Ther. 2021, 27, 311.e1–311.e10. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Vidriales, M.B.; López-Berges, C.; Díaz-Mediavilla, J.; Gutiérrez, N.; Cañizo, C.; Ramos, F.; Calmuntia, M.J.; Pérez, J.J.; González, M.; et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood 2001, 98, 1746–1751. [Google Scholar] [CrossRef]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010, 116, 354–365. [Google Scholar] [CrossRef]
- Lausen, B.; Schumacher, M. Maximally Selected Rank Statistics. Biometrics 1992, 48, 73. [Google Scholar] [CrossRef]
- Kiyoi, H.; Ohno, R.; Ueda, R.; Saito, H.; Naoe, T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene 2002, 21, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.E.; Hills, R.; Kottaridis, P.D.; Srirangan, S.; Wheatley, K.; Burnett, A.K.; Linch, D.C. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): An analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood 2005, 106, 3658–3665. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Döhner, K.; Krauter, J.; Fröhling, S.; Corbacioglu, A.; Bullinger, L.; Habdank, M.; Späth, D.; Morgan, M.; Benner, A.; et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N. Engl. J. Med. 2008, 358, 1909–1918. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Yamaguchi, H.; Najima, Y.; Usuki, K.; Ueki, T.; Oh, I.; Mori, S.; Kawata, E.; Uoshima, N.; Kobayashi, Y.; et al. Prognostic impact of low allelic ratio FLT3-ITD and NPM1 mutation in acute myeloid leukemia. Blood Adv. 2018, 2, 2744–2754. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Utuama, O.; Mukhtar, F.; Pham, Y.T.H.; Dabo, B.; Manani, P.; Moser, J.; Michael-Asalu, A.; Tran, C.T.; Le, L.C.; Le, T.V.; et al. Racial/ethnic, age and sex disparities in leukemia survival among adults in the United States during 1973-2014 period. PLoS ONE 2019, 14, e0220864. [Google Scholar] [CrossRef]
- Stone, R.M.; Larson, R.A.; Döhner, H. Midostaurin in FLT3-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2017, 377, 1903. [Google Scholar] [CrossRef]
- Rücker, F.G.; Du, L.; Luck, T.J.; Benner, A.; Krzykalla, J.; Gathmann, I.; Voso, M.T.; Amadori, S.; Prior, T.W.; Brandwein, J.M.; et al. Molecular landscape and prognostic impact of FLT3-ITD insertion site in acute myeloid leukemia: RATIFY study results. Leukemia 2021, 36, 90–99. [Google Scholar] [CrossRef]
| Variable | FLT3 DIT NO DETECTED | FLT3 RATIO < 0.25 | FLT3 RATIO 0.251–0.50 | FLT3 RATIO 0.51–0.80 | FLT3 RATIO > 0.80 | Significance |
|---|---|---|---|---|---|---|
| Age at diagnosis | ||||||
| Mean | 53.6 | 53.1 | 53.8 | 53.11 | 52.9 | p = NS |
| Range | 12.3–81.2 | 19.9–76.8 | 17.7–75.6 | 17.1–77.6 | 13.8–81 | |
| Sexo | ||||||
| Female | 1043 | 36 | 44 | 56 | 90 | p = 0.008 |
| Male | 1278 | 39 | 38 | 48 | 67 | |
| ECOG | ||||||
| 0 | 833 | 26 | 37 | 41 | 35 | p = 0.006 |
| 1 | 838 | 29 | 25 | 26 | 70 | |
| 2 | 240 | 11 | 7 | 18 | 22 | |
| 3 | 49 | 1 | 4 | 5 | 6 | |
| 4 | 14 | 0 | 0 | 1 | 2 | |
| WBC count (×1000/mL) | ||||||
| Mean | 28.28 | 48.86 | 49.46 | 92.5 | 101.06 | p < 0.001 |
| Range | 0.6–340 | 0.7–365 | 0.31–298 | 1.7–347 | 1.4–434 | |
| Cytogenetic Risk | ||||||
| Low Risk | 228 | 2 | 3 | 1 | 0 | p < 0.001 |
| Intermediate Risk | 1314 | 59 | 61 | 76 | 130 | |
| High Risk | 535 | 5 | 7 | 9 | 10 | |
| NPM1 mutation | ||||||
| Presence | 509 | 44 | 45 | 63 | 102 | p < 0.001 |
| Absence | 1592 | 29 | 33 | 34 | 49 | |
| OUTCOME | FLT3 DIT NO DETECTED | FLT3 RATIO < 0.25 | FLT3 RATIO 0.251–0.50 | FLT3 RATIO 0.51–0.80 | FLT3 RATIO > 0.80 | Significance |
| Complete Remission, % (n) | 71 (1539) | 69 (52) | 75 (61) | 55 (57) | 63 (99) | p = 0.057 |
| Resistance Disease, % (n) | 22 (240) | 20 (15) | 15 (12) | 29 (29) | 27 (43) | p = NS |
| Early Death, % (n) | 7 (64) | 11 (8) | 10 (8) | 16 (17) | 10 (15) | p = NS |
| Relapse Disease, % (n) | 35 (801) | 27 (20) | 37 (30) | 27 (28) | 40 (63) | p = NS |
| OS (median, CI), months | 20.4 (18.0–22.7) | 18.6 (9.8–27.5) | 14.8 (12.8–16.8) | 13.5 (7.8–19.3) | 11.0 (8.9–13.5) | p < 0.001 |
| RFS (median, CI), months | 34.1 (22.3–45.9) | 32.7 (13.1–52.2) | 22.9 (0–46.3) | 90.7 (5.3–176) | 32.4 (24–40.7) | p < 0.001 |
| Variable | Responders (n = 1901) | NO Responders (n = 996) | Significance | ||
|---|---|---|---|---|---|
| ncases | % of responders | ncases | % of responders | ||
| Sexo | |||||
| Female | 928 | 48.82 | 425 | 42.67 | p = 0.002 |
| Male | 973 | 51.18 | 571 | 57.33 | |
| Edad | |||||
| Mean (SD) | 52.49 (13.98) | 55.68 (14.36) | p < 0.001 | ||
| WBC count (×1000/mL) | |||||
| Mean (SD) | 35.82 (57.07) | 42.89 (63.01) | p = 0.004 | ||
| Cytogenetic Risk | |||||
| Low Risk | 208 | 12.21 | 30 | 3.48 | p < 0.001 |
| Intermediate Risk | 1226 | 71.95 | 522 | 60.56 | |
| High Risk | 270 | 15.85 | 310 | 35.96 | |
| NPM1 mutation | |||||
| Presence | 634 | 36.88 | 192 | 21.38 | p < 0.001 |
| Absence | 1085 | 63.12 | 706 | 78.62 | |
| FLT3 ITD mutation | |||||
| Presence | 368 | 19.33 | 211 | 21.16 | p = NS |
| Absence | 1536 | 80.67 | 786 | 78.84 | |
| FLT3 ITD ratio levels | |||||
| No mutation | 1539 | 85.26 | 785 | 83.69 | p = 0.057 |
| <0.25 | 49 | 2.71 | 26 | 2.77 | |
| 0.25-0.50 | 61 | 3.38 | 21 | 2.24 | |
| 0.501-0.80 | 57 | 3.16 | 47 | 5.01 | |
| >0.80 | 99 | 5.48 | 59 | 6.29 | |
| FLT3 ITD ratio > 0.5 | |||||
| Presence | 164 | 9.09 | 109 | 11.62 | p = 0.035 |
| Absence | 1641 | 90.91 | 829 | 88.38 | |
| FLT3 ITD ratio > 0.8 | |||||
| Presence | 99 | 5.48 | 59 | 6.29 | p = NS |
| Absence | 1706 | 94.52 | 879 | 93.71 | |
| Variable | OR | Significance | Lower CI | Upper CI |
|---|---|---|---|---|
| Age | 0.980 | p < 0.001 | 0.973 | 0.987 |
| WBC (×1000/mL) | 0.996 | p < 0.001 | 0.994 | 0.998 |
| Cytogenetic risk | ||||
| Low risk vs. intermediate risk | 0.341 | p < 0.001 | 0.222 | 0.523 |
| Low risk vs. High risk | 0.145 | p < 0.001 | 0.093 | 0.226 |
| NPM1 mutation | ||||
| Absence vs. presence | 2.865 | p < 0.001 | 2.235 | 3.674 |
| Ratio FLT3–ITD > 0.5 | ||||
| Absence vs. presence | 0.617 | p = 0.005 | 0.441 | 0.862 |
| A. Factors associated with death. Cox multivariate for OS | ||||
| Variable | HR | Significance | Lower CI | Upper CI |
| Gender (male vs. female) | 0.860 | p = 0.007 | 0.772 | 0.960 |
| Age (continuous variable) | 1.020 | p < 0.001 | 1.015 | 1.024 |
| WBC (×1000/mL) (continuous variable) | 1.002 | p < 0.001 | 1.001 | 1.003 |
| Cytogenetic risk | ||||
| Low risk vs. intermediate risk | 1.596 | p < 0.001 | 1.264 | 2.016 |
| Low risk vs. high risk | 3.267 | p < 0.001 | 2.558 | 4.172 |
| FLT3–ITD ratio levels | ||||
| Neg. vs. <0.25 | 1.404 | p = NS | 0.983 | 2.005 |
| Neg. vs. 0.25–0.50 | 1.190 | p = NS | 0.866 | 1.634 |
| Neg. vs. 0.51–0.80 | 1.475 | p = 0.009 | 1.104 | 1.972 |
| Neg. vs. >0.80 | 1.644 | p < 0.001 | 1.305 | 2.072 |
| Consolidation (no transplant; autotransplant; allogeneic transplant) | ||||
| No transplant vs. autotransplant | 0.372 | p < 0.001 | 0.311 | 0.445 |
| No transplant vs. allogeneic transplant | 0.321 | p < 0.001 | 0.273 | 0.377 |
| B. Factors associated with relapse. Cox multivariate for RFS. | ||||
| Variable | HR | Significance | Lower CI | Upper CI |
| WBC (×1000/mL) (continuous variable) | 1.001 | p = 0.038 | 1.000 | 1.003 |
| Cytogenetic risk | ||||
| Low risk vs. intermediate risk | 1.740 | p < 0.001 | 1.331 | 2.275 |
| Low risk vs. high risk | 2.847 | p < 0.001 | 2.118 | 3.826 |
| FLT3–ITD ratio levels | ||||
| Neg. vs. <0.25 | 1.143 | p = NS | 0.713 | 1.833 |
| Neg. vs. 0.25–0.50 | 1.366 | p = NS | 0.921 | 2.027 |
| Neg. vs. 0.501–0.80 | 0.969 | p = NS | 0.628 | 1.495 |
| Neg. vs. >0.80 | 2.104 | p < 0.001 | 1.562 | 2.833 |
| Consolidation (no transplant; autotransplant; allogeneic transplant) | ||||
| No transplant vs. autotransplant | 0.589 | p < 0.001 | 0.491 | 0.706 |
| No transplant vs. allogeneic transplant | 0.291 | p < 0.001 | 0.239 | 0.354 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, R.; Carreño-Tarragona, G.; Barragán, E.; Boluda, B.; Larráyoz, M.J.; Chillón, M.C.; Carrillo-Cruz, E.; Bilbao, C.; Sánchez-García, J.; Bernal, T.; et al. Impact of FLT3–ITD Mutation Status and Its Ratio in a Cohort of 2901 Patients Undergoing Upfront Intensive Chemotherapy: A PETHEMA Registry Study. Cancers 2022, 14, 5799. https://doi.org/10.3390/cancers14235799
Ayala R, Carreño-Tarragona G, Barragán E, Boluda B, Larráyoz MJ, Chillón MC, Carrillo-Cruz E, Bilbao C, Sánchez-García J, Bernal T, et al. Impact of FLT3–ITD Mutation Status and Its Ratio in a Cohort of 2901 Patients Undergoing Upfront Intensive Chemotherapy: A PETHEMA Registry Study. Cancers. 2022; 14(23):5799. https://doi.org/10.3390/cancers14235799
Chicago/Turabian StyleAyala, Rosa, Gonzalo Carreño-Tarragona, Eva Barragán, Blanca Boluda, María J. Larráyoz, María Carmen Chillón, Estrella Carrillo-Cruz, Cristina Bilbao, Joaquín Sánchez-García, Teresa Bernal, and et al. 2022. "Impact of FLT3–ITD Mutation Status and Its Ratio in a Cohort of 2901 Patients Undergoing Upfront Intensive Chemotherapy: A PETHEMA Registry Study" Cancers 14, no. 23: 5799. https://doi.org/10.3390/cancers14235799
APA StyleAyala, R., Carreño-Tarragona, G., Barragán, E., Boluda, B., Larráyoz, M. J., Chillón, M. C., Carrillo-Cruz, E., Bilbao, C., Sánchez-García, J., Bernal, T., Martinez-Cuadron, D., Gil, C., Serrano, J., Rodriguez-Medina, C., Bergua, J., Pérez-Simón, J. A., Calbacho, M., Alonso-Domínguez, J. M., Labrador, J., ... Montesinos, P. (2022). Impact of FLT3–ITD Mutation Status and Its Ratio in a Cohort of 2901 Patients Undergoing Upfront Intensive Chemotherapy: A PETHEMA Registry Study. Cancers, 14(23), 5799. https://doi.org/10.3390/cancers14235799









