The Prognostic Role of True Radical Resection in Perihilar Cholangiocarcinoma after Improved Evaluation of Radial Margin Status
Abstract
Simple Summary
Abstract
1. Background
2. Patients and Methods
2.1. Study Population
2.2. Preoperative Management
2.3. Surgery
2.4. Pathological Evaluation
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Our Cohort
3.2. Histopathological Findings
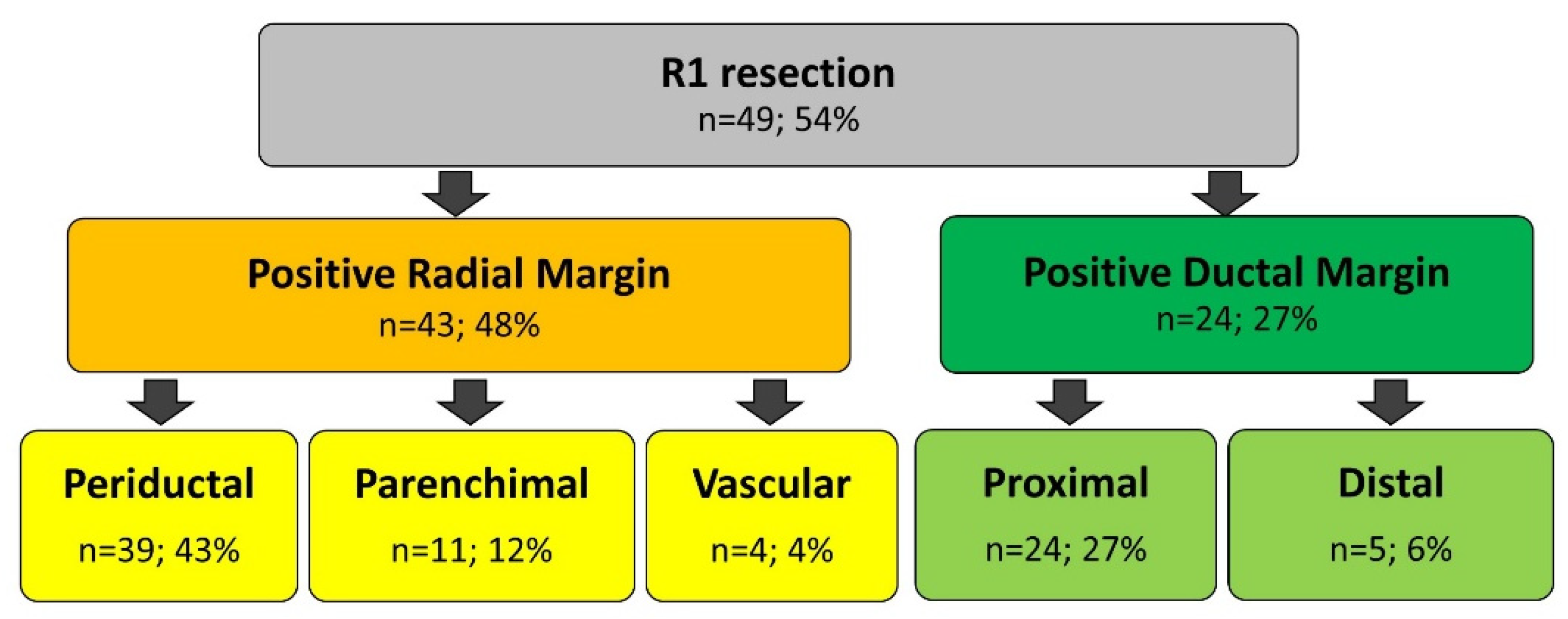
3.3. Surgical Margins
3.4. Short- and Long-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of Surgical Treatment for Perihilar Cholangiocarcinoma: A Single-Center 34-Year Review of 574 Consecutive Resections. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Wiggers, J.K.; Gonen, M.; Doussot, A.; Allen, P.J.; Besselink, M.G.H.; Blumgart, L.H.; Busch, O.R.C.; D’Angelica, M.I.; DeMatteo, R.P.; et al. Survival after Resection of Perihilar Cholangiocarcinoma-Development and External Validation of a Prognostic Nomogram. Ann. Oncol. 2015, 26, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Rassam, F.; Roos, E.; van Lienden, K.P.; van Hooft, J.E.; Klümpen, H.J.; van Tienhoven, G.; Bennink, R.J.; Engelbrecht, M.R.; Schoorlemmer, A.; Beuers, U.H.W.; et al. Modern Work-up and Extended Resection in Perihilar Cholangiocarcinoma: The AMC Experience. Langenbeck’s Arch. Surg. 2018, 403, 289–307. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Zhao, Z.; Wei, K.; Meng, W.; Li, X. The Clinicopathological Factors Associated with Prognosis of Patients with Resectable Perihilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Medicine 2018, 97, e11999. [Google Scholar] [CrossRef]
- Shinohara, K.; Ebata, T.; Shimoyamo, Y.; Mizuni, T.; Yokoyama, Y.; Yamaguchi, Ã.J.; Onoe, Ã.S.; Watanabe, N.; Nagino, M. A Study on Radial Margin Status in Resected Perihilar Cholangiocarcinoma. Ann. Surg. 2019, 273, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, D.; Farges, O.; Fuks, D.; Trouillet, N.; Pruvot, F.R.; Regimbeau, J. Assessment of Pathology Reports on Hilar Cholangiocarcinoma: The Results of a Nationwide, Multicenter Survey Performed by the AFC-HC-2009 Study Group. J. Hepatol. 2012, 56, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.; Franken, L.C.; Soer, E.C.; Van Hooft, J.E.; Takkenberg, R.B.; Klümpen, H.; Wilmink, J.W.; Van De Vijver, M.J.; Van Gulik, T.M.; Verheij, J. Lost in Translation: Confusion on Resection and Dissection Planes Hampers the Interpretation of Pathology Reports for Perihilar Cholangiocarcinoma. Virchows Arch. 2019, 475, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Megías, V.M. Pathological Aspects of so Called “Hilar Cholangiocarcinoma”. World J. Gastrointest. Oncol. 2013, 5, 159. [Google Scholar] [CrossRef]
- Komaya, K.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Mizuno, T.; Yamaguchi, J.; Nagino, M. Recurrence after Curative-Intent Resection of Perihilar Cholangiocarcinoma: Analysis of a Large Cohort with a Close Postoperative Follow-up Approach. Surgery 2018, 163, 732–738. [Google Scholar] [CrossRef]
- Nuzzo, G.; Giuliante, F.; Ardito, F.; Giovannini, I.; Aldrighetti, L.; Belli, G.; Gugielmi, A.; Ruzzenente, A.; Dalla Valle, R. Improvement in Perioperative and Long-Term Outcome After Surgical Treatment of Hilar Cholangiocarcinoma. Arch. Surg. 2012, 147, 26. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.C.; Coelen, R.J.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J.; et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-One-Year Experience with 564 Patients at a Single Institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Hirano, S.; Ambo, Y.; Tanaka, E.; Okushiba, S.; Morikawa, T.; Katoh, H. Forty Consecutive Resections of Hilar Cholangiocarcinoma with No Postoperative Mortality and No Positive Ductal Margins: Results of a Prospective Study. Ann. Surg. 2004, 240, 95–101. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ohtsuka, M.; Miyakawa, S.; Nagino, M.; Yamamoto, M.; Kokudo, N. Classification of Biliary Tract Cancers Established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3rd English Edition. J. Hepatobiliary Pancreat Sci. 2015, 22, 181–196. [Google Scholar] [CrossRef]
- Burt, A.; Venancio, A.; Bedossa, P.; Clouston, A.; Guido, M.; Burt, A.D.; Kakar, S.; Ng, I.; Park, Y.N.; Reeves, H. Data Set for the Reporting of Intrahepatic Cholangiocarcinoma, Perihilar Cholangiocarcinoma and Hepatocellular Carcinoma: Recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology 2018, 73, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Sakata, J.; Katada, T.; Hirose, Y.; Soma, D.; Prasoon, P.; Miura, K.; Kobayashi, T. Surgical Management of Carcinoma in Situ at Ductal Resection Margins in Patients with Extrahepatic Cholangiocarcinoma. Ann. Gastroenterol. Surg. 2018, 2, 359–366. [Google Scholar] [CrossRef]
- Stremitzer, S.; Stift, J.; Laengle, J.; Schwarz, C.; Kaczirek, K.; Jones, R.P.; Quinn, L.M.; Fenwick, S.W.; Diaz-Nieto, R.; Poston, G.J.; et al. Prognosis and Circumferential Margin in Patients with Resected Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2021, 28, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Bosma, A. Surgical Pathology of Cholangiocarcinoma of the Liver Hilus (Klatskin Tumor). Semin. Liver Dis. 1990, 10, 85–90. [Google Scholar] [CrossRef]
- Gerhards, M.F.; Van Gulik, T.M.; Bosma, A.; Ten Hoopen-Neumann, H.; Verbeek, P.C.M.; Gonzalez Gonzalez, D.; De Wit, L.T.; Gouma, D.J. Long-Term Survival after Resection of Proximal Bile Duct Carcinoma (Klatskin Tumors). World J. Surg. 1999, 23, 91–96. [Google Scholar] [CrossRef]
- D’amico, F.E.; Mescoli, C.; Caregari, S.; Pasquale, A.; Billato, I.; Alessandris, R.; Lanari, J.; Bassi, D.; Boetto, R.; D’Amico, F.; et al. Impact of Positive Radial Margin on Recurrence and Survival in Perihilar Cholangiocarcinoma. Cancers 2022, 14, 1680. [Google Scholar] [CrossRef]
- Liang, L.; Li, C.; Jia, H.-D.; Diao, Y.-K.; Xing, H.; Pawlik, T.M.; Lau, W.Y.; Shen, F.; Huang, D.-S.; Zhang, C.-W.; et al. Prognostic Factors of Resectable Perihilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis of High-Quality Studies. Ther. Adv. Gastrointest. Endosc. 2021, 14, 2631774521993065. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.A.; Al-Saffar, H.A.; Fernández Moro, C.; Shtembari, S.; Danielsson, O.; Sparrelid, E.; Sturesson, C. Redefining Resection Margins and Dissection Planes in Perihilar Cholangiocarcinoma—Radical Resection Is a Rare Event. Virchows Arch. 2021, 480, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Seyama, Y.; Kubota, K.; Sano, K.; Noie, T.; Takayama, T.; Kosuge, T.; Makuuchi, M. Long-Term Outcome of Extended Hemihepatectomy for Hilar Bile Duct Cancer with No Mortality and High Survival Rate. Ann. Surg. 2003, 238, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Nassour, I.; Mokdad, A.A.; Porembka, M.R.; Choti, M.A.; Polanco, P.M.; Mansour, J.C.; Minter, R.M.; Wang, S.C.; Yopp, A.C. Adjuvant Therapy Is Associated with Improved Survival in Resected Perihilar Cholangiocarcinoma: A Propensity Matched Study. Ann. Surg. Oncol. 2018, 25, 1193–1201. [Google Scholar] [CrossRef]
- Im, J.H.; Choi, G.H.; Lee, W.J.; Han, D.H.; Park, S.W.; Bang, S.; Choi, H.J.; Seong, J. Adjuvant Radiotherapy and Chemotherapy Offer a Recurrence and Survival Benefit in Patients with Resected Perihilar Cholangiocarcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 2435–2445. [Google Scholar] [CrossRef]




| Variables | R0 | R1 | p Value | RM− | RM+ | p Value |
|---|---|---|---|---|---|---|
| (n = 41) | (n = 49) | (n = 47) | (n = 43) | |||
| Age, years | 69 (58–77) | 70 (65–76) | 0.279 | 70 (62–76) | 70 (63–76) | 0.475 |
| Gender, male | 22 (54) | 38 (78) | 0.017 | 27 (51) | 33 (77) | 0.052 |
| CEA ng/mL | 2 (2–2.7) | 2 (1.6–3.4) | 0.967 | 2 (2–2.7) | 2 (1.7–4) | 0.481 |
| CA19–9 U/mL | 81 (26–538) | 429 (93–1194) | 0.012 | 75 (27–587) | 439 (156–1151) | 0.014 |
| Preoperative biliary drainage | ||||||
| transhepatic | 25 (61) | 29 (59) | 0.863 | 28 (60) | 26 (60) | 0.931 |
| endoscopic | 8 (20) | 13 (27) | 0.433 | 11 (23) | 10 (23) | 0987 |
| Bismuth classification | 0.069 | 0.093 | ||||
| Type II | 4 (10) | 0 (0) | 4 (9) | 0 (0) | ||
| Type IIIa | 6 (15) | 13 (27) | 9 (19) | 10 (23) | ||
| Type IIIb | 18 (44) | 17 (35) | 21 (45) | 14 (33) | ||
| Type IV | 13 (32) | 19 (39) | 13 (28) | 19 (44) | ||
| PVE | 5 (12) | 8 (16) | 7 (15) | 6 (14) | ||
| Type of resection | 0.285 | 0.349 | ||||
| Left-sides hepatectomy | 29 (68) | 27 (55) | 32 (68) | 24 (56) | ||
| Right-sides hepatectomy | 11 (29) | 19 (39) | 14 (30) | 16 (37) | ||
| Mesohepatectomy | 1 (2) | 3 (6) | 1 (2) | 3 (7) | ||
| Vascular resection | 4 (10) | 11 (22) | 0.108 | 5 (11) | 10 (23) | 0.109 |
| Histopathological tumor grade | 0.047 | 0.153 | ||||
| Well/moderately | 33 (80) | 30 (61) | 36 (77) | 27 (63) | ||
| Poorly/undifferentiated | 8 (20) | 19 (39) | 11 (23) | 16 (37) | ||
| Satellitosis | 1 (2) | 8 (16) | 0.029 | 1 (2) | 8 (19) | 0.009 |
| Tumor diameter, cm | 2 (1.5–3) | 3 (2–4) | 0.025 | 2 (1.5–3.5) | 3 (2–4) | 0.044 |
| AJCC pT classification | 0.094 | 0.202 | ||||
| T1/T2 | 24 (59) | 20 (41) | 26 (55) | 18 (42) | ||
| T3/T4 | 17 (41) | 29 (59) | 21 (45) | 25 (58) | ||
| Perineural invasion | 39 (95) | 45 (92) | 0.534 | 43 (91) | 41 (95) | 0.463 |
| Ductal margin positivity | 0 (0) | 24 (49) | <0.0001 | 6 (13) | 18 (42) | 0.002 |
| Lymph node metastasis | 21 (51) | 30 (61) | 0.340 | 25 (53) | 26 (60) | 0.487 |
| Lymph node harvasted | 8 (6–13) | 10 (7–16) | 0.074 | 8 (5–13) | 10 (7–16) | 0.082 |
| Major complication (Dindo ≥ 3) | 9 (22) | 20 (41) | 0.129 | 11 (23) | 18 (42) | 0.128 |
| 90-day/in-hospital Mortality | 1 (2) | 4 (8) | 0.238 | 1 (2) | 4 (9) | 0.138 |
| Adjuvant chemo/radiotherapy | 23 (56) | 32 (65) | 0.372 | 29 (62) | 26 (60) | 0.904 |
| Variables | OS (%) | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| n | 1-Year | 3-Year | 5-Year | p Value | HR (95% CI) | p Value | |
| Gender | 0.049 | ||||||
| Female | 29 | 96 | 74 | 54 | |||
| Male | 56 | 89 | 53 | 28 | |||
| Bismuth type IV | 0.875 | ||||||
| No | 53 | 94 | 60 | 40 | |||
| Yes | 32 | 79 | 58 | 21 | |||
| Side of hepatectomy * | 0.489 | ||||||
| Left | 54 | 90 | 61 | 37 | |||
| Right | 28 | 87 | 54 | 22 | |||
| Combined vascular resection | 0.211 | ||||||
| No | 73 | 89 | 63 | 38 | |||
| Yes | 12 | 80 | 41 | 21 | |||
| Tumor diameter, cm | 0.213 | ||||||
| <3 | 45 | 90 | 65 | 39 | |||
| ≥3 | 40 | 86 | 54 | 33 | |||
| Histopathological tumor grade | 0.042 | 0.045 | |||||
| Well/moderately | 60 | 95 | 68 | 35 | 1 | ||
| Poorly/undifferentiated | 25 | 72 | 37 | 37 | 2.07 (1.02–4.21) | ||
| Perineural invasion | 0.405 | ||||||
| No | 6 | 63 | 31 | / | |||
| Yes | 79 | 90 | 60 | 37 | |||
| AJCC pT classification | 0.086 | ||||||
| T1/T2 | 42 | 92 | 71 | 59 | |||
| T3/T4 | 43 | 84 | 50 | 22 | |||
| Residual disease status | 0.012 | 0.009 | |||||
| R0 | 40 | 97 | 72 | 46 | 1 | ||
| R1 | 45 | 81 | 49 | 27 | 2.68 (1.27–5.63) | ||
| Lymph node | 0.015 | 0.008 | |||||
| N0 | 36 | 93 | 73 | 63 | 1 | ||
| N+ | 49 | 84 | 49 | 20 | 2.73 (1.29–5.75) | ||
| Dindo classification ≥ 3 | 0.960 | ||||||
| No | 56 | 91 | 61 | 33 | |||
| Yes | 29 | 82 | 55 | / | |||
| Adjuvant therapy | 0.823 | ||||||
| No | 30 | 85 | 63 | 38 | |||
| Yes | 55 | 90 | 58 | 35 | |||
| Variables | RFS (%) | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| n | 1-Year | 3-Year | 5-Year | p Value | HR (95% CI) | p Value | |
| Gender | 0.976 | ||||||
| Female | 29 | 62 | 30 | / | |||
| Male | 56 | 64 | 27 | 9 | |||
| Bismuth type IV | 0.583 | ||||||
| No | 53 | 62 | 23 | / | |||
| Yes | 32 | 66 | 35 | 14 | |||
| Side of hepatectomy * | 0.548 | ||||||
| Left | 54 | 60 | 29 | / | |||
| Right | 28 | 75 | 20 | / | |||
| Combined vascular resection | 0.122 | ||||||
| No | 73 | 64 | 33 | 15 | |||
| Yes | 12 | 62 | / | / | |||
| Tumor diameter, cm | 0.064 | ||||||
| <3 | 45 | 70 | 38 | 14 | |||
| ≥3 | 40 | 56 | 19 | / | |||
| Histopathological tumor grade | 0.227 | ||||||
| Well/moderately | 60 | 69 | 26 | 12 | |||
| Poorly/undifferentiated | 25 | 51 | 33 | / | |||
| Perineural invasion | 0.932 | ||||||
| No | 6 | 50 | 25 | / | |||
| Yes | 79 | 65 | 29 | 12 | |||
| AJCC pT classification | 0.014 | 0.030 | |||||
| T1/T2 | 42 | 67 | 48 | / | 1 | ||
| T3/T4 | 43 | 61 | 11 | 4 | 1.85 (1.05–3.24) | ||
| Residual disease status | 0.004 | 0.009 | |||||
| R0 | 40 | 77 | 45 | 19 | 1 | ||
| R1 | 45 | 52 | 14 | / | 2.14 (1.20–3.83) | ||
| Lymph node | 0.069 | ||||||
| N0 | 36 | 67 | 38 | 33 | |||
| N+ | 49 | 61 | 20 | / | |||
| Dindo classification ≥ 3 | 0.884 | ||||||
| No | 56 | 66 | 27 | 13 | |||
| Yes | 29 | 59 | 38 | / | |||
| Adjuvant therapy | 0.076 | ||||||
| No | 30 | 63 | 38 | 31 | |||
| Yes | 55 | 64 | 22 | / | |||
| RM Clearance | RM− | RM+ | 5-Year OS RM−/RM+ | p Value | 5-Year RFS RM−/RM+ | p Value | R0 | R1 | 5-Year OS R0/R1 | p Value | 5-Year RFS R0/R1 | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 mm | 68 (76%) | 22 (24%) | 39/26% | 0.012 | 14/0% | 0.053 | 50 (56%) | 40 (44%) | 25/42% | 0.015 | 20/0% | 0.003 |
| <1 mm | 47 (52%) | 43 (48%) | 48/19% | 0.013 | 17/0% | 0.031 | 40 (44%) | 50 (56%) | 28/46% | 0.014 | 19/0% | 0.006 |
| <2 mm | 8 (9%) | 82 (91%) | 50/32% | 0.279 | 17/0% | 0.393 | 8 (9%) | 82 (91%) | 32/50% | 0.279 | 17/0% | 0.393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bellis, M.; Mastrosimini, M.G.; Conci, S.; Pecori, S.; Campagnaro, T.; Castelli, C.; Capelli, P.; Scarpa, A.; Guglielmi, A.; Ruzzenente, A. The Prognostic Role of True Radical Resection in Perihilar Cholangiocarcinoma after Improved Evaluation of Radial Margin Status. Cancers 2022, 14, 6126. https://doi.org/10.3390/cancers14246126
De Bellis M, Mastrosimini MG, Conci S, Pecori S, Campagnaro T, Castelli C, Capelli P, Scarpa A, Guglielmi A, Ruzzenente A. The Prognostic Role of True Radical Resection in Perihilar Cholangiocarcinoma after Improved Evaluation of Radial Margin Status. Cancers. 2022; 14(24):6126. https://doi.org/10.3390/cancers14246126
Chicago/Turabian StyleDe Bellis, Mario, Maria Gaia Mastrosimini, Simone Conci, Sara Pecori, Tommaso Campagnaro, Claudia Castelli, Paola Capelli, Aldo Scarpa, Alfredo Guglielmi, and Andrea Ruzzenente. 2022. "The Prognostic Role of True Radical Resection in Perihilar Cholangiocarcinoma after Improved Evaluation of Radial Margin Status" Cancers 14, no. 24: 6126. https://doi.org/10.3390/cancers14246126
APA StyleDe Bellis, M., Mastrosimini, M. G., Conci, S., Pecori, S., Campagnaro, T., Castelli, C., Capelli, P., Scarpa, A., Guglielmi, A., & Ruzzenente, A. (2022). The Prognostic Role of True Radical Resection in Perihilar Cholangiocarcinoma after Improved Evaluation of Radial Margin Status. Cancers, 14(24), 6126. https://doi.org/10.3390/cancers14246126








