Simple Summary
Macrophages exert a dual role in tumor progression. Macrophage-centered immunotherapies, including immune checkpoints (ICs) stimulation and inhibition, have demonstrated promising clinical perspectives. However, due to immune or metastatic heterogeneity, different cancer patients respond differently to these immunotherapies. Therefore, it is necessary for researchers to further discover and understand novel ICs on macrophages in the tumor microenvironment to design personalized immunomodulatory therapies to overcome therapy resistance. Based on this, we more comprehensively summarize a large number of IC and other receptor-ligands between macrophages and tumor cells, as well as their biology and role in anti-cancer immunity.
Abstract
Immunotherapy, especially immune checkpoint blocking, has become the primary anti-tumor treatment in recent years. However, the current immune checkpoint inhibitor (ICI) therapy is far from satisfactory. Macrophages are a key component of anti-tumor immunity as they are a common immune cell subset in tumor tissues and act as a link between innate and adaptive immunity. Hence, understanding the regulation of macrophage activation in tumor tissues by receptor-ligand interaction will provide promising macrophage-targeting strategies to complement current adaptive immunity-based immunotherapy and traditional anti-tumor treatment. This review aims to offer a systematic summary of the current advances in number, structure, expression, biological function, and interplay of immune checkpoint and other receptor-ligand between macrophages and tumor cells.
1. Introduction
Cancer cells evade immune surveillance to avoid recognition and elimination by the immune system. A significant mechanism is through the immune checkpoint (IC) pathways. Immune checkpoints are comprised of stimulatory and inhibitory receptors that bind to their natural ligands and can control the type, intensity, and period of the immune response to maintain physiological homeostasis. However, cancer cells can dampen the immune response through over-expressing inhibitory immune checkpoint molecules [1]. Based on this immune evasion mechanism, immunotherapy, which activates stimulatory immune checkpoints or blocks inhibitory immune checkpoints to boost anti-tumor immune responses, serves as a supplement to tumor therapy after surgery, radiotherapy, and chemotherapy [2].
In the beginning, researchers paid more attention to investigating the adaptive immune system, especially T cells, such as Programmed Cell Death 1/Programmed Cell Death Ligand 1 (PD-1/PD-L1), Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4) [3]. To date, 16 different immunomodulators have been approved by the FDA for more than 12 types of cancer [4] (Table 1). In addition, clinical concerns have been identified that only a minority of patients would benefit from IC therapy [5], most possibly due to immune or metastatic heterogeneity [6]. Thus, to identify and target novel immune checkpoint receptor-ligand pathways with better response rates, other components of the tumor microenvironment (TME) have been further investigated.

Table 1.
Immunomodulators that were approved by FDA.
Tumor-associated macrophages (TAMs), a crucial component of anti-tumor innate immunity, are among the most abundant population in TME [7]. The capability of macrophages is significant for bridging innate and adaptive immunity, such as phagocytosis of pathogens and damaged cells, presentation of antigens, and secretion of cytokines [8]. TAMs are classified into two major subpopulations: classically activated macrophages (M1) and alternatively activated macrophages (M2). In contrast to M1, which have anti-tumor effects, M2 are involved to some extent in tumor progression and metastasis [9] (Figure 1). Several immune checkpoints have been shown to be dysregulated in TAMs and promote cancer evasion. For example, CD47–signal regulatory protein α (SIRPα) axis has been proven critical for the tumor to escape from macrophage-mediated phagocytosis [10]. Additional IC may be identified and therapeutically targeted to provide effective and individualized immunomodulatory therapies to eradicate tumor cells. In this review, we will provide a comprehensive summary of the current advances in the number, structure, expression, biological function, and interaction of immune checkpoint and other receptor-ligand between macrophages and tumor cells, which may lead to the development of new approaches to combating macrophage-associated therapeutic resistance.
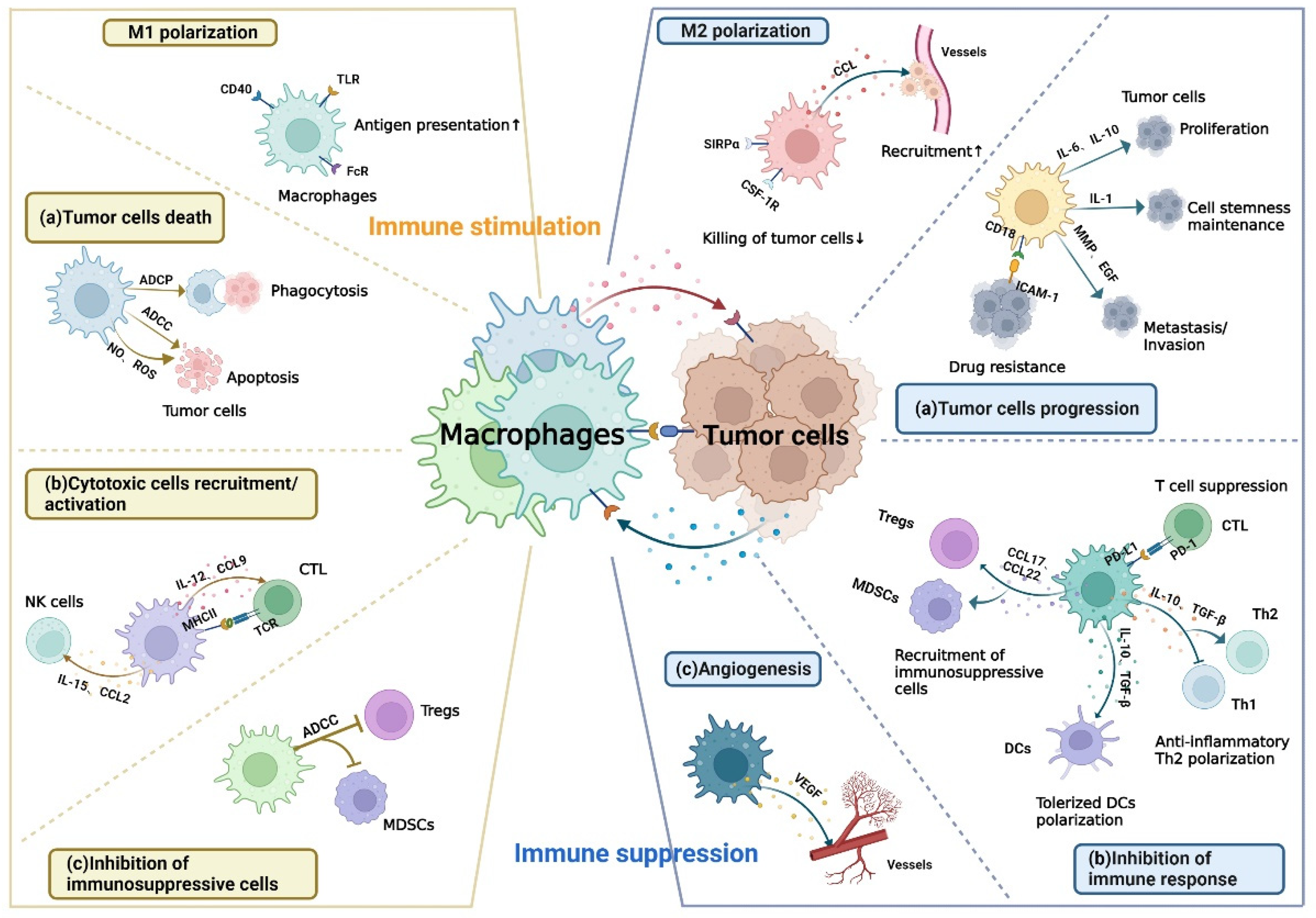
Figure 1.
The dual role of macrophages in TME. Left: immune stimulation. Signals from tumor cells can induce M1 polarization with the increase in antigen-presenting capacity. M1 macrophages exert anti-tumor effects in three ways include (a) phagocytosing tumor cells and triggering apoptosis of them; (b) secreting cytokines to recruit and activate CTL or NK cells and presenting tumor antigens to CTL; (c) inhibiting immunosuppressive cells, including Tregs and MDSCs. Right: immune suppression. Signals from tumor cells can also induce M2 polarization with the decrease in the killing capacity of tumor cells. M2 macrophages can produce chemokines to recruit macrophages to TME and contribute to tumor immune escape in three ways include (a) promoting tumor proliferation, metastasis, invasion as well as drug resistance and maintaining tumor cell stemness; (b) inhibiting pro-inflammatory response by suppressing CTL, inducing the polarization of anti-inflammatory Th2 and tolerized DCs, and recruiting immunosuppressive cells; (c) enhancing angiogenesis to promote tumor progression. ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; CCL, C–C motif chemokine ligand; CTL, cytotoxic T lymphocytes; CSF, colony-stimulating factor; DCs, dendritic cells; EGF, epidermal growth factor; FcR, Fc receptor; ICAM, intercellular adhesion molecule; IL, interleukins; MDSCs, myeloid-derived suppressor cells; MHC, major histocompatibility complex; MMP, matrix metalloproteinase; NO, nitric oxide; NK, natural killer; PD, programmed cell death; ROS, reactive oxygen species; SIRPα, signal regulatory protein α; TGF, transforming growth factor; Th, helper T cell; TLR, toll-like receptor; Tregs, regulatory T cells; VEGF, vascular endothelial growth factor.
2. Immune Checkpoint Receptor-Ligand Interaction Dependent on Cell-Cell Contact
Macrophages have a dual role in TME. On the one hand, as innate immunocytes, macrophages recognize malignant cells and clear them by phagocytosis, recruitment and activation of other immune cells, and secretion of pro-inflammation cytokines (Figure 2). On the other hand, researchers have found that macrophages can also contribute to cancer progression and therapeutic resistance (Figure 3). In this section, we will discuss the ligand-receptor pairs that are dependent on tumor-macrophages contacts.
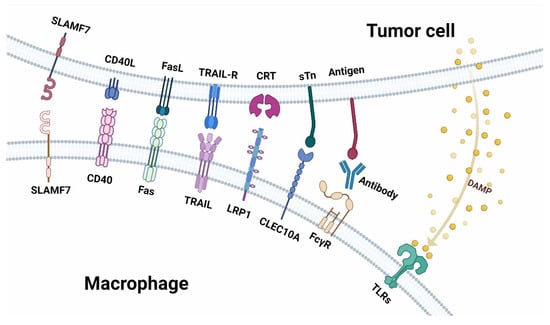
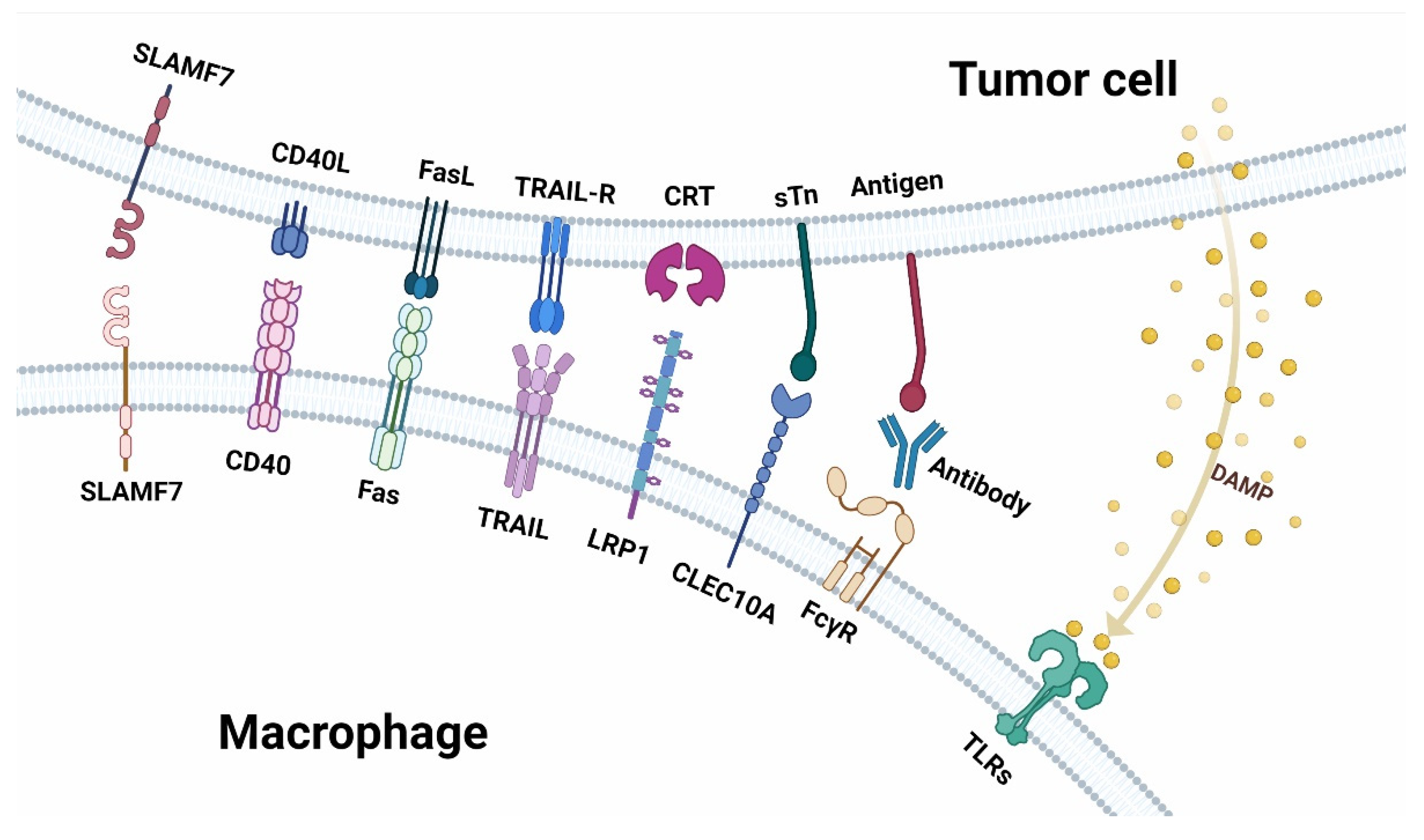
Figure 2.
Receptor-ligand interaction between macrophages and tumor cells in anti-tumor immunity. Top: the ligands expressed on tumor cells and the molecules secreted by tumor cells. Below: are the receptors expressed on macrophages.
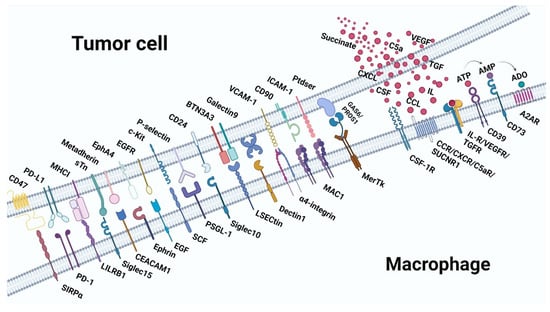
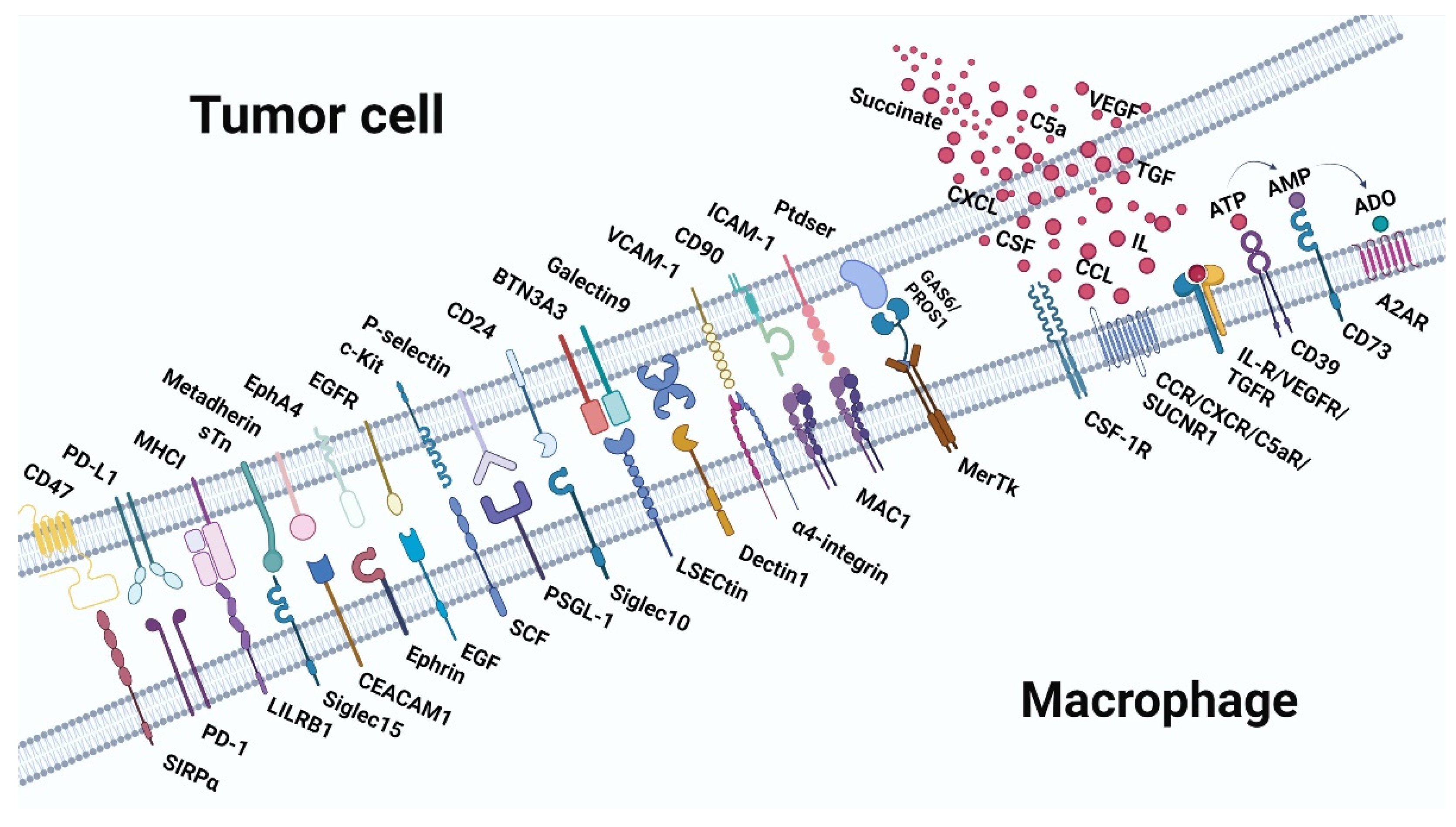
Figure 3.
Receptor-ligand interaction between macrophages and tumor cells in pro-tumor immunity. Top: the ligands expressed on tumor cells and the molecules secreted by tumor cells. Below: are the receptors expressed on macrophages. A2AR, adenosine 2A receptor; ADO, adenosine; AMP, adenosine monophosphate; ATP, adenosine triphosphate.
2.1. Rreceptor-Ligand Interaction in Anti-Tumor Immunity
2.1.1. FcγR-Antibody-Antigen Axis
Fcγ receptor (FcγR) belongs to a family of cell surface receptors that bind to the Fc domain of immunoglobulins and is expressed in various hematopoietic cells, including macrophages, B cells, and monocytes. FcγR consists of six members (FcγRI, FcγRIIA, FcγRIIB, FcγRIIC, FcγRIIIA, and FcγRIIIB) [11], of which most are phagocytosis-activating receptors with the immunoreceptor tyrosine-based activation motif (ITAM), except FcγRIIB, which is the only phagocytosis-inhibitory receptor with the immunoreceptor tyrosine-based inhibitory motif (ITIM) [12]. FcγRs can identify antigens in tumors through binding to antibodies and play a key role in killing or phagocytosis of tumor cells via antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP), respectively [13]. Interestingly, although M2 are traditionally considered pro-tumor, it may exert stronger phagocytosis than M1 to play an anti-tumor role mediated by antibodies such as rituximab in the lymphoma microenvironment [14,15].
When bound to antibodies, the FcγR domain on macrophages activates downstream signals. Accordingly, the tyrosine residues in the ITAM will be phosphorylated by SRC family kinases, which results in the recruitment and activation of the spleen tyrosine kinase (SYK) family, which transduces downstream signals that eventually lead to actin cytoskeleton rearrangement, the formation of phagocytic synapses between macrophages and tumor cells, and phagocytosis of target cells [12]. Furthermore, monoclonal antibody-based strategies have shown prominent effects in eliminating cancer cells, such as acute lymphocytic leukemia [16], ovarian cancer [17], and breast cancer [18]. In addition, FcγR-deficient mice that lacked the FcγRI and FcγRIII exhibited weaker functions of macrophages in arresting and engulfing cancer cells. On the other hand, attenuated binding of antibodies to FcγRIIB demonstrated elevated phagocytosis of cancer cells in lymphoma and leukemia mouse models [16]. Moreover, Morrissey et al. designed a family of chimeric antigen receptors with CAR structures for phagocytosis (CAR-P), which can trigger enhanced engulfment of macrophages to tumor cells [19].
2.1.2. CD40-CD40L
CD40 (also known as Bp50 and TNFRSF5), a cell surface type I costimulatory glycoprotein, contains an extracellular domain, a cytoplasmic tail, and a transmembrane domain belonging to the tumor necrosis factor (TNF) family. CD40 is ubiquitously expressed in hematopoietic and nonhematopoietic cells, especially B cells and myeloid cell lineage [20]. CD40L (also known as gp39, CD154, and TNFSF5), identified as the natural ligand of CD40, is a 50-kDa type II membrane glycoprotein that is also broadly expressed in various cells [20], including tumor cells [21]. The CD40/CD40L axis actives not only the RAFs and NF-κB signaling but also the Janus kinase 3 (JAK3) and signal transducer and activator of transcription (STAT) pathway. It is involved in the maturation, survival, and cytokine production of antigen-presenting cells (APCs) to lead to the activation of T cells [22].
CD40 agonists have been proven effective in stimulating TAMs to degrade fibrosis required for tumors and elicit effective T-cell responses, thereby forming a pro-inflammatory environment [23]. In contrast to agonist therapy, increasing the CD40/CD40L interaction between tumor cells and macrophages is also a promising treatment for cancer. For example, Eriksson et al. demonstrated that after the transfer of trimerized membrane-bound extracellular CD40L (TMZ-CD40L) in pancreatic cancer cells, the TMZ-CD40L-expressing tumor cells could enhance the infiltration of T cells and M1 macrophages in tumors, ultimately inducing anti-tumor immunity [24]. A similar effect has been verified in murine lung cancer [25] and bladder cancer models [26].
2.1.3. Fas-FasL
Fas (also known as CD95 or APO-1) is a 45-kDa type I transmembrane protein that belongs to the TNF receptor superfamily. Widely present in cells or tissues, Fas is made up of a transmembrane domain, an intracellular death domain (DD), and an N-terminal domain containing three cysteine-rich domains (CRDs) [27]. FasL, the ligand of Fas, exists in two types. The membrane-bound FasL is a 40-kDa type II transmembrane protein with a TNF homology domain (THD) that binds to Fas, whereas the soluble FasL (sFasL) is a 26-kDa protein fragment lacking the transmembrane and cytosolic domains [28]. Compared to Fas, FsaL is mainly expressed in lymphocytes, macrophages, and NK cells [29].
Mechanistically, upon binding of FasL to Fas, the adapter molecule Fas-associated death domain (FADD) is recruited to aggregation, and then FADD interacts with procaspase-8 by the death effector domain (DED) so as to form the death-inducing signaling complex (DISC). DISC initiates the activation of the caspase cascade that contributes to DNA fragmentation and cell-programmed death [29]. However, some Fas+ tumor cells can escape FasL-mediated apoptosis through downregulating Fas expression while increasing the expression of FasL or by serving as a novel Fas-resistant subpopulation due to immune-based selective pressure [30]. Thus, the role of this crosstalk in TME deserves further exploration.
2.1.4. TRAIL-TRAIL-R
TNF-related apoptosis-inducing ligand (TRAIL, also known as TNFSF10 and APO2L) is a typical member of the TNF family, a 33 kDa acid type II transmembrane protein. Furthermore, the cysteine proteases can cleave the extracellular domain of TRAIL to produce a 20 kDa soluble TRAIL. TRAIL is expressed in various immune cells, including monocytes, DCs, and macrophages [31]. Its ligand, TRAIL-R, can be classified into two groups: the full-length intracellular DD-containing receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5), the alternative receptors TRAIL-R3 (DCR1), TRAIL-R4 (DCR2) and osteoprotegerin (OPG), both of which are distributed in various tissues [32]. The TRAIL-TRAIL-R axis is involved in spermatogenesis, the pathogenesis of pulmonary arterial hypertension (PAH), and tumor immune surveillance [31].
Upon TRAIL binding, the TRAIL-R manipulates apoptosis through a death-inducing signaling pathway, similar to Fas/FasL axis [32]. Moreover, targeting the TRAIL-TRAIL-R axis in TME has been confirmed to exert a profound effect on inhibiting tumor progression. For instance, TRAIL-R agonist-induced collapse of vascular integrity and apoptosis in tumors in a mouse fibrosarcoma model [33]. Although many TRAIL-based agents have already reached the clinical stage, further studies are needed to overcome TRAIL resistance in cancer therapy [32].
2.1.5. SLAMF7-SLAMF7 Axis
Signaling lymphocytic activation molecule receptor family 7 (SLAMF7, also known as CRACC, CS1, CD319) is a member of the SLAM family. It is a single-pass transmembrane protein with an extracellular tyrosine-rich Ig-like domain and cytoplasmic tail, which carries an immune receptor tyrosine-based switch motif (ITSM) [34] and is usually expressed on hematopoietic cells [35]. SLAMF7 can mediate the development and function of lymphocytes cells and APCs, the generation of memory cells, the lytic viability of NK cells, the production of cytokines, the inhibition of MHC-independent cells, and the aggregation of platelet [36].
Significantly, as a homotypic receptor, SLAMF7 on macrophages can bind to SLAMF7 on cancer cells and act synergistically with macrophage-1 (MAC1) antigen, which is known to interact with two ITAM-containing receptors, FcRγ and DAP12 [37], and then induce cytoskeletal reorganization to phagocytosis tumor cells through SRC kinase, SYK, and BTK, rather than enhancing adhesion to targets cells [38]. Additionally, SLAMF7 knockout macrophages were defective in CD47 blockade-induced phagocytosis of mouse hematopoietic tumor cell lines [38]. This finding demonstrated that SLAM family receptors are required for tumor phagocytosis during the blockade of the SIRPα-CD47 axis. However, this view has been contradicted lately by Yuan et al., who presented that SLAMF7 expression on cancer cells is not required for phagocytosis [39]. Therefore, additional research is required to determine if SLAMF7 specifically interacts with the effects of CD47-targeting hematopoietic treatment.
2.1.6. LRP1-CRT
Low-density lipoprotein receptor-related protein-1 (LRP1, also known as CD91 or α2M) is a 600-kDa transmembrane protein composed of a 515-kDa heavy (α) subunit and an 85-kDa light (β) subunit, the latter containing two YXXL motifs and two NPXY motifs, belonging to multifunctional LDL receptor (LDLR) gene family [40]. It is widely expressed on various cell surfaces, including smooth muscle cells [41], endothelial cells [42], and macrophages [43], and is involved in a variety of biological processes, such as inflammation, tissue remodeling, and clearance of extracellular molecules [44]. Calreticulin (CRT) acts as a recognition ligand of LRP expressed on the engulfing cell, which was discovered by Shyra J et al. in 2005 [45]. CRT is a member of the endoplasmic reticulum lectin chaperone protein family with a relative molecular mass of 46-kDa. It is highly species-conserved and mainly located in the endoplasmic reticulum (ER), which then binds to newly synthesized proteins to mediate their folding and glycosylation [46]. CRT is not only found on the surface of apoptotic cells but also in living cells. For example, the expression of CRT on cancer cells can mediate the uptake by APCs [47].
Increased expression of CRT on tumor cell membranes has been reported to bind to LRP1 on macrophages and initiate phagocytosis and immune response in target cells by triggering antigen presentation from APCs to T cells [45]. Furthermore, because CRT is also present in normal cells, the existence of the SIRPα-CD47 axis prevents the inadvertent uptake of phagocytes by these cells and, in turn, the anti-CD47 antibody-induced phagocytosis requires the interaction of target cell CRT with LRP on phagocytic cells [45]. Indeed, blockade or knockdown of CRT had comparable effects in suppressing the anti-CD47 antibody-mediated phagocytosis of cancer cells and abolishing their immunogenicity in mice [48].
2.1.7. CLEC10A-sTn Axis
C-type lectin domain family 10 member A (CLEC10A, also known as CD301, MGL) was first identified back in 1996 [49]. It is a type II transmembrane receptor that is a member of the C-type lectin family and uses a common carbohydrate recognition domain to recognize glycan compounds in a Ca2+-dependent manner [50]. It is mainly expressed in intermediate monocytes, DCs, and macrophages [51]. Interestingly, CLEC10A expression is significantly lower in the majority of tumors than in normal tissues, which is linked to poor prognosis and cancer progression [52]. CLEC10A plays an active part in many biological processes, such as adaptive and innate immune responses [50]. Currently, several researchers have focused on the ability of CLEC10A to promote the anti-tumor activity of immune cells, and it has been reported that CLEC10A plays an indispensable role in increasing antigen-specific CD8+ T cell activation [53]. Beyond this, as a well-known epitope associated with human cancer, sialyl-Tn (sTn) Ag has been identified by C-type lectin expressed on macrophages as early as 16 years ago [49].
Naghmeh’s team verified transmembrane CLEC10A expressed on macrophages or dendritic cells interacts well with tumor-associated sTn (Neu5Acα2,6-Tn and Neu5Gcα2,6-Tn) in the micromolar range [54]. Unfortunately, the precise mechanism and implications of this interaction need to be clarified.
2.2. Rreceptor-Ligand Interaction in Pro-Tumor Immunity
2.2.1. SIRPα-CD47 Axis
Signal regulatory protein alpha (SIRPα) is the first member of the SIRP family, which was identified in the 1990s, has an extracellular immunoglobulin-like (Ig-like) region and a cytoplasmic ITIM [55] and is mainly expressed on myeloid cells, including macrophages [56]. It is, therefore, an inhibitory receptor that has the ability to suppress intracellular signaling. The extracellular domain of CD47, which also contains five transmembrane domains and COOH-terminal splice variant tails [57], was initially discovered as a SIRP ligand in 1999 [58]. CD47 was initially discovered in ovarian cancer cells [59] but expressed in all cells and overexpressed in cancer cells as a “self-marker,” delivering an inhibitory “don’t eat me” signal by engaging in SIRPα, thereby avoiding phagocytosis by macrophages [60]. In addition, CD47 can participate in regulating cellular activities, including cell-cell fusion, cytokine production, migration, and activation [61,62].
When CD47 binds to SIRPα on the surface of macrophages, it leads to SIRPα cellular ITIM phosphorylation, which then recruits Src homology 2 (SH2) domain-containing protein tyrosine phosphatases 1 or 2 (SHP1/2) and activates them [63]. SHP1/2 dephosphorylates and inhibits myosin IIA, resulting in disruption of cytoskeleton rearrangement and phagocytosis activity, which in turn maintains homeostasis in vivo [64]. However, this pathway has also been reported to inhibit phagocytic synapses between macrophages and tumor cells in almost all tumor types, thereby promoting tumor immune escape [65]. Nowadays, substantial evidence supports that the checkpoint inhibitors of SIRPα-CD47 can suppress tumor growth by skewing tumor-associated macrophages toward the M1 phenotype from the M2 phenotype, which restores the phagocytic activities of macrophages [66]. Anyway, disruption of the SIRPα-CD47 axis can also activate adaptive immune responses, including amplifying the presentation of tumor-derived antigens to CD8+ T cell [67], or enhancing NK cell-modulated ADCC and complement-dependent cytotoxicity (CDC), thus alleviating suppression of the innate immune system [68]. However, because of its binding to CD47 on the surface of red blood cells (RBC), anti-CD47 treatment can lead to phagocytosis of RBC by macrophages and non-specific hemagglutination, which interferes with RBC antibody screening [69].
2.2.2. PD1-PD-L1 Axis
Programmed cell death protein 1 (PD-1, also known as CD279) belongs to the B7 family and is a 55-kDa transmembrane protein first identified in 1992. It contains an extracellular IgV-like domain and a cytoplasmic tail with an ITIM domain [70]. PD-1 is constitutively expressed in immune cells, such as activated T cells, dendritic cells (DCs), and macrophages [71]. Moreover, it serves as an inhibitory receptor to control immune response. It has been shown that the level of PD-1+ TAMs increased during tumor progression in murine models, but the other subset of PD-1– TAMs populations, which exhibit stronger phagocytic activity, decreased in this process [72]. Its ligand-programmed cell death protein ligand 1 (PD-L1, also known as CD274) is distributed not only on tumor cells and immune cells but also on other cells, such as vascular endothelial cells and pancreatic islet cells [73]. Moreover, in the 2000s, this checkpoint ligand was considered an important protein for immune evasion [74].
Mechanistically, when engaged with PD-L1, the ITIM domain of PD-1 expressed on macrophages will be activated and phosphorylated, which then mediates the activation of downstream pathways, such as SHP1 and SHP2, ultimately promoting anti-phagocytosis [72]. Nevertheless, the precise mechanism of the PD-1-PD-L1 axis in TAMs is poorly understood. Mediavilla et al. found that disruption of this axis leads to M1 polarization and activation of CD8+ T cells [72]. Additionally, PD-1 blockade increases tumor infiltration by anti-tumor macrophages while downregulating the number of pro-tumor macrophages, which then induces phagocyte-mediated anti-tumor immunity [75].
2.2.3. LILRB1-MHC-I Axis
As a member of the leukocyte immunoglobulin-like receptor family, Leukocyte immunoglobulin-like receptor B1 (LILRB1) is distributed on the majority of immune cells [76] and osteoclasts [77], while paired immunoglobulin-like receptor B (PIR-B) is the only mouse receptor orthologous to the human LILRB family [78]. Both of them contain extracellular Ig-like regions and an intracellular ITIM motif that recruit SHP1 and SHP2, thus transducing an inhibitory signal [79]. Major histocompatibility complex I (MHCI) was considered the primary ligand of LILRB1 until 2003 [80], consisting of a heavy α-chain and β2-microglobulin (β2M). Moreover, it is species-specific, expressed on nucleated cells, and presents antigens to T cells [81].
In 2018, Barkal and his colleagues found that direct binding of LILRB1 to the β2M subunit of cancer cell MHC-I inhibits phagocytosis and disrupts immune surveillance by transducing intracellular inhibitory signals in TAMs [82]. However, interference with the MHC-I-LILRB1 axis through antibody blockade or genetic manipulation can drive tumor cells to be engulfed both in vitro and in vivo. The PIR-B-deficient macrophages showed an M1-like signature and increased proinflammatory cytokine release [83]. Furthermore, it is worth noting that MHC-I has the capacity to present specific peptides of tumor cells to T cells. Thus, it is optimized to target the β2-M or inhibit LILRB1 rather than disturb the binding of MHC-I-T cells to avoid affecting the cytotoxicity of CD8+T cells.
2.2.4. MerTK-PROS1/GAS6-PtdSer Axis
MerTK is a component of the Tyro3-Axl-MerTK (TAM) family of receptor tyrosine kinases (RTKs) and contains two extracellular fibronectin type III (FNIII), two Ig-like domains, as well as a conserved kinase domain that characterized by the unusual KWIAIES sequence [84]. It is widely expressed or co-expressed by various cells and is involved in many physiological processes, such as tissue repair, clearance of apoptotic material, innate immune control, and platelet aggregation [85]. The two cognate molecules, protein S (PROS1) and growth arrest-specific protein 6 (GAS6), were identified as the ligands of TAM in 1995 [86]. PROS1 and GAS6 are produced by tumor cells and nonneoplastic cells in TME, which function as bridging molecules that are the physical link between MerTK and Phosphatidylserine (PtdSer) [87]. PtdSer, a kind of glycerophospholipid, is expressed on the inner leaflet of the phospholipid bilayer of normal cells and flips to the outer leaflet of the membrane on the apoptotic cells, providing an “eat me” signal to phagocytes [88].
MERTK on TAMs activated and started downstream signaling cascades after indirectly binding to PtdSer on dying tumor cells. This action led to the rearrangement of the actin cytoskeleton and the phagocytosis of macrophages, which strengthened the clearance of apoptotic tumor cells [89]. However, studies have shown that this interaction could induce macrophage polarization to M2 anti-inflammatory phenotype, secreting tissue repair-promoting cytokines, including IL-10 and TGF-β, ultimately facilitating immune escape [89]. The intervention of the MerTK-PROS1/GAS6-PtdSer axis may be a potent candidate for developing anti-tumor therapy. A study on murine MC38 colon cancer found that MerTK blockade induced an accumulation of apoptotic cells in cancers, the production of local type I IFN, and an increase in tumor immunogenicity, thus indicating the potential of cancer immunotherapy [90].
2.2.5. Siglec15-sTn Axis
Sialic Acid-binding Immunoglobulin-like Lectin 15 (Siglec15), a member of the Siglec family of glycan-recognition proteins, is a vertebrate cell-surface receptor that can recognize sialylated glycans. Siglec15 is composed of two Ig-like domains, a transmembrane domain containing a lysine residue and a short intracellular tail. Moreover, it is linked to the signal adaptor molecule DNAX activation protein of 12 kDa (DAP12), which has an ITAM that activates immune cells [91]. The expression of Siglec15 was found to be restricted to myeloid cells, including macrophages and DCs in the spleen and lymph nodes [92]. In addition, it has been demonstrated that Siglec15 preferentially recognizes and binds to the sialyl-Tn antigen (sTn). sTn is a tumoral short O-glycan structure abnormally expressed in various tumors and associated with tumor metastasis [93].
Takamiya et al. first indicated that Siglec15 on TAMs can recognize sTn antigen on tumor cells and then transduces a signal to SYK through binding determinant Lys274 of DAP12 and enhance TGF-β secretion, which gradually facilitates tumor growth and metastasis. Additionally, THP-1 cells treated with SYK inhibitor or substitution of the Siglec-15 Lys274 to Ala suppress TGF-β secretion due to blocked interaction between Siglec15 and DAP12 [94].
2.2.6. Siglec10-CD24 Axis
Sialic acid-binding immunoglobulin-like lectin 10 (Siglec10) is described as a member of the Ig-like lectin family, which can bind sialic acid and contains an intracellular ITIM domain [95]. In the meanwhile, it modulates cell-cell contacts and transmits inhibitory signals after being distributed to several immune cells, including macrophages and dendritic cells [96]. Sialoglycoprotein CD24 (also known as heat-stable antigen or small-cell lung cancer cluster-4 antigen) has been reported to connect with Siglec10 so as to mediate innate immunity [97]. CD24 is a highly glycosylated cell-surface protein and can be detected in various cell types, including cancer cells [98], exerting an inhibitory effect in autoimmune diseases, noxious inflammation, and cancer biology [99,100,101].
A single-cell RNA sequencing data has revealed that CD24 was overexpressed on a broad spectrum of tumors, and plenty of TAMs expressed a high level of Siglec10 [102]. Recently, many groups have discovered the anti-phagocytic signal of the Siglec10-CD24 axis in triple-negative breast cancer (TNBC) [102], mantle cell lymphoma (MCL) [103], and ovarian cancer [104]. The effect of Siglec10/CD24 takes place in a similar signaling pathway to PD1/PDL1 [105]. Furthermore, CD24 mAb treatment or CD24 deletion in tumor cell lines or mice can improve tumor cell phagocytosis, control tumor development, and lengthen life in vitro or in vivo by recovering the macrophages’ capacity [102].
2.2.7. Ephrin-EphA4 Axis
Ephrin and its membrane-binding ligand Erythropoietin-producing hepatocyte kinase (Eph) are the largest receptor tyrosine kinases (RTKs) family. Ephrin and Eph both can be divided into two subclasses: EphrinA, EphrinB, and EphA, EphB, with an N-terminal extracellular molecular binding region, a transmembrane domain, and an intracellular kinase domain, the later one comprises of a juxta membrane (JM) domain, a tyrosine kinase domain (KD), sterile alpha motif (SAM) and PDZ binding motif (PDZBM) [106]. Their capacity to create signals with the property of bidirectional transduction, i.e., mediating both forward and backward signal cascades, was discovered to be expressed typically on a wide variety of cells [107]. This critical cell communication system plays widespread roles in cytoskeleton formation, cell adhesion, and movement, affecting neuronal development and skeletal stability [108].
Lu et al. demonstrated that EphA4 on CSCs can interact with TAMs by directly binding to Ephrin, subsequently stimulating Src and NF-κB and inducing a broad spectrum of cytokines secretion to sustain the stem cell state of the tumor [109]. However, it is worth noting that the role of the Ephrin-EphA4 axis in macrophages needs further study.
2.2.8. EGF-EGFR Axis
Epidermal growth factor receptor (EGFR, also known as ErbB) is a membrane surface protein with tyrosine kinase activity, which contains an extracellular ligand-binding region, a single transmembrane region consisting of two repeated cysteine-rich regions, and an intracellular sequence containing tyrosine protein kinases and self-phosphorylation sites [110]. It is widely expressed in human epidermal and stromal cells but is highly expressed in various human cancer cells [111]. Epidermal growth factor (EGF), one of the seven ligands of EGFR, only binds to EGFR, whereas other EGFR ligands can bind to diverse members of the EGFR family [112]. The EGF-EGFR axis plays a crucial part in the physiological processes of cell growth, proliferation, and differentiation via RAS/RAF/ErK, PI3K/AKT, or Ral/c-Src/STAT pathway [113,114,115].
EGF-EGFR interaction was once thought to constitute a paracrine loop. However, it has been reported that EGFR can be activated through cell-cell interaction with its ligands [116,117]. Recently, Sevgi et al. used a multidisciplinary approach to investigate the interaction between BCC and macrophages and confirmed that this interaction is a juxtacrine loop. Moreover, cell-to-cell contact was required for EGF to stimulate EGFR on BCC [118]. Which fundamental mechanism controls this relationship, though, is still a matter of debate.
2.2.9. SCF- c-Kit Axis
Stem cell factor receptor (c-Kit, also known as CD117), a member of the class III RTKs family, contains an extracellular (EC) ligand-binding domain, a single transmembrane (TM) region, and a juxtamembrane (JM) region [119]. C-Kit is a well-known proto-oncogene, and the encoded protein is expressed in several human tumor histotypes, including breast cancer [120], lung cancer [121], glioma [122], and leukemia [123]. The Stem Cell Factor (SCF), the counter receptor of c-Kit, is a type II homodimer of two four-helix bundles containing two distinct isoforms: soluble SCF (‘sSCF) and membrane-bound SCF (mSCF) [124].
SCF can stimulate c-Kit to mediate many cellular processes, such as proliferation, adhesion, differentiation, survival, and migration [125], via activating multiple downstream signaling pathways, such as Ras/Erk, JAK/STAT, and PI3K [126]. A recent study discovered that c-Kit, which is produced by ovarian CSC, interacts with cell-anchored, soluble SCF, which is expressed by myeloid cells such as macrophages or non-myeloid infiltrating cells, to promote the formation of tumors. Furthermore, this research also demonstrated SCF/c-Kit is a complex juxtacrine/paracrine circuit that is involved in enhancing ovarian CSC stemness properties. In vitro, the tyrosine kinase inhibitor imatinib can dampen the effect of activating the Akt pathway in c-Kit+ cells [127].
2.2.10. CEACAM1-Metadherin Axis
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) belongs to the immunoglobulin superfamily, consisting of an extracellular domain, a transmembrane domain, and a cytoplasmic signaling domain with either a long (L) ITIM-containing domain or short (S) domain lack of ITIMs [128]. CEACAM1 is distributed on a variety of myeloid cells and lymphocytes. Furthermore, it plays a vital role in cell-to-cell adhesion, cell signaling pathway, and other complex biological processes such as inflammatory response and tumor progression [129,130]. A pluripotent oncogene known as Metadherin (MTDH, also known as AEG-1), which has been implicated in driving cancer metastasis, can directly interact with CEACAM1 [131]. Metadherin has also been discovered in other parts of cells, including the nucleus and endoplasmic reticulum [132].
More recently, Sally et al. identified a cell surface metadherin/CEACAM1-CCL3 positive feedback loop that can increase the formation of polyploid tumor cells and limit the efficacy of therapies in an isogenic tumor cell pair model of highly metastatic (HM) and non-metastatic (NM) tumor cells that resemble the spontaneous human ovarian cancer metastasis model. In addition, Metadherin on cancer cell surface communicated with CEACAM1 on macrophages to secrete C–C motif chemokine ligand 3 (CCL3), which promotes various tumor types of metastasis [131]. Since CEACAM1 has been reported could inhibit cell proliferation, and cytotoxicity of T cells and NK cells in melanoma, a new anti-CEACAM1 antibody (MRG1) was used to bind to the N domain of CEACAM1, restoring the susceptibility of melanoma cells to attacked by T cells [133].
2.2.11. P-Selectin-PSGL-1 Axis
P-selectin sometimes referred to as SELP, is a cysteine-rich glycoprotein with a molecular mass of roughly 140,000 and is one of the essential members of the selectin family. It is expressed on the surface of activated platelets and endothelial cells [134]. Meanwhile, PSGL-1 is one of the ligands of P-selectin, ~120 kDa, with a homodimer structure linked by disulfide bonds, which is expressed chiefly on white cells, rarely on platelets, and strongly upregulated in M2-polarizing macrophages [135,136].
P-selectin interacts with PSGL-1 on macrophages, then accelerates leukocyte recruitment and migration to damaged endothelial cells. Moreover, it can mediate the mutual effect of leukocytes, endothelial cells, and platelets [137,138]. However, multiple myeloma (MM) gene expression profile data revealed that all investigated MM cell lines and primary MM cells showed PSGL-1, while all macrophages exhibited P-selectin. Meanwhile, P-selectin/PSGL-1 axis played a crucial part in macrophage-mediated MM drug resistance [139]. Therapeutically, PSGL-1 mAb can repolarize M2 macrophages to the M1-like phenotype and induce an inflammatory microenvironment, thus enhancing anti-tumor activity in myeloma cells and humanized mouse models [137,140].
2.2.12. LSECtin-BTN3A3 Axis
Liver and lymph node sinusoidal endothelial cells lectin (LSECtin) is a transmembrane protein with a CRD that binds to extracellular ligands and belongs to the C-type lectin receptor family [95]. It was initially cloned by Liu et al. in 2004 and mainly expressed in hepatic sinusoidal endothelial cells, Kupffer cells, peripheral DCs, and macrophages [141]. LSECtin can combine with the Ebola virus, SARS-CoV, fibrillar virus, and hepatitis C virus [142,143,144]. Additionally, it can negatively regulate hepatic T-cell immunity [145]. Liu et al. also first identified BTN3A3 as the receptor for LSECtin [146]. BTN3A3 is one of the B7-related butyrophilins (BTN) family members, contains extracellular IgV and IgC domains, a transmembrane domain, and an intracellular B30.2 domain that show some structural features of the B7 family as well as distributed in diverse types of tumor cells [147]. The single-nucleotide polymorphisms (SNPs) in BTN3A3 demonstrated that it is related to the increase in susceptibility to ovarian [148].
According to research, BTN3A3 on TAMs might directly connect with LSECtin on BCC, which would afterward cause STAT3 to become phosphorylated and promote the expression of genes responsible for stemness. Based on this result, either macrophage-specific ablation of LSECtin or silencing of BTN3A3 in mice bearing human tumor xenografts can block the LSECtin-BTN3A3 axis, decreasing CSC frequency and tumor growth ultimately [146].
2.2.13. Dectin1-Galectin9 Axis
Dendritic cell-associated C-type lectin1 (Dectin1) is a type II membrane protein with a molecular mass of 28 kDa that is encoded by CLEC7A, containing an ITAM on the intracellular tail. It is a member of the pattern-recognition receptors (PRR) C-type lectin family and is mostly expressed on myeloid cells [149]. Furthermore, it acts as a transmembrane signal receptor to mediate various functions of cells, including binding to eubacteria, inducing cytokine, and producing chemokine [150]. Subsequently, Galectin9 has been found to ligate Dectin1 in pancreatic ductal adenocarcinoma (PDA) [151]. Galectin9 belongs to the galactoside–binding family of lectins containing two domains, namely the N-terminal sugar recognition domain (N-CRD) and C-terminal sugar recognition region (C-CRD) and is expressed by lymphocytes and other cells [152]. Galectin9 plays a decisive role in physiological and pathological processes, such as disrupting the anti-cancer activity of cytotoxic lymphocytes [153] and activating anti-microbial innate immune responses in response to T cell suppression [154].
Especially, Galectin9 can activate Dectin1 on macrophages to promote pancreatic carcinoma and peritumoral immune tolerance, and the Dectin1–Galectin9 axis plays a decisive role in the education of T cells toward immunogenic or tolerogenic phenotypes in PDA. The immune-suppressive character of PDA-infiltrating T cells is reversed by Dectin1 blockade, which also results in anti-tumor immunity against PDA [151].
2.2.14. α4-Integrin-VCAM-1 Axis
Integrin is a glycoproteins family that forms heterodimeric receptors for ECM molecules and includes one α subunit and one β subunit [155]. Moreover, it is distributed on many cell types of the hematopoietic lineage, such as T lymphocytes and macrophages, promoting adhesion to target cells [156]. Incidentally, α4-integrin belongs to this family. As a ligand of integrin and a member of the immunoglobulin superfamily, vascular cell adhesion molecule-1 (VCAM-1, also known as CD106) was first discovered in 1989 [157]. VCAM-1 predominantly binds to α4β1-integrin (also known as VLA-4) but only binds weakly to α4β7-integrin [158]. VCAM-1 is involved in numerous pathophysiological processes, including immunological disorders [159] and cardiovascular diseases [160]. However, several studies have also discovered aberrant VCAM-1 expression in various malignancies, including gastric, breast, and melanomas [161,162,163].
The binding of α4-integrin and VCAM-1 plays a major part in regulating leukocyte adhesion and transendothelial migration during inflammation [164]. VCAM-1-expressing tumor cells attract, ligate and activate α4-integrin-expressing osteoclast precursor cells to establish bone metastasis sites in breast cancer with bone metastasis. Furthermore, previous research also reported that VCAM-1 tethers metastasis-associated macrophages (MAMs) to cancer cells via α4 integrin and transduces a survival signal, which can protect cancer cells from apoptosis [165]. Meanwhile, this protumor function of VCAM-1 can be eliminated by the α4-integrin blockade and delay cancer bone metastasis [165,166].
2.2.15. MAC1-CD90 Axis
Macrophage Antigen-1 (MAC1) is a heterodimeric complement receptor consisting of CD11b (α subunit) and CD18 (β subunit) integrins, which is expressed on different leukocyte subsets, including macrophages [167]. MAC1 is a key in triggering phagocytosis of complement fragment C3bi-opsonized particles [168], such as pathogens and apoptotic cells, in addition to promoting myeloid cell adhesion and migration [37,169]. CD90 (also known as Thy-1) is a glycosyl phosphatidyl inositol (GPI)-anchored cell surface protein with a molecular weight of 25~37 kDa, belonging to the IgSF [170]. Additionally, CD90 has been revealed to be a novel ligand for MAC1 by Wetzel and his colleagues [171]. CD90 is now known to be expressed in both cancer cells and normal tissue cells, such as endothelial cells, according to a recent report [172]. More importantly, CD90 can mediate cell adhesion and serves as a marker of cancer stem cells (CSC) [173].
Along with these, it has been shown that MAC1/CD90 can mediate the interaction between breast cancer stem cells (BCSCs) and TAMs in a contact-dependent way. However, CD90 provides the physical connection required for other cell-surface receptors, such as Ephrin/EphA4 juxtacrine signaling, since it lacks an intracellular domain [109]. Some studies revealed that knockdown or interference of CD90 by shRNA or miR-125a/b in TAMs, respectively, will result in suppression of cell proliferation and stem cell properties, the twofold to a threefold reduction in cytokine mRNA, finally avoiding tumor development [109,174].
2.2.16. MAC1-ICAM-1 Axis
Intercellular adhesion molecule-1 (ICAM-1, also known as CD54) is a 90 kDa single-strand glycoprotein that belongs to the IgSF [175]. It is mainly distributed on the surface of endothelial cells, monocytes, lymphocytes, and cancer cells facilitating not just cell-cell adhesion and recognition but also the migration of immune cells such as leukocytes [176]. ICAM-1, thus, plays a crucial part in physiological processes such as cell signal transmission and activation, cell tissue development and differentiation, immunological response [177,178], and pathological processes, for example, tumor metastasis [179].
The previous study has shown that ICAM-1 is another ligand of MAC-1. According to the earlier study, MAC-1 on neutrophils binds to ICAM-1-expressing cells to mediate stable adhesion [180]. Additionally, it has been reported that ICAM-1 on TAMs could bind CD18 on Myeloma cells to regulate drug resistance [139], and this contact-dependent interaction tended to expand M2 infiltration and release cells from tumor cell aggregates [181]. The ICAM-1/CD18 axis in TME can form a positive autocrine feedback loop in ovarian cancer models, thereby promoting tumor cell proliferation, migration, spheroid formation, and peritoneal implantation. Subsequently, antibody blockade of ICAM-1 in macrophages blunted spheroid formation and ovarian cancer progression in these models [182].
3. Immune Checkpoint Receptor-Ligand Interaction Independent of Cell-Cell Contact
In addition to cell-cell contact, tumor cells can release plenty of molecules with associated receptors on macrophages (Figure 1 and Figure 2), such as CSF-1, Chemokines, and IL-10, resulting in the reprogramming of macrophages.
3.1. TLRs
Toll-like receptors (TLRs) are the best-characterized PRRs that control innate immunity. TLRs on macrophages are capable of detecting a wide range of molecules, including damage-associated molecular patterns (DAMP) generated from tumors, viruses, lipopolysaccharide (LPS), and bacterial nucleic acids, resulting in the activation of multiple signaling. These activated signal pathways can skew macrophages toward the M1-like phenotype, increase the secretion of proinflammation cytokines such as TNF-α and IFN-α, and upregulate the recruitment and activation of T cells to limit tumor progression [183]. Thus, targeting TLRs of TAMs may be a promising anti-tumor therapy. The majority of investigations, which use TLR agonists for facilitating immune responses against tumors, have focused on TLR3 [184], TLR4 [185], TLR7/8 [186], and TLR9 [187]. However, tumor-associated DAMP can also bind to TLRs expressed on macrophages to promote chemoresistance and metastasis [188]. Therefore, more research must be conducted to identify the precise contact mechanism between tumor cells and TAMs.
3.2. CSF-1R
Colony-stimulating factor-1 receptor (CSF-1R), restrictedly expressed on macrophages and frontier monocytes, is a major growth and differentiation factor belonging to the tyrosine kinase family. The CSF-1/CSF-1R axis is essential for monocyte recruitment, survival, and polarization [189]. In order to maintain an immunosuppressive environment, CSF-1 (also known as M-CSF) may be abundantly secreted from a variety of tumors and regulate the function of monocytes [190]. CSF-1R inhibitors can reverse the chemoresistance of breast cancer cell lines, increase the antigen presentation capacity of macrophages to enhance T cell responses, and reduce M2-like macrophage infiltration [191,192]. Numerous CSF-1R inhibitors and antibodies have been created and evaluated in clinical settings [193].
3.3. Chemokine Receptor
The chemokine receptor and ligand crosstalk between TAMs and tumor cells, such as C-C motif chemokine receptor 2 (CCR2)/C-C motif chemokine ligand 2 (CCL2), CCR5/CCL5, C-X-C motif chemokine 1(CXCR1)/CXCR2/CXCL8 (also known as IL-8), CCR4/CXCL12 (also known as SDF-1), CCR6/CCL20 (also known as MIP-3α), plays a crucial role in recruiting monocytes to infiltrate tumors, promoting macrophage polarization toward the M2-like phenotype and protecting tumor cells from chemotherapy-induced apoptosis [194,195]. Thus, it may be a promising anti-tumor strategy to target the chemokine receptor/ligand axis. Additionally, it has been demonstrated in several tumor types that blocking this macrophage signaling using inhibitors or antibodies might prevent the recruitment of M2-like TAMs, increase the functionality of tumor-infiltrating CD8+ T cells, and promote the response to immunotherapy [196].
3.4. Interleukin Receptor
The interaction between tumor-derived interleukins (ILs) and macrophages develop a network that regulates the efficacy of immunotherapy against cancer [197]. The two members of the tumor-derived IL family that have received the most research attention are IL-1 and IL-10, both of which are crucial in the development of tumors. As an immunosuppressive cytokine, IL-1 can bind to IL-1R on macrophages and recruit the signaling adaptor MyD88 that activates NF-κB, which maintains the expression of immunosuppressive genes in macrophages and the significant promotion of tumors in mouse melanoma models [198]. In colorectal cancer, IL-10 generated a similar immunosuppressive impact through the CaKMII-ERK1/2-STAT3 pathway [199]. Clinical study has shown that antibody treatments that target IL-1 or IL-10 are successful therapeutically [200].
3.5. ATP/Adenosine Receptor
The ectonucleotidases CD39 and CD73 are expressed in various immune cells and non-immune cells, including macrophages and tumor cells. Extracellular ATP (eATP), which is produced by stressed or dying tumor cells, may be bounded by CD39 in TME, which then dephosphorylates eATP to AMP. Sequentially, CD73 converts AMP to adenosine, which dampens the effect on the innate and adaptive immune response via activating adenosine receptors (ARs) expressed on immune cells [201,202]. Additionally, multiple groups have found an increase in anti-tumor responses in CD39-deficient mice [203], CD73-deficient mice [204], and AR-deficient mice [205] experiments, making this axis become a potential therapeutic target in cancer immunotherapy.
3.6. C5aR
C5a, a complement anaphylatoxin, is released from various cells, including tumors and myeloid cells, functioning through binding to its receptors C5aR on myeloid innate immune cells, such as macrophages, DCs, and neutrophils [206]. Traditionally, C5a has been considered a proinflammatory factor and is involved in multiple ways to sustain the inflammatory response. For example, C5a can activate macrophages to release proinflammatory molecules and trigger an oxidative burst [206]. The function of C5a in TME has been found to be dose-dependent, however. In turn, the elevated levels of C5a prevent complement activation and are linked to tumor immune escape [207]. A study on murine breast cancer has revealed that C5aR–mediated alveolar macrophages could inhibit Th1 cells but increase Th2 cells with lower tumoricidal activity, which facilitates lung metastatic burden [208]. Therapeutically, a combined PD-1/C5a blockade significantly reduces tumor growth and metastasis in lung cancer [209]. C3a has also emerged as a potential tumor growth mediator, while research is lacking [206].
3.7. VEGFR
Vascular endothelial growth factor (VEGF), one member of the growth factors family, can bind to VEGFR expressed on earliest hematopoietic progenitors and played a crucial role in the TME [210]. For instance, the VEGF/VEGFR axis may prevent immune cells from differentiating and functioning properly, hence promoting the metastasis of neoplasms [211]. Nevertheless, although known TAMs can be attracted by VEGF secreted from tumors into the tumor site, the precise mechanism of the VEGF/VEGFR axis between tumors and TAMs is unclear [210]. In preclinical glioblastoma models, it has been demonstrated that simultaneous suppression of angiopoietin-2 and VEGFR increases survival time via diverse mechanisms, including the normalization of vascular function, reduction in tumor burden, and modification of macrophage phenotype [212].
3.8. TGF-βR
Transforming growth factor β (TGF-β) is an immunosuppressive cytokine secreted by tumor cells and immune cells, which binds to TGF-R on a variety of cells and has a dual function in TME. TGF-β slows the metastasis of tumors in the first stages of their development. However, excessive TGF-β will prevent effector T cells from maturing and from producing cytokines [213]. Furthermore, it has been demonstrated that TGFβ derived from the tumor may skew macrophage to an M2-like phenotype, aiding in the development of an immunosuppressive TME [214]. By now, TGF-βR1 inhibitors have shown safety and tolerability in the clinical trial of advanced hepatocellular carcinoma [215].
3.9. SUCNR1
Succinate receptor (SUCNR1) is one kind of the G protein-coupled receptor (GPCR), highly distributed in mouse tissues, such as the kidney, liver, and spleen. After being stimulated by succinate, SUCNR1 may increase inositol trisphosphate (IP3) levels, calcium mobilization, extracellular signal-regulated kinase (ERK) activation, and the generation of nitric oxide (NO) and prostaglandin E2 (PGE2) in the human embryonic kidney (HEK) 293 cells [216]. Recently, Wu et al. have found that tumor-derived succinate could trigger SUCNR1-PI3K-hypoxia-inducible factor 1 alpha (HIF-1 alpha) axis to polarize macrophages into anti-inflammatory phenotype, which accelerates tumor progression in vitro and in vivo studies [217]. Targeting the succinate/SUCNR1 axis between tumor cells and TAMs may be a prospective anti-tumor method in the future.
4. Conclusions
This review summarizes the immune checkpoint and other receptor-ligand interaction between macrophages and tumor cells, which plays an indispensable role in anti-tumor immunity or pro-tumor immunity, such as SIRPα-CD47, PD1-PD-L1, FcγR-Antibody-Antigen, CSF-1/CSF-1R axis. In TME, tumor cells can suppress the growth, survival, antigen-presenting, and phagocytic ability of macrophages by governing multiple inhibitory and stimulatory signals. Therefore, the discovery and target of immune checkpoints are essential to restore macrophage anti-tumor functions. Meanwhile, this TAMs-targeted approach seems promising from a clinical perspective (Table 2). For example, some SIRPα-CD47 targeted clinical trials have entered phase III and manifested great effects in the treatment of cancer patients.

Table 2.
Partial clinical trials of TAM-targeted immunotherapeutic strategies.
However, some problems remain unsolved: (1) Very little is known about the downstream crossover signal transmission pathway of IC on TAMs or tumor cells, although there has been a lot of bioinformatic analysis of IC expression profiles [218,219]. (2) Lack of insight into the regulatory network between TAMs and other immune cells under immunosuppressive conditions. (3) Due to immune or metastatic heterogeneity, different cancer types respond differently to the same immune checkpoint inhibitor (ICI). (4) Only a minority of macrophages-targeted drugs were approved by FDA (Table 3).

Table 3.
Macrophages-targeted antibodies that were approved by FDA.
In conclusion, macrophage-oriented therapy is a potential new approach to fighting cancer without a shadow of a doubt. In addition, it is necessary to identify novel checkpoint receptor-ligand, elucidate the interaction mode between macrophages and tumor cells as well as other components in TME, and conduct more relevant clinical studies to design new strategies and combination regimens with improved specificity profiles to overcome resistance and eradicate cancer.
Author Contributions
Writing—original draft preparation, Y.Y.; figures and tables designed, Y.Y.; writing—review and editing, P.L.; supervision, W.Z. and P.L.; funding acquisition, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC) (871386269, to PL) and startup funding from Tongji Hospital (to PL).
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Cancer Immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Agrawal, M.Y.; Kaushik, I.; Ramachandran, S.; Srivastava, S.K. Immune checkpoint proteins: Signaling mechanisms and molecular interactions in cancer immunotherapy. Semin. Cancer Biol. 2022, 86, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Vandsemb, E.N.; Herbst, R.S.; Chen, L. Adaptive immune resistance at the tumour site: Mechanisms and therapeutic opportunities. Nat. Rev. Drug Discov. 2022, 21, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Qin, L.; Liao, Z.; Song, J.; Yuan, C.; Liu, Y.; Wang, Y.; Xu, H.; Zhang, Q.; Pei, Y.; et al. Microenvironment characterization and multi-omics signatures related to prognosis and immunotherapy response of hepatocellular carcinoma. Exp. Hematol. Oncol. 2020, 9, 34. [Google Scholar] [CrossRef]
- Lopez-Yrigoyen, M.; Cassetta, L.; Pollard, J.W. Macrophage targeting in cancer. Ann. N. Y. Acad. Sci. 2020, 1499, 18–41. [Google Scholar] [CrossRef]
- Molgora, M.; Colonna, M. Turning enemies into allies—Reprogramming tumor-associated macrophages for cancer therapy. Med 2021, 2, 666–681. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, J.; Di Lu, D.; Xu, X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 75. [Google Scholar] [CrossRef]
- Feng, M.; Jiang, W.; Kim, B.Y.S.; Zhang, C.C.; Fu, Y.-X.; Weissman, I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 568–586. [Google Scholar] [CrossRef]
- Yu, J.; Song, Y.; Tian, W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J. Hematol. Oncol. 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Bakema, J.E.; Van Egmond, M. Fc receptor-dependent mechanisms of monoclonal antibody therapy of cancer. Curr. Top. Microbiol. Immunol. 2014, 382, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Leidi, M.; Gotti, E.; Bologna, L.; Miranda, E.; Rimoldi, M.; Sica, A.; Roncalli, M.; Palumbo, G.A.; Introna, M.; Golay, J. M2 Macrophages Phagocytose Rituximab-Opsonized Leukemic Targets More Efficiently than M1 Cells In Vitro. J. Immunol. 2009, 182, 4415–4422. [Google Scholar] [CrossRef]
- Taskinen, M.; Karjalainen-Lindsberg, M.-L.; Nyman, H.; Eerola, L.-M.; Leppä, S. A High Tumor-Associated Macrophage Content Predicts Favorable Outcome in Follicular Lymphoma Patients Treated with Rituximab and Cyclophosphamide-Doxorubicin-Vincristine-Prednisone. Clin. Cancer Res. 2007, 13, 5784–5789. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Jan, M.; Weissman-Tsukamoto, R.; Zhao, F.; Park, C.Y.; Weissman, I.L.; Majeti, R. Therapeutic Antibody Targeting of CD47 Eliminates Human Acute Lymphoblastic Leukemia. Cancer Res. 2010, 71, 1374–1384. [Google Scholar] [CrossRef]
- Liu, R.; Wei, H.; Gao, P.; Yu, H.; Wang, K.; Fu, Z.; Ju, B.; Zhao, M.; Dong, S.; Li, Z.; et al. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget 2017, 8, 39021–39032. [Google Scholar] [CrossRef]
- Cheung, A.; Opzoomer, J.; Ilieva, K.M.; Gazinska, P.; Hoffmann, R.M.; Mirza, H.; Marlow, R.; Francesch-Domenech, E.; Fittall, M.; Rodriguez, D.D.; et al. Anti-Folate Receptor Alpha–Directed Antibody Therapies Restrict the Growth of Triple-negative Breast Cancer. Clin. Cancer Res. 2018, 24, 5098–5111. [Google Scholar] [CrossRef]
- Morrissey, M.A.; Williamson, A.P.; Steinbach, A.M.; Roberts, E.W.; Kern, N.; Headley, M.B.; Vale, R.D. Chimeric antigen receptors that trigger phagocytosis. eLife 2018, 7, e36688. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef]
- Baxendale, A.J.; Dawson, C.W.; E Stewart, S.; Mudaliar, V.; Reynolds, G.; Gordon, J.; Murray, P.G.; Young, L.S.; Eliopoulos, A.G. Constitutive activation of the CD40 pathway promotes cell transformation and neoplastic growth. Oncogene 2005, 24, 7913–7923. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Cheng, X.; Truong, B.; Sun, L.; Yang, X.; Wang, H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol. Ther. 2020, 219, 107709. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef]
- Eriksson, E.; Moreno, R.; Milenova, I.; Liljenfeldt, L.; Dieterich, L.C.; Christiansson, L.; Karlsson, H.; Ullenhag, G.; Mangsbo, S.M.; Dimberg, A.; et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther. 2016, 24, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Imaizumi, K.; Kawabe, T.; Wakayama, H.; Horio, Y.; Sekido, Y.; Hara, T.; Hashimoto, N.; Takahashi, M.; Shimokata, K.; et al. Induction of antitumor immunity by transduction of CD40 ligand gene and interferon-γ gene into lung cancer. Cancer Gene Ther. 2001, 8, 421–429. [Google Scholar] [CrossRef][Green Version]
- Liljenfeldt, L.; Dieterich, L.C.; A Dimberg, A.; Mangsbo, S.M.; I Loskog, A.S. CD40L gene therapy tilts the myeloid cell profile and promotes infiltration of activated T lymphocytes. Cancer Gene Ther. 2014, 21, 95–102. [Google Scholar] [CrossRef]
- Oehm, A.; Behrmann, I.; Falk, W.; Pawlita, M.; Maier, G.; Klas, C.; Li-Weber, M.; Richards, S.; Dhein, J.; Trauth, B. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J. Biol. Chem. 1992, 267, 10709–10715. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, T.; Golstein, P.; Nagata, S. Molecular cloning and expression of the fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75, 1169–1178. [Google Scholar] [CrossRef]
- Villa-Morales, M.; Fernández-Piqueras, J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 85–101. [Google Scholar] [CrossRef]
- Scott, I.A. Positive and negative consequences of Fas/Fas ligand interactions in the antitumor response. Front. Biosci. 2005, 10, 809–821. [Google Scholar] [CrossRef]
- De Looff, M.; De Jong, S.; Kruyt, F.A.E. Multiple Interactions Between Cancer Cells and the Tumor Microenvironment Modulate TRAIL Signaling: Implications for TRAIL Receptor Targeted Therapy. Front. Immunol. 2019, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.S.; Yang, A.; Yang, B.; Couto, S.; Stern, H.; Gogineni, A.; Pitti, R.; Marsters, S.; Weimer, R.M.; Singh, M.; et al. Proapoptotic Activation of Death Receptor 5 on Tumor Endothelial Cells Disrupts the Vasculature and Reduces Tumor Growth. Cancer Cell 2012, 22, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, M.A.; Mor, A. The SLAM family receptors: Potential therapeutic targets for inflammatory and autoimmune diseases. Autoimmun. Rev. 2018, 17, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, P.L.; Mueller, K.L.; Qi, H.; Cannons, J.L. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat. Rev. Immunol. 2009, 9, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Claus, M.; Urlaub, D.; Fasbender, F.; Watzl, C. SLAM family receptors in natural killer cells–Mediators of adhesion, activation and inhibition via cis and trans interactions. Clin. Immunol. 2018, 204, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Abram, C.L.; Lowell, C.A. The Ins and Outs of Leukocyte Integrin Signaling. Annu. Rev. Immunol. 2009, 27, 339–362. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, M.-C.; Guo, H.; Davidson, D.; Mishel, S.; Lu, Y.; Rhee, I.; Pérez-Quintero, L.-A.; Zhang, S.; Cruz-Munoz, M.-E.; et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature 2017, 544, 493–497. [Google Scholar] [CrossRef]
- He, Y.; Bouwstra, R.; Wiersma, V.R.; De Jong, M.; Lourens, H.J.; Fehrmann, R.; De Bruyn, M.; Ammatuna, E.; Huls, G.; Van Meerten, T.; et al. Cancer cell-expressed SLAMF7 is not required for CD47-mediated phagocytosis. Nat. Commun. 2019, 10, 533. [Google Scholar] [CrossRef]
- Yang, T.; Williams, B.O. Low-Density Lipoprotein Receptor-Related Proteins in Skeletal Development and Disease. Physiol. Rev. 2017, 97, 1211–1228. [Google Scholar] [CrossRef]
- Calvier, L.; Boucher, P.; Herz, J.; Hansmann, G. LRP1 Deficiency in Vascular SMC Leads to Pulmonary Arterial Hypertension That Is Reversed by PPARγ Activation. Circ. Res. 2019, 124, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhao, Z.; Sagare, A.P.; Wu, Y.; Wang, M.; Owens, N.C.; Verghese, P.B.; Herz, J.; Holtzman, D.M.; Zlokovic, B.V. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol. Neurodegener. 2018, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.; Zhu, L.; Tavori, H.; Huynh, K.; Giunzioni, I.; Stafford, J.; Linton, M.F.; Fazio, S. Deletion of Macrophage Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1) Accelerates Atherosclerosis Regression and Increases C-C Chemokine Receptor Type 7 (CCR7) Expression in Plaque Macrophages. Circulation 2018, 138, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Wujak, L.; Schnieder, J.; Schaefer, L.; Wygrecka, M. LRP1: A chameleon receptor of lung inflammation and repair. Matrix Biol. 2018, 68-69, 366–381. [Google Scholar] [CrossRef]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.-A.; Michalak, M.; Henson, P.M. Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through trans-Activation of LRP on the Phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef]
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and cancer. Cell Res. 2021, 31, 5–16. [Google Scholar] [CrossRef]
- Schcolnik-Cabrera, A.; Oldak, B.; Juárez, M.; Cruz-Rivera, M.; Flisser, A.; Mendlovic, F. Calreticulin in phagocytosis and cancer: Opposite roles in immune response outcomes. Apoptosis 2019, 24, 245–255. [Google Scholar] [CrossRef]
- Chao, M.P.; Jaiswal, S.; Weissman-Tsukamoto, R.; Alizadeh, A.A.; Gentles, A.J.; Volkmer, J.; Weiskopf, K.; Willingham, S.B.; Raveh, T.; Park, C.Y.; et al. Calreticulin Is the Dominant Pro-Phagocytic Signal on Multiple Human Cancers and Is Counterbalanced by CD47. Sci. Transl. Med. 2010, 2, 63ra94. [Google Scholar] [CrossRef]
- Suzuki, N.; Yamamoto, K.; Toyoshima, S.; Osawa, T.; Irimura, T. Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. Its unique carbohydrate binding specificity for Tn antigen. J. Immunol. 1996, 156, 128–135. [Google Scholar]
- van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Sweet preferences of MGL: Carbohydrate specificity and function. Trends Immunol. 2008, 29, 83–90. [Google Scholar] [CrossRef] [PubMed]
- van Kooyk, Y.; Ilarregui, J.M.; van Vliet, S.J. Novel insights into the immunomodulatory role of the dendritic cell and macrophage-expressed C-type lectin MGL. Immunobiology 2015, 220, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Zhang, L.; Li, J.; Liao, L.; Huang, L.; Yang, J. Immunological role and prognostic potential of CLEC10A in pan-cancer. Am. J. Transl. Res. 2022, 14, 2844–2860. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, Y.; Lin, X.; Wang, H.; Yong, L.; Zhang, H.; Cai, F. CLEC10A can serve as a potential therapeutic target and its level correlates with immune infiltration in breast cancer. Oncol. Lett. 2022, 24, 285. [Google Scholar] [CrossRef] [PubMed]
- Mortezai, N.; Behnken, H.N.; Kurze, A.-K.; Ludewig, P.; Buck, F.; Meyer, B.; Wagener, C. Tumor-associated Neu5Ac-Tn and Neu5Gc-Tn antigens bind to C-type lectin CLEC10A (CD301, MGL). Glycobiology 2013, 23, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Chen, J. SIRPα–CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Adams, S.; Van Der Laan, L.J.; Vernon-Wilson, E.; De Lavalette, C.R.; A Döpp, E.; Dijkstra, C.D.; Simmons, D.L.; Berg, T.K.V.D. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 1998, 161, 1853–1859. [Google Scholar]
- Bian, H.-T.; Shen, Y.-W.; Zhou, Y.-D.; Nagle, D.G.; Guan, Y.-Y.; Zhang, W.-D.; Luan, X. CD47: Beyond an immune checkpoint in cancer treatment. Biochim. Biophys. Acta 2022, 1877, 188771. [Google Scholar] [CrossRef]
- Seiffert, M.; Cant, C.; Chen, Z.; Rappold, I.; Brugger, W.; Kanz, L.; Brown, E.J.; Ullrich, A.; Bühring, H.-J. Human Signal-Regulatory Protein Is Expressed on Normal, But Not on Subsets of Leukemic Myeloid Cells and Mediates Cellular Adhesion Involving Its Counterreceptor CD47. Blood 1999, 94, 3633–3643. [Google Scholar] [CrossRef]
- Campbell, I.G.; Freemont, P.S.; Foulkes, W.; Trowsdale, J. An Ovarian Tumor Marker with Homology to Vaccinia Virus Contains an IgV-like Region and Multiple Transmembrane Domains. Cancer Res. 1992, 52, 5416–5420. [Google Scholar]
- Russ, A.; Hua, A.B.; Montfort, W.R.; Rahman, B.; Bin Riaz, I.; Khalid, M.U.; Carew, J.S.; Nawrocki, S.T.; Persky, D.; Anwer, F. Blocking “don’t eat me” signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev. 2018, 32, 480–489. [Google Scholar] [CrossRef]
- Liu, Y.; Merlin, D.; Burst, S.L.; Pochet, M.; Madara, J.L.; Parkos, C.A. The Role of CD47 in Neutrophil Transmigration. J. Biol. Chem. 2001, 276, 40156–40166. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, M.I.; Lindberg, F.P.; Kersh, G.J.; Allen, P.M.; Brown, E.J. Costimulation of T Cell Activation by Integrin-associated Protein (CD47) Is an Adhesion-dependent, CD28-independent Signaling Pathway. J. Exp. Med. 1997, 185, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.N.; Brown, M.H. The SIRP family of receptors and immune regulation. Nat. Rev. Immunol. 2006, 6, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.K.; Discher, D.E. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 2008, 180, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Eladl, E.; Tremblay-LeMay, R.; Rastgoo, N.; Musani, R.; Chen, W.; Liu, A.; Chang, H. Role of CD47 in Hematological Malignancies. J. Hematol. Oncol. 2020, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Dougan, M.; Ingram, J.R.; Ho, C.C.M.; Kauke, M.J.; Almo, S.C.; Ploegh, H.L.; Garcia, K.C. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2646–E2654. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Agbor-Enoh, S.; Jang, M.K.; Alawi, M.; Saygi, C.; Gravina, A.; Tediashvili, G.; Nguyen, V.Q.; Liu, Y.; et al. The SIRPα–CD47 immune checkpoint in NK cells. J. Exp. Med. 2021, 218, e20200839. [Google Scholar] [CrossRef]
- Velliquette, R.W.; Aeschlimann, J.; Kirkegaard, J.; Shakarian, G.; Lomas-Francis, C.; Westhoff, C.M. Monoclonal anti-CD47 interference in red cell and platelet testing. Transfusion 2018, 59, 730–737. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2017, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Qi, Q.; Qian, X.; Han, J.; Zhu, X.; Zhang, Q.; Xia, R. The role of PD-1/PD-L1 axis and macrophage in the progression and treatment of cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Dhupkar, P.; Gordon, N.; Stewart, J.; Kleinerman, E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018, 7, 2654–2664. [Google Scholar] [CrossRef]
- Colonna, M.; Nakajima, H.; Navarro, F.; López-Botet, M. A novel family of Ig-like receptors for HLA class I molecules that modulate function of lymphoid and myeloid cells. J. Leukoc. Biol. 1999, 66, 375–381. [Google Scholar] [CrossRef]
- Mori, Y.; Tsuji, S.; Inui, M.; Sakamoto, Y.; Endo, S.; Ito, Y.; Fujimura, S.; Koga, T.; Nakamura, A.; Takayanagi, H.; et al. Inhibitory Immunoglobulin-Like Receptors LILRB and PIR-B Negatively Regulate Osteoclast Development. J. Immunol. 2008, 181, 4742–4751. [Google Scholar] [CrossRef]
- Munitz, A.; Cole, E.T.; Beichler, A.; Groschwitz, K.; Ahrens, R.; Steinbrecher, K.; Willson, T.; Han, X.; Denson, L.; Rothenberg, M.E.; et al. Paired Immunoglobulin-Like Receptor B (PIR-B) Negatively Regulates Macrophage Activation in Experimental Colitis. Gastroenterology 2010, 139, 530–541. [Google Scholar] [CrossRef]
- Cella, M.; Nakajima, H.; Facchetti, F.; Hoffmann, T.; Colonna, M. ILT Receptors at the Interface Between Lymphoid and Myeloid. Cells 2000, 251, 161–166. [Google Scholar] [CrossRef]
- E Willcox, B.; Thomas, L.M.; Bjorkman, P.J. Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat. Immunol. 2003, 4, 913–919. [Google Scholar] [CrossRef]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Barkal, A.A.; Weiskopf, K.; Kao, K.S.; Gordon, S.R.; Rosental, B.; Yiu, Y.Y.; George, B.M.; Markovic, M.; Ring, N.G.; Tsai, J.M.; et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 2018, 19, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Pan, P.-Y.; Eisenstein, S.; Divino, C.M.; Lowell, C.A.; Takai, T.; Chen, S.-H. Paired Immunoglobin-like Receptor-B Regulates the Suppressive Function and Fate of Myeloid-Derived Suppressor Cells. Immunity 2011, 34, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Linger, R.M.; Keating, A.K.; Earp, H.S.; Graham, D.K. TAM Receptor Tyrosine Kinases: Biologic Functions, Signaling, and Potential Therapeutic Targeting in Human Cancer. Adv. Cancer Res. 2008, 100, 35–83. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.K.; DeRyckere, D.; Davies, K.D.; Earp, H.S. The TAM family: Phosphatidylserine-sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 2014, 14, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Conn, G.; Goret, M.; Lai, C.; Bruno, J.; Radzlejewski, C.; Mattsson, K.; Fisher, J.; Gies, D.R.; Jones, P.F.; et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 1995, 80, 661–670. [Google Scholar] [CrossRef]
- Aehnlich, P.; Powell, R.; Peeters, M.; Rahbech, A.; Straten, P.T. TAM Receptor Inhibition–Implications for Cancer and the Immune System. Cancers 2021, 13, 1195. [Google Scholar] [CrossRef]
- Wang, J.; Yu, C.; Zhuang, J.; Qi, W.; Jiang, J.; Liu, X.; Zhao, W.; Cao, Y.; Wu, H.; Qi, J.; et al. The role of phosphatidylserine on the membrane in immunity and blood coagulation. Biomark. Res. 2022, 10, 4. [Google Scholar] [CrossRef]
- Burstyn-Cohen, T.; Maimon, A. TAM receptors, Phosphatidylserine, inflammation, and Cancer. Cell Commun. Signal. 2019, 17, 156. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, M.; Zhang, G.; Liang, W.-C.; Lin, W.; Wu, Y.; Piskol, R.; Ridgway, J.; McNamara, E.; Huang, H.; et al. Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity 2020, 52, 357–373.e9. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Q.; Sanmanmed, M.F.; Wang, J. Siglec-15 as an Emerging Target for Next-generation Cancer Immunotherapy. Clin. Cancer Res. 2021, 27, 680–688. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Tabuchi, Y.; Nakamura, K.; Nakamura, M. Siglec-15: An immune system Siglec conserved throughout vertebrate evolution. Glycobiology 2007, 17, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, R.; Ohtsubo, K.; Takamatsu, S.; Taniguchi, N.; Angata, T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF- secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 2012, 23, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Varki, A. Discovery, classification, evolution and diversity of Siglecs. Mol. Asp. Med. 2022, 101117. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.C.; Macauley, M.S.; Kawasaki, N. Siglecs as sensors of self in innate and adaptive immune responses. Ann. N. Y. Acad. Sci. 2012, 1253, 37–48. [Google Scholar] [CrossRef]
- Li, N.; Zhang, W.; Wan, T.; Zhang, J.; Chen, T.; Yu, Y.; Wang, J.; Cao, X. Cloning and Characterization of Siglec-10, a Novel Sialic Acid Binding Member of the Ig Superfamily, from Human Dendritic Cells. J. Biol. Chem. 2001, 276, 28106–28112. [Google Scholar] [CrossRef]
- Altevogt, P.; Sammar, M.; Hüser, L.; Kristiansen, G. Novel insights into the function ofCD24: A driving force in cancer. Int. J. Cancer 2020, 148, 546–559. [Google Scholar] [CrossRef]
- Toubai, T.; Hou, G.; Mathewson, N.; Liu, C.; Wang, Y.; Oravecz-Wilson, K.; Cummings, E.; Rossi, C.; Evers, R.; Sun, Y.; et al. Siglec-G–CD24 axis controls the severity of graft-versus-host disease in mice. Blood 2014, 123, 3512–3523. [Google Scholar] [CrossRef]
- Kristiansen, G.; Sammar, M.; Altevogt, P. Tumour Biological Aspects of CD24, A Mucin-Like Adhesion Molecule. Histochem. J. 2003, 35, 255–262. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Tang, J.; Zheng, P.; Liu, Y. CD24 and Siglec-10 Selectively Repress Tissue Damage–Induced Immune Responses. Science 2009, 323, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Aroldi, A.; Mauri, M.; Parma, M.; Terruzzi, E.; Fedele, M.; Perfetti, P.; Cocito, F.; Mologni, L.; Chiarle, R.; Piazza, R.; et al. CD24/Siglec-10 “Don’t Eat Me” Signal Blockade Is a Potential Immunotherapeutic Target in Mantle-Cell Lymphoma. Blood 2021, 138, 2276. [Google Scholar] [CrossRef]
- Bradley, C.A. CD24—a novel ‘don’t eat me’ signal. Nat. Rev. Drug Discov. 2019, 18, 747. [Google Scholar] [CrossRef]
- Stanczak, M.A.; Läubli, H. Siglec receptors as new immune checkpoints in cancer. Mol. Asp. Med. 2022, 101112. [Google Scholar] [CrossRef]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Rudno-Rudzińska, J.; Kielan, W.; Frejlich, E.; Kotulski, K.; Hap, W.; Kurnol, K.; Dzierżek, P.; Zawadzki, M.; Hałoń, A.; 2-nd Department of General and Oncological Surgery. A review on Eph/ephrin, angiogenesis and lymphangiogenesis in gastric, colorectal and pancreatic cancers. Chin. J. Cancer Res. 2017, 29, 303–312. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph-Ephrin Bidirectional Signaling in Physiology and Disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef]
- Lu, H.; Clauser, K.R.; Tam, W.L.; Frose, J.; Ye, X.; Eaton, E.N.; Reinhardt, F.; Donnenberg, V.S.; Bhargava, R.; Carr, S.A.; et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2014, 16, 1105–1117. [Google Scholar] [CrossRef]
- Koseska, A.; Bastiaens, P.I. Processing Temporal Growth Factor Patterns by an Epidermal Growth Factor Receptor Network Dynamically Established in Space. Annu. Rev. Cell Dev. Biol. 2020, 36, 359–383. [Google Scholar] [CrossRef]
- Kumar, R.; George, B.; Campbell, M.R.; Verma, N.; Paul, A.M.; Melo-Alvim, C.; Ribeiro, L.; Pillai, M.R.; da Costa, L.M.; Moasser, M.M. HER family in cancer progression: From discovery to 2020 and beyond. Adv. Cancer Res. 2020, 147, 109–160. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.; Cohen, S. Epidermal growth factor. J. Biol. Chem. 1990, 265, 7709–7712. [Google Scholar] [CrossRef] [PubMed]
- Vouri, M.; Hafizi, S. TAM Receptor Tyrosine Kinases in Cancer Drug Resistance. Cancer Res. 2017, 77, 2775–2778. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C. EGF receptor ligands. Exp. Cell Res. 2003, 284, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Denning, M.F.; Dlugosz, A.A.; Cheng, C.; Dempsey, P.J.; Coffey, R.J., Jr.; Threadgill, D.W.; Magnuson, T.; Yuspa, S.H. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp. Dermatol. 2000, 9, 192–199. [Google Scholar] [CrossRef]
- Singh, A.B.; Tsukada, T.; Zent, R.; Harris, R.C. Membrane-associated HB-EGF modulates HGF-induced cellular responses in MDCK cells. J. Cell Sci. 2004, 117, 1365–1379. [Google Scholar] [CrossRef]
- Iwamoto, R.; Handa, K.; Mekada, E. Contact-dependent Growth Inhibition and Apoptosis of Epidermal Growth Factor (EGF) Receptor-expressing Cells by the Membrane-anchored Form of Heparin-binding EGF-like Growth Factor. J. Biol. Chem. 1999, 274, 25906–25912. [Google Scholar] [CrossRef]
- Onal, S.; Turker-Burhan, M.; Bati-Ayaz, G.; Yanik, H.; Pesen-Okvur, D. Breast cancer cells and macrophages in a paracrine-juxtacrine loop. Biomaterials 2020, 267, 120412. [Google Scholar] [CrossRef]
- Pathania, S.; Pentikäinen, O.T.; Singh, P.K. A holistic view on c-Kit in cancer: Structure, signaling, pathophysiology and its inhibitors. Biochim. Biophys. Acta 2021, 1876, 188631. [Google Scholar] [CrossRef]
- Han, Z.-B.; Ren, H.; Zhao, H.; Chi, Y.; Chen, K.; Zhou, B.; Liu, Y.-J.; Zhang, L.; Xu, B.; Liu, B.; et al. Hypoxia-inducible factor (HIF)-1 directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF). Carcinogenesis 2008, 29, 1853–1861. [Google Scholar] [CrossRef]
- Levina, V.; Marrangoni, A.; Wang, T.; Parikh, S.; Su, Y.; Herberman, R.; Lokshin, A.; Gorelik, E. Elimination of Human Lung Cancer Stem Cells through Targeting of the Stem Cell Factor–c-kit Autocrine Signaling Loop. Cancer Res. 2010, 70, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Stanulla, M.; Welte, K.; Hadam, M.R.; Pietsch, T. Coexpression of stem cell factor and its receptor c-Kit in human malignant glioma cell lines. Acta Neuropathol. 1995, 89, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lin, J.; Gao, L.; Deng, A.; Lu, X.; Li, Y.; Wang, L.; Yu, L. High Expression of c-kit mRNA Predicts Unfavorable Outcome in Adult Patients with t(8;21) Acute Myeloid Leukemia. PLoS ONE 2015, 10, e0124241. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Anton, A.; Gras, D.; Bourdin, A.; Dubreuil, P.; Chanez, P. KIT as a therapeutic target for non-oncological diseases. Pharmacol. Ther. 2018, 197, 11–37. [Google Scholar] [CrossRef]
- Lennartsson, J.; Rönnstrand, L. Stem Cell Factor Receptor/c-Kit: From Basic Science to Clinical Implications. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef]
- Rönnstrand, L. Signal transduction via the stem cell factor receptor/c-Kit. Cell. Mol. Life Sci. 2004, 61, 2535–2548. [Google Scholar] [CrossRef]
- Mazzoldi, E.L.; Pavan, S.; Pilotto, G.; Leone, K.; Pagotto, A.; Frezzini, S.; Nicoletto, M.O.; Amadori, A.; Pastò, A. A juxtacrine/paracrine loop between C-Kit and stem cell factor promotes cancer stem cell survival in epithelial ovarian cancer. Cell Death Dis. 2019, 10, 142. [Google Scholar] [CrossRef]
- Kim, W.M.; Huang, Y.-H.; Gandhi, A.; Blumberg, R.S. CEACAM1 structure and function in immunity and its therapeutic implications. Semin. Immunol. 2019, 42, 101296. [Google Scholar] [CrossRef]
- Müller, M.; Klaile, E.; Vorontsova, O.; Singer, B.B.; Öbrink, B. Homophilic adhesion and CEACAM1-S regulate dimerization of CEACAM1-L and recruitment of SHP-2 and c-Src. J. Cell Biol. 2009, 187, 569–581. [Google Scholar] [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef]
- To, S.K.Y.; Tang, M.K.S.; Tong, Y.; Zhang, J.; Chan, K.K.L.; Ip, P.P.C.; Shi, J.; Wong, A.S.T. A Selective β −Catenin-Metadherin/CEACAM1-CCL3 Axis Mediates Metastatic Heterogeneity upon Tumor–Macrophage Interaction. Adv. Sci. 2022, 9, 2103230. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Q. Astrocyte elevated gene-1 and breast cancer (Review). Oncol. Lett. 2011, 2, 399–405. [Google Scholar] [CrossRef]
- Ortenberg, R.; Sapir, Y.; Raz, L.; Hershkovitz, L.; Ben Arav, A.; Sapoznik, S.; Barshack, I.; Avivi, C.; Berkun, Y.; Besser, M.J.; et al. Novel Immunotherapy for Malignant Melanoma with a Monoclonal Antibody That Blocks CEACAM1 Homophilic Interactions. Mol. Cancer Ther. 2012, 11, 1300–1310. [Google Scholar] [CrossRef]
- McEver, R.P.; Beckstead, J.H.; Moore, K.L.; Marshall-Carlson, L.; Bainton, D.F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J. Clin. Investig. 1989, 84, 92–99. [Google Scholar] [CrossRef]
- Tinoco, R.; Otero, D.C.; Takahashi, A.A.; Bradley, L.M. PSGL-1: A New Player in the Immune Checkpoint Landscape. Trends Immunol. 2017, 38, 323–335. [Google Scholar] [CrossRef]
- Frenette, P.S.; Denis, C.; Weiss, L.; Jurk, K.; Subbarao, S.; Kehrel, B.; Hartwig, J.H.; Vestweber, D.; Wagner, D.D. P-Selectin Glycoprotein Ligand 1 (Psgl-1) Is Expressed on Platelets and Can Mediate Platelet–Endothelial Interactions in Vivo. J. Exp. Med. 2000, 191, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Zuchtriegel, G.; Uhl, B.; Puhr-Westerheide, D.; Pörnbacher, M.; Lauber, K.; Krombach, F.; Reichel, C.A. Platelets Guide Leukocytes to Their Sites of Extravasation. PLOS Biol. 2016, 14, e1002459. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, J.; Qian, J.; Qiu, P.; Hanabuchi, S.; Lu, Y.; Wang, Z.; Liu, Z.; Li, H.; He, J.; et al. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia 2012, 27, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Phennicie, R.; Kauffman, K.; Nowakowska, D.; Zafari, M.; Komoroski, V.; Sazinsky, S.; Wahle, J.; Manfra, D.; Vaidya, S.; et al. 862 Targeting PSGL-1, a novel macrophage checkpoint, repolarizes suppressive macrophages, induces an inflammatory tumor microenvironment, and suppresses tumor growth. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Liu, W.; Tang, L.; Zhang, G.; Wei, H.; Cui, Y.; Guo, L.; Gou, Z.; Chen, X.; Jiang, D.; Zhu, Y.; et al. Characterization of a Novel C-type Lectin-like Gene, LSECtin. J. Biol. Chem. 2004, 279, 18748–18758. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, B.; Kuai, X.; Xing, G.; Yang, J.; Chen, J.; Tang, L.; Zhang, L.; He, F. C-type lectin LSECtin interacts with DC-SIGNR and is involved in hepatitis C virus binding. Mol. Cell. Biochem. 2009, 327, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Powlesland, A.S.; Fisch, T.; Taylor, M.; Smith, D.F.; Tissot, B.; Dell, A.; Pöhlmann, S.; Drickamer, K. A Novel Mechanism for LSECtin Binding to Ebola Virus Surface Glycoprotein through Truncated Glycans. J. Biol. Chem. 2008, 283, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Gramberg, T.; Hofmann, H.; Möller, P.; Lalor, P.; Marzi, A.; Geier, M.; Krumbiegel, M.; Winkler, T.; Kirchhoff, F.; Adams, D.; et al. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology 2005, 340, 224–236. [Google Scholar] [CrossRef]
- Liu, B.; Wang, M.; Wang, X.; Zhao, D.; Liu, D.; Liu, J.; Chen, P.-J.; Yang, D.; He, F.; Tang, L. Liver Sinusoidal Endothelial Cell Lectin Inhibits CTL-Dependent Virus Clearance in Mouse Models of Viral Hepatitis. J. Immunol. 2013, 190, 4185–4195. [Google Scholar] [CrossRef]
- Liu, D.; Lu, Q.; Wang, X.; Wang, J.; Lu, N.; Jiang, Z.; Hao, X.; Li, J.; Liu, J.; Cao, P.; et al. LSECtin on tumor-associated macrophages enhances breast cancer stemness via interaction with its receptor BTN3A3. Cell Res. 2019, 29, 365–378. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Huang, W.; Wang, Y.; Fan, S. Prognostic and Therapeutic Significance of BTN3A Proteins in Tumors. J. Cancer 2021, 12, 4505–4512. [Google Scholar] [CrossRef]
- Peedicayil, A.; Vierkant, R.A.; Hartmann, L.C.; Fridley, B.L.; Fredericksen, Z.S.; White, K.L.; Elliott, E.A.; Phelan, C.M.; Tsai, Y.-Y.; Berchuck, A.; et al. Risk of Ovarian Cancer and Inherited Variants in Relapse-Associated Genes. PLoS ONE 2010, 5, e8884. [Google Scholar] [CrossRef]
- Taylor, P.; Tsoni, S.V.; Willment, J.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 2006, 8, 31–38. [Google Scholar] [CrossRef]
- Tone, K.; Stappers, M.H.; Willment, J.A.; Brown, G.D. C-type lectin receptors of the Dectin-1 cluster: Physiological roles and involvement in disease. Eur. J. Immunol. 2019, 49, 2127–2133. [Google Scholar] [CrossRef]
- Daley, D.; Mani, V.R.; Mohan, N.; Akkad, N.; Ochi, A.; Heindel, D.W.; Lee, K.B.; Zambirinis, C.P.; Pandian, G.S.B.; Savadkar, S.; et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 2017, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Kanwar, Y.S. Identification and Characterization of Galectin-9, a Novel β-Galactoside-binding Mammalian Lectin. J. Biol. Chem. 1997, 272, 6078–6086. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.G.; Yasinska, I.M.; Sakhnevych, S.S.; Fiedler, W.; Wellbrock, J.; Bardelli, M.; Varani, L.; Hussain, R.; Siligardi, G.; Ceccone, G.; et al. The Tim-3-galectin-9 Secretory Pathway is Involved in the Immune Escape of Human Acute Myeloid Leukemia Cells. EBioMedicine 2017, 22, 44–57. [Google Scholar] [CrossRef]
- Schlichtner, S.; Meyer, N.H.; Yasinska, I.M.; Aliu, N.; Berger, S.M.; Gibbs, B.F.; Fasler-Kan, E.; Sumbayev, V.V. Functional role of galectin-9 in directing human innate immune reactions to Gram-negative bacteria and T cell apoptosis. Int. Immunopharmacol. 2021, 100, 108155. [Google Scholar] [CrossRef]
- Lietha, D.; Izard, T. Roles of Membrane Domains in Integrin-Mediated Cell Adhesion. Int. J. Mol. Sci. 2020, 21, 5531. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef]
- Osborn, L.; Hession, C.; Tizard, R.; Vassallo, C.; Luhowskyj, S.; Chi-Rosso, G.; Lobb, R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 1989, 59, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Kong, D.-H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, M.F.; Ortiz-Quintero, J.; Garrido-Moreno, V.; Sanhueza-Olivares, F.; Guerrero-Moncayo, A.; Chiong, M.; Castro, P.F.; García, L.; Gabrielli, L.; Corbalán, R.; et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166170. [Google Scholar] [CrossRef]
- Gupta, G.P.; Nguyen, D.X.; Chiang, A.C.; Bos, P.D.; Kim, J.Y.; Nadal, C.; Gomis, R.R.; Manova-Todorova, K.; Massagué, J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 2007, 446, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Ruco, L.P.; De Laat, P.A.; Matteucci, C.; Bernasconi, S.; Sciacca, F.M.; Van Der Kwast, T.H.; Hoogsteden, H.C.; Uccini, S.; Mantovani, A.; Versnel, M.A. Expression of ICAM-1 and VCAM-1 in human malignant mesothelioma. J. Pathol. 1996, 179, 266–271. [Google Scholar] [CrossRef]
- Ding, Y.-B.; Chen, G.-Y.; Xia, J.-G.; Zang, X.-W.; Yang, H.-Y.; Yang, L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J. Gastroenterol. 2003, 9, 1409–1414. [Google Scholar] [CrossRef]
- Elices, M.J.; Osborn, L.; Takada, Y.; Crouse, C.; Luhowskyj, S.; Hemler, M.E.; Lobb, R.R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/Fibronectin binding site. Cell 1990, 60, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mu, E.; Wei, Y.; Riethdorf, S.; Yang, Q.; Yuan, M.; Yan, J.; Hua, Y.; Tiede, B.J.; Lu, X.; et al. VCAM-1 Promotes Osteolytic Expansion of Indolent Bone Micrometastasis of Breast Cancer by Engaging α4β1-Positive Osteoclast Progenitors. Cancer Cell 2011, 20, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.H.-F.; Massagué, J. Macrophage Binding to Receptor VCAM-1 Transmits Survival Signals in Breast Cancer Cells that Invade the Lungs. Cancer Cell 2011, 20, 538–549. [Google Scholar] [CrossRef]
- Rosetti, F.; Mayadas, T.N. The many faces of Mac-1 in autoimmune disease. Immunol. Rev. 2015, 269, 175–193. [Google Scholar] [CrossRef]
- Lamers, C.; Plüss, C.J.; Ricklin, D. The Promiscuous Profile of Complement Receptor 3 in Ligand Binding, Immune Modulation, and Pathophysiology. Front. Immunol. 2021, 12, 662164. [Google Scholar] [CrossRef]
- Asada, M.; Furukawa, K.; Kantor, C.; Gahmberg, C.G.; Kobata, A. Structural study of the sugar chains of human leukocyte cell adhesion molecules CD11/CD18. Biochemistry 1991, 30, 1561–1571. [Google Scholar] [CrossRef]
- Low, M.G.; Kincade, P.W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature 1985, 318, 62–64. [Google Scholar] [CrossRef]
- Wetzel, A.; Chavakis, T.; Preissner, K.T.; Sticherling, M.; Haustein, U.-F.; Anderegg, U.; Saalbach, A. Human Thy-1 (CD90) on Activated Endothelial Cells Is a Counterreceptor for the Leukocyte Integrin Mac-1 (CD11b/CD18). J. Immunol. 2004, 172, 3850–3859. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.; Dai, Y.D.; Tong, M.; Chan, Y.P.; Kwan, P.S.; Fu, L.; Qin, Y.R.; Tsao, S.W.; Lung, H.L.; Lung, M.L.; et al. A CD90+ Tumor-Initiating Cell Population with an Aggressive Signature and Metastatic Capacity in Esophageal Cancer. Cancer Res. 2013, 73, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.V.; Saygin, C.; Braley, C.; Wiechert, A.C.; Karunanithi, S.; Crean-Tate, K.; Abdul-Karim, F.W.; Michener, C.M.; Rose, P.G.; Lathia, J.D.; et al. Thy-1 predicts poor prognosis and is associated with self-renewal in ovarian cancer. J. Ovarian Res. 2019, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Xiao, S.; Li, Y.; Chen, Q. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J. Cell. Biochem. 2018, 120, 3046–3055. [Google Scholar] [CrossRef]
- Staunton, D.E.; Marlin, S.D.; Stratowa, C.; Dustin, M.L.; Springer, T.A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell 1988, 52, 925–933. [Google Scholar] [CrossRef]
- Singh, M.; Thakur, M.; Mishra, M.; Yadav, M.; Vibhuti, R.; Menon, A.M.; Nagda, G.; Dwivedi, V.P.; Dakal, T.C.; Yadav, V. Gene regulation of intracellular adhesion molecule-1 (ICAM-1): A molecule with multiple functions. Immunol. Lett. 2021, 240, 123–136. [Google Scholar] [CrossRef]
- Salvador, A.M.; Nevers, T.; Velázquez, F.; Aronovitz, M.; Wang, B.; Molina, A.A.; Jaffe, I.Z.; Karas, R.H.; Blanton, R.M.; Alcaide, P. Intercellular Adhesion Molecule 1 Regulates Left Ventricular Leukocyte Infiltration, Cardiac Remodeling, and Function in Pressure Overload–Induced Heart Failure. J. Am. Hear. Assoc. 2016, 5, e003126. [Google Scholar] [CrossRef]
- Lawson, C.; Wolf, S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 2009, 61, 22–32. [Google Scholar] [CrossRef]
- Regev, O.; Kizner, M.; Roncato, F.; Dadiani, M.; Saini, M.; Castro-Giner, F.; Yajuk, O.; Kozlovski, S.; Levi, N.; Addadi, Y.; et al. ICAM-1 on Breast Cancer Cells Suppresses Lung Metastasis but Is Dispensable for Tumor Growth and Killing by Cytotoxic T Cells. Front. Immunol. 2022, 13, 849701. [Google Scholar] [CrossRef]
- Lynam, E.; Sklar, L.A.; Taylor, A.D.; Neelamegham, S.; Edwards, B.S.; Smith, C.W.; Simon, S.I. β2 -Integrins mediate stable adhesion in collisional interactions between neutrophils and ICAM-1-expressing cells. J. Leukoc. Biol. 1998, 64, 622–630. [Google Scholar] [CrossRef]
- Bai, J.; Adriani, G.; Dang, T.-M.; Tu, T.-Y.; Penny, H.-X.L.; Wong, S.-C.; Kamm, R.D.; Thiery, J.-P. Contact-dependent carcinoma aggregate dispersion by M2a macrophages via ICAM-1 and β2 integrin interactions. Oncotarget 2015, 6, 25295–25307. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J. Clin. Investig. 2016, 126, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Medzhitov, R. Toll-like receptors and cancer. Nat. Rev. Cancer 2008, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Seya, T.; Takeda, Y.; Matsumoto, M. A Toll-like receptor 3 (TLR3) agonist ARNAX for therapeutic immunotherapy. Adv. Drug Deliv. Rev. 2019, 147, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Boushehri, M.A.S.; Lamprecht, A. TLR4-Based Immunotherapeutics in Cancer: A Review of the Achievements and Shortcomings. Mol. Pharm. 2018, 15, 4777–4800. [Google Scholar] [CrossRef]
- Michaelis, K.A.; Norgard, M.A.; Zhu, X.; Levasseur, P.R.; Sivagnanam, S.; Liudahl, S.M.; Burfeind, K.G.; Olson, B.; Pelz, K.R.; Ramos, D.M.A.; et al. Publisher Correction: The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 2019, 10, 5257. [Google Scholar] [CrossRef]
- Kapp, K.; Volz, B.; Oswald, D.; Wittig, B.; Schmidt, M. Abstract 2622: Two new TLR9 agonists for cancer immunotherapy: Combination with checkpoint inhibitors. Cancer Res. 2017, 77, 2622. [Google Scholar] [CrossRef]
- Jang, G.-Y.; Lee, J.W.; Kim, Y.S.; Lee, S.E.; Han, H.D.; Hong, K.-J.; Kang, T.H.; Park, Y.-M. Interactions between tumor-derived proteins and Toll-like receptors. Exp. Mol. Med. 2020, 52, 1926–1935. [Google Scholar] [CrossRef]
- Hume, D.A.; MacDonald, K. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012, 119, 1810–1820. [Google Scholar] [CrossRef]
- Achkova, D.; Maher, J. Role of the colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer. Biochem. Soc. Trans. 2016, 44, 333–341. [Google Scholar] [CrossRef]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R Blockade Reprograms Tumor-Infiltrating Macrophages and Improves Response to T-cell Checkpoint Immunotherapy in Pancreatic Cancer Models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef] [PubMed]
- Paulus, P.; Stanley, E.R.; Schafer, R.; Abraham, D.; Aharinejad, S. Colony-Stimulating Factor-1 Antibody Reverses Chemoresistance in Human MCF-7 Breast Cancer Xenografts. Cancer Res. 2006, 66, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Al-Ameen, S.K.; Al-Koumi, D.M.; Hamad, M.G.; Jalal, N.A.; Oh, C.-H. Recent Advances of Colony-Stimulating Factor-1 Receptor (CSF-1R) Kinase and Its Inhibitors. J. Med. Chem. 2018, 61, 5450–5466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Vilgelm, A.E.; Richmond, A. Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front. Immunol. 2019, 10, 333. [Google Scholar] [CrossRef]
- Argyle, D.; Kitamura, T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front. Immunol. 2018, 9, 2629. [Google Scholar] [CrossRef]
- Mortezaee, K.; Majidpoor, J. Roles for macrophage-polarizing interleukins in cancer immunity and immunotherapy. Cell. Oncol. 2022, 45, 333–353. [Google Scholar] [CrossRef]
- Pan, W.; Zhu, S.; Qu, K.; Meeth, K.; Cheng, J.; He, K.; Ma, H.; Liao, Y.; Wen, X.; Roden, C.; et al. The DNA Methylcytosine Dioxygenase Tet2 Sustains Immunosuppressive Function of Tumor-Infiltrating Myeloid Cells to Promote Melanoma Progression. Immunity 2017, 47, 284–297.e5. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, C.; Wang, S.; Shi, D.; Wei, C.; Song, J.; Lin, X.; Dou, R.; Bai, J.; Xiang, Z.; et al. Wnt5a-induced M2 polarization of tumor-associated macrophages via IL-10 promotes colorectal cancer progression. Cell Commun. Signal. 2020, 18, 51. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, T.; Hu, R.; Zhu, R.; Li, C.; Ruan, Y.; Xie, X.; Li, Y. Next frontier in tumor immunotherapy: Macrophage-mediated immune evasion. Biomark. Res. 2021, 9, 72. [Google Scholar] [CrossRef]
- Moesta, A.K.; Li, X.-Y.; Smyth, M.J. Targeting CD39 in cancer. Nat. Rev. Immunol. 2020, 20, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Da, M.; Chen, L.; Enk, A.; Ring, S.; Mahnke, K. The Multifaceted Actions of CD73 During Development and Suppressive Actions of Regulatory T Cells. Front. Immunol. 2022, 13, 914799. [Google Scholar] [CrossRef] [PubMed]
- Perrot, I.; Michaud, H.-A.; Giraudon-Paoli, M.; Augier, S.; Docquier, A.; Gros, L.; Courtois, R.; Déjou, C.; Jecko, D.; Becquart, O.; et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019, 27, 2411–2425.e9. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Divisekera, U.; Duret, H.; Sparwasser, T.; Teng, M.W.; Darcy, P.K.; Smyth, M.J. CD73-Deficient Mice Have Increased Antitumor Immunity and Are Resistant to Experimental Metastasis. Cancer Res. 2011, 71, 2892–2900. [Google Scholar] [CrossRef]
- Ohta, A.; Gorelik, E.; Prasad, S.J.; Ronchese, F.; Lukashev, D.; Wong, M.K.K.; Huang, X.; Caldwell, S.; Liu, K.; Smith, P.; et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13132–13137. [Google Scholar] [CrossRef]
- Ajona, D.; Ortiz-Espinosa, S.; Pio, R. Complement anaphylatoxins C3a and C5a: Emerging roles in cancer progression and treatment. Semin. Cell Dev. Biol. 2019, 85, 153–163. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Daugan, M.V.; Petitprez, F.; Sautès-Fridman, C.; Fridman, W.H. Context-dependent roles of complement in cancer. Nat. Cancer 2019, 19, 698–715. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chintala, N.K.; Vadrevu, S.K.; Patel, J.; Karbowniczek, M.; Markiewski, M.M. Pulmonary Alveolar Macrophages Contribute to the Premetastatic Niche by Suppressing Antitumor T Cell Responses in the Lungs. J. Immunol. 2015, 194, 5529–5538. [Google Scholar] [CrossRef]
- Ajona, D.; Ortiz-Espinosa, S.; Moreno, H.; Lozano, T.; Pajares, M.J.; Agorreta, J.; Bértolo, C.; Lasarte, J.J.; Vicent, S.; Hoehlig, K.; et al. A Combined PD-1/C5a Blockade Synergistically Protects against Lung Cancer Growth and Metastasis. Cancer Discov. 2017, 7, 694–703. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhao, H.; Ren, X.-B. Relationship of VEGF/VEGFR with immune and cancer cells: Staggering or forward? Cancer Biol. Med. 2016, 13, 206–214. [Google Scholar] [CrossRef]
- Gavalas, N.G.; Tsiatas, M.; Tsitsilonis, O.; Politi, E.; Ioannou, K.; Ziogas, A.C.; Rodolakis, A.; Vlahos, G.; Thomakos, N.; Haidopoulos, D.; et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br. J. Cancer 2012, 107, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.E.; Kirkpatrick, N.D.; Huang, Y.; Farrar, C.T.; Marijt, K.A.; Kloepper, J.; Datta, M.; Amoozgar, Z.; Seano, G.; Jung, K.; et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl. Acad. Sci. USA 2016, 113, 4470–4475. [Google Scholar] [CrossRef] [PubMed]
- Flavell, R.A.; Sanjabi, S.; Wrzesinski, S.H.; Licona-Limón, P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010, 10, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2012, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Santoro, A.; Kelley, R.K.; Gane, E.; Costentin, C.E.; Gueorguieva, I.; Smith, C.; Cleverly, A.; Lahn, M.M.; Raymond, E.; et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019, 39, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; O’Neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227.e5. [Google Scholar] [CrossRef]
- Dobosz, P.; Stempor, P.A.; Roszik, J.; Herman, A.; Layani, A.; Berger, R.; Avni, D.; Sidi, Y.; Leibowitz-Amit, R. Checkpoint Genes at the Cancer Side of the Immunological Synapse in Bladder Cancer. Transl. Oncol. 2020, 13, 193–200. [Google Scholar] [CrossRef]
- Stempor, P.; Avni, D.; Leibowitz, R.; Sidi, Y.; Stępień, M.; Dzieciątkowski, T.; Dobosz, P. Comprehensive Analysis of Correlations in the Expression of miRNA Genes and Immune Checkpoint Genes in Bladder Cancer Cells. Int. J. Mol. Sci. 2021, 22, 2553. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).