HER2-Low Status Is Not Accurate in Breast Cancer Core Needle Biopsy Samples: An Analysis of 5610 Consecutive Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
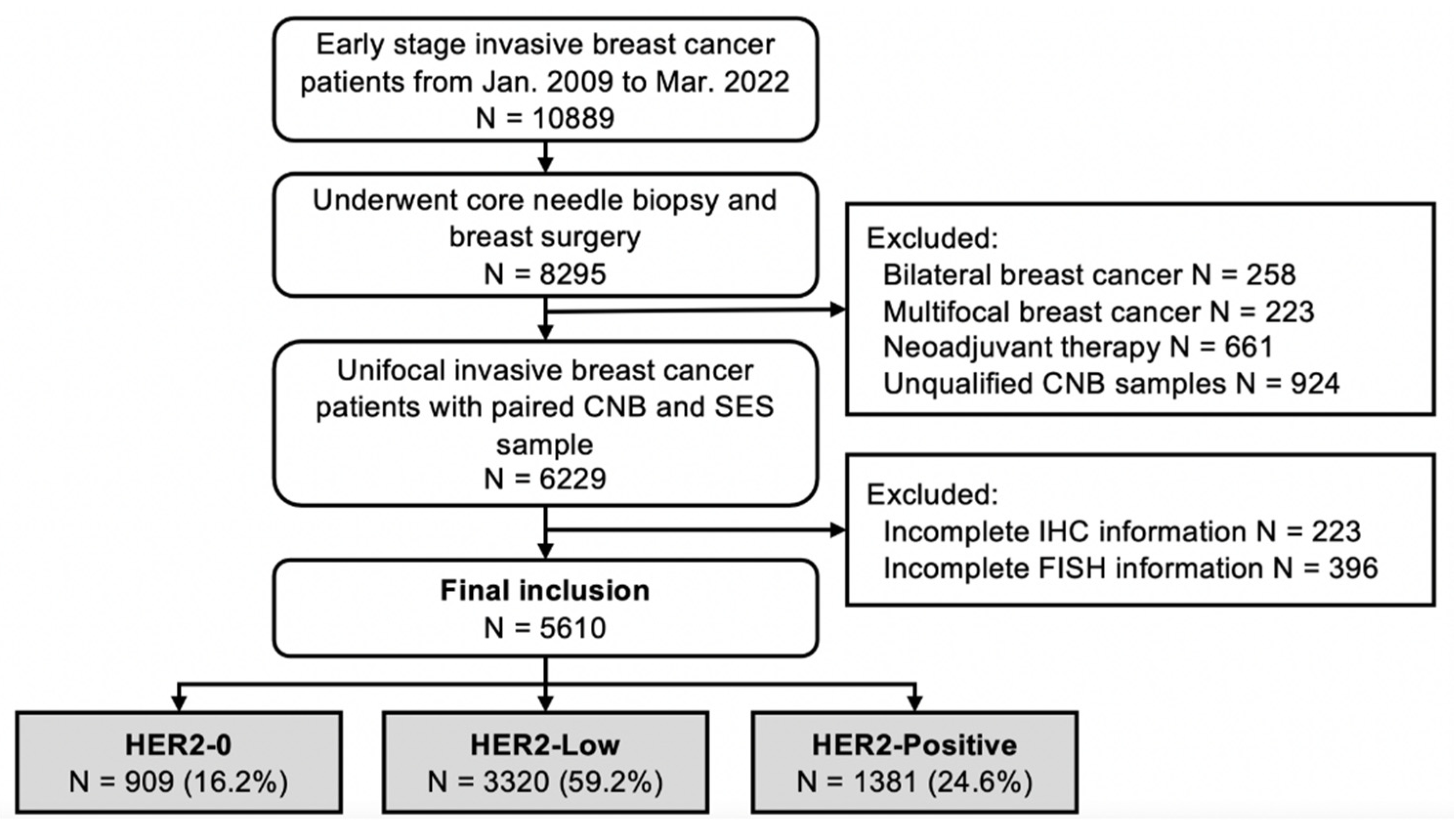
2.1. Study Population
2.2. Histopathological Evaluation and HER2 Testing Algorithms
2.3. Statistical Analysis
3. Results
3.1. Patient Cohorts and Baseline Characteristics
3.2. Biomarker Status Changes from CNB to SES Samples
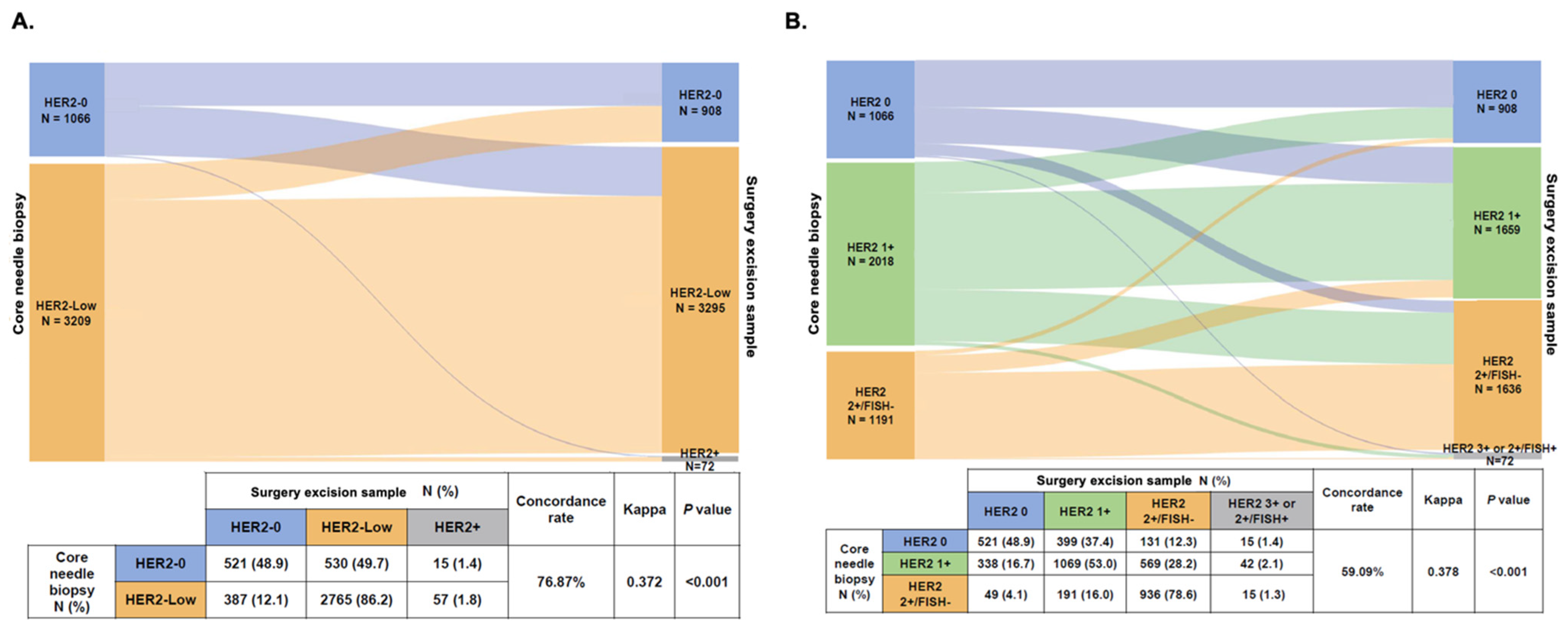
3.3. HER2 Status Changes from CNB to SES Samples
3.4. Clinicopathological Features Associated with HER2 Status Change
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Hamilton, E.; Tolaney, S.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Banerji, U.; van Herpen, C.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.; Boni, V.; Rolfo, C.; de Vries, E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modi, S.; Park, H.; Murthy, R.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.Q.; Feng, J.; Fang, J.; Chen, X.; Lu, Y.; Tong, Y. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1022. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Baez-Navarro, X.; Salgado, R.; Denkert, C.; Lennerz, J.K.; Penault-Llorca, F.; Viale, G.; Bartlett, J.M.S.; van Deurzen, C.H.M. Selecting patients with HER2-low breast cancer: Getting out of the tangle. Eur. J. Cancer 2022, 175, 187–192. [Google Scholar] [CrossRef]
- Shu, L.; Tong, Y.; Li, Z.; Chen, X.; Shen, K. Can HER2 1+ Breast Cancer Be Considered as HER2-Low Tumor? A Comparison of Clinicopathological Features, Quantitative HER2 mRNA Levels, and Prognosis among HER2-Negative Breast Cancer. Cancers 2022, 14, 4250. [Google Scholar] [CrossRef]
- Wolff, A.; Hammond, M.; Schwartz, J.; Hagerty, K.; Allred, D.; Cote, R.; Dowsett, M.; Fitzgibbons, P.; Hanna, W.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef]
- Shanmugalingam, A.; Hitos, K.; Hegde, S.; Al-Mashat, A.; Pathmanathan, N.; Edirimmane, S.; Hughes, T.M.; Ngui, N.K. Concordance between core needle biopsy and surgical excision for breast cancer tumor grade and biomarkers. Breast Cancer Res. Treat. 2022, 193, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, Y.; Gu, Z.; Shen, K. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2012, 134, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, L.; Mao, Y.; Zhu, S.; Wu, J.; Huang, O.; Li, Y.; Chen, W.; Wang, J.; Yuan, Y.; et al. Preoperative core needle biopsy is accurate in determining molecular subtypes in invasive breast cancer. BMC Cancer 2013, 13, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, K.H.; Wolff, A.C. ERBB2-Low Breast Cancer-Is It a Fact or Fiction, and Do We Have the Right Assay? JAMA Oncol. 2022, 8, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Bicchierai, G.; Saieva, C.; De Benedetto, D.; Desideri, I.; Becherini, C.; Abdulcadir, D.; Vanzi, E.; Boeri, C.; Gabbrielli, S.; et al. Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: Single-institution experience and review of published literature. Eur. J. Surg. Oncol. 2017, 43, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, J.; Huang, O.; He, J.; Zhu, L.; Li, Y.; Chen, W.; Fei, X.; Chen, X.; Shen, K. Clinicopathological Features and Disease Outcome in Breast Cancer Patients with Hormonal Receptor Discordance between Core Needle Biopsy and Following Surgical Sample. Ann. Surg. Oncol. 2019, 26, 2779–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Katerji, H.; Turner, B.; Audeh, W.; Hicks, D. HER2-low breast cancers: Incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. 2022, 35, 1075–1082. [Google Scholar] [CrossRef]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; De Bartolo, D.; Vernaci, G.; et al. HER2-low-positive breast cancer: Evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer 2022, 8, 66. [Google Scholar] [CrossRef]
- Lin, L.; Sirohi, D.; Coleman, J.; Gulbahce, H. American Society of Clinical Oncology/College of American Pathologists 2018 Focused Update of Breast Cancer HER2 FISH Testing GuidelinesResults From a National Reference Laboratory. Am. J. Clin. Pathol. 2019, 152, 479–485. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, X.; Fei, X.; Lin, L.; Wu, J.; Huang, O.; He, J.; Zhu, L.; Chen, W.; Li, Y.; et al. Can breast cancer patients with HER2 dual-equivocal tumours be managed as HER2-negative disease? Eur. J. Cancer 2018, 89, 9–18. [Google Scholar] [CrossRef]
- Lu, Y.; Tong, Y.; Chen, X.; Shen, K. Association of Biomarker Discrepancy and Treatment Decision, Disease Outcome in Recurrent/Metastatic Breast Cancer Patients. Front. Oncol. 2021, 11, 638619. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Gandini, S.; Nicolo, E.; Trillo, P.; Giugliano, F.; Zagami, P.; Vivanet, G.; Bellerba, F.; Trapani, D.; Marra, A.; et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur. J. Cancer 2022, 163, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Hammond, M.; Allison, K.; Harvey, B.; Mangu, P.; Bartlett, J.; Bilous, M.; Ellis, I.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J.; Panel, M. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.; Geyer, C.; Rastogi, P.; Costantino, J.; Atkins, J.; Crown, J.; Polikoff, J.; Boileau, J.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. (NCCN) Clinical Practice Guidelines in Oncology. Breast Cancer, Version 4. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 21 June 2022).
- Dieci, M.V.; Miglietta, F. HER2: A never ending story. Lancet Oncol. 2021, 22, 1051–1052. [Google Scholar] [CrossRef]
- Hamilton, E.; Shastry, M.; Shiller, S.M.; Ren, R. Targeting HER2 heterogeneity in breast cancer. Cancer Treat. Rev. 2021, 100, 102286. [Google Scholar] [CrossRef]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. HER2-Low Breast Cancer: Molecular Characteristics and Prognosis. Cancers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Kalvala, J.; Parks, R.M.; Green, A.R.; Cheung, K.L. Concordance between core needle biopsy and surgical excision specimens for Ki-67 in breast cancer—A systematic review of the literature. Histopathology 2022, 80, 468–484. [Google Scholar] [CrossRef]
- Franchet, C.; Djerroudi, L.; Maran-Gonzalez, A.; Abramovici, O.; Antoine, M.; Becette, V.; Berghian, A.; Blanc-Fournier, C.; Brabencova, E.; Charafe-Jauffret, E.; et al. 2021 update of the GEFPICS’ recommendations for HER2 status assessment in invasive breast cancer in France. Ann. Pathol. 2021, 41, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Koopman, T.; van der Vegt, B.; Dijkstra, M.; Bart, J.; Duiker, E.; Wisman, G.B.A.; de Bock, G.H.; Hollema, H. HER2 immunohistochemistry in endometrial and ovarian clear cell carcinoma: Discordance between antibodies and with in-situ hybridisation. Histopathology 2018, 73, 852–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, A.; Liu, M.; Bellizzi, A.; Brock, J.; Fadare, O.; Hanley, K.; Harigopal, M.; Jorns, J.; Kuba, M.; Ly, A.; et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol. 2022, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.; Shou, J.; Massarweh, S.; Schiff, R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 865s–870s. [Google Scholar] [CrossRef]
- Ithimakin, S.; Day, K.; Malik, F.; Zen, Q.; Dawsey, S.; Bersano-Begey, T.; Quraishi, A.; Ignatoski, K.; Daignault, S.; Davis, A.; et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: Implications for efficacy of adjuvant trastuzumab. Cancer Res. 2013, 73, 1635–1646. [Google Scholar] [CrossRef] [Green Version]
- Ballard, M.; Jalikis, F.; Krings, G.; Schmidt, R.; Chen, Y.; Rendi, M.; Dintzis, S.; Jensen, K.; West, R.; Sibley, R.; et al. ‘Non-classical’ HER2 FISH results in breast cancer: A multi-institutional study. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. 2017, 30, 227–235. [Google Scholar] [CrossRef]
- Chen, M.; Chen, W.; Liu, D.; Chen, W.; Shen, K.; Wu, J.; Zhu, L. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: Features of HER2-low breast cancer. Breast Cancer 2022, 29, 844–853. [Google Scholar] [CrossRef]
- Bove, S.; Comes, M.C.; Lorusso, V.; Cristofaro, C.; Didonna, V.; Gatta, G.; Giotta, F.; La Forgia, D.; Latorre, A.; Pastena, M.I.; et al. A ultrasound-based radiomic approach to predict the nodal status in clinically negative breast cancer patients. Sci. Rep. 2022, 12, 7914. [Google Scholar] [CrossRef]
- Fanizzi, A.; Pomarico, D.; Paradiso, A.; Bove, S.; Diotaiuti, S.; Didonna, V.; Giotta, F.; La Forgia, D.; Latorre, A.; Pastena, M.I.; et al. Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study. Cancers 2021, 13, 352. [Google Scholar] [CrossRef]


| Characteristics | Overall Population N = 5610 (%) | HER2-0 # N = 1066 (%) | HER2-Low # N = 3209 (%) | HER2-Positive # N = 1335 (%) | p Value |
|---|---|---|---|---|---|
| Age, years (median, range) | 56.0 (22–95) | 57.0 (22–91) | 57.0 (24–92) | 54.0 (23–95) | <0.001 |
| <55 | 2543 (45.3) | 473 (44.4) | 1393 (43.4) | 677 (50.7) | |
| ≥55 | 3067 (54.7) | 593 (55.6) | 1816 (56.6) | 658 (49.3) | |
| BMI, kg/m2 | 0.019 | ||||
| <24 | 3464 (61.7) | 646 (60.6) | 1950 (60.8) | 868 (65.0) | |
| ≥24 | 2146 (38.3) | 420 (39.4) | 1259 (39.2) | 467 (35.0) | |
| Menstruation | 0.626 | ||||
| Pre/peri-menopausal | 2034 (36.3) | 395 (37.1) | 1144 (35.6) | 495 (37.1) | |
| Post-menopausal | 3576 (63.7) | 671 (62.9) | 2065 (64.4) | 840 (62.9) | |
| Time to surgery | 0.172 | ||||
| <1 week | 4950 (88.4) | 950 (89.2) | 2809 (87.7) | 1191 (89.4) | |
| ≥1 week | 645 (11.5) | 114 (10.7) | 391 (12.2) | 140 (10.5) | |
| NA | 15 (0.1) | 2 (0.1) | 9 (0.1) | 4 (0.1) | |
| Histology * | <0.001 | ||||
| IDC | 4952 (88.3) | 880 (82.6) | 2828 (88.1) | 1244 (93.2) | |
| Non-IDC | 658 (11.7) | 186 (17.4) | 381 (11.9) | 91 (6.8) | |
| Grade * | <0.001 | ||||
| I | 209 (3.7) | 54 (5.1) | 151 (4.7) | 4 (0.3) | |
| II | 2748 (49.0) | 483 (45.3) | 1823 (56.8) | 442 (33.1) | |
| III | 2238 (39.9) | 427 (40.1) | 973 (30.3) | 838 (62.8) | |
| NA | 415 (7.4) | 102 (9.6) | 262 (8.2) | 51 (3.8) | |
| Tumor size, cm | <0.001 | ||||
| ≤2 | 2815 (50.2) | 555 (52.1) | 1714 (53.4) | 546 (40.6) | |
| >2 | 2790 (49.7) | 510 (47.8) | 1493 (46.5) | 787 (59.0) | |
| NA | 5 (0.1) | 1 (0.1) | 2 (0.1) | 2 (0.1) | |
| Nodal status | <0.001 | ||||
| Negative | 3414 (60.9) | 699 (65.6) | 1984 (61.8) | 731 (54.8) | |
| Positive | 2183 (38.9) | 362 (34.0) | 1219 (38.0) | 602 (45.1) | |
| NA | 13 (0.2) | 5 (0.5) | 6 (0.2) | 2 (0.1) | |
| LVI | <0.001 | ||||
| Yes | 879 (15.7) | 155 (14.5) | 438 (13.6) | 286 (21.4) | |
| No | 4731 (84.3) | 911 (85.5) | 2771 (86.4) | 1049 (78.6) | |
| ER # | <0.001 | ||||
| Positive | 4152 (74.0) | 761 (71.4) | 2707 (84.4) | 684 (51.2) | |
| Negative | 1458 (26.0) | 305 (28.6) | 502 (15.6) | 651 (48.8) | |
| PR # | <0.001 | ||||
| Positive | 3547 (63.2) | 677 (63.5) | 2385 (74.3) | 485 (36.3) | |
| Negative | 2063 (36.8) | 389 (36.5) | 824 (25.7) | 850 (63.7) | |
| Ki67 #, % | <0.001 | ||||
| <20 | 1869 (33.3) | 365 (34.2) | 1356 (42.3) | 148 (11.1) | |
| ≥20 | 3741 (66.7) | 701 (65.8) | 1853 (57.7) | 1187 (88.9) | |
| Molecular subtype # | <0.001 | ||||
| Luminal-A | 1275 (22.7) | 263 (24.7) | 1012 (31.5) | 0 (0.0) | |
| Luminal-B/HER2-Negative | 2222 (39.6) | 503 (47.2) | 1719 (53.6) | 0 (0.0) | |
| Luminal-B/HER2-Positive | 726 (12.9) | 0 (0.0) | 0 (0.0) | 726 (54.4) | |
| HER2-amplified | 609 (10.9) | 0 (0.0) | 0 (0.0) | 609 (45.6) | |
| TNBC | 778 (13.9) | 300 (28.1) | 478 (14.9) | 0 (0.0) |
| CNB Lesion | SES Lesion | Concordance Rate | Kappa | p Value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| ER | 96.10% | 0.898 | <0.001 | ||
| Positive | 4062 | 90 | |||
| Negative | 129 | 1329 | |||
| PR | 92.70% | 0.845 | <0.001 | ||
| Positive | 3296 | 251 | |||
| Negative | 158 | 1905 | |||
| HER2 | 98.26% | 0.952 | <0.001 | ||
| Positive | 1335 | 26 | |||
| Negative a | 72 | 4203 | |||
| Ki67, % | ≥20 | <20 | 81.07% | 0.598 | <0.001 |
| <20 | 309 | 1560 | |||
| ≥20 | 2988 | 753 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhu, S.; Tong, Y.; Fei, X.; Jiang, W.; Shen, K.; Chen, X. HER2-Low Status Is Not Accurate in Breast Cancer Core Needle Biopsy Samples: An Analysis of 5610 Consecutive Patients. Cancers 2022, 14, 6200. https://doi.org/10.3390/cancers14246200
Lu Y, Zhu S, Tong Y, Fei X, Jiang W, Shen K, Chen X. HER2-Low Status Is Not Accurate in Breast Cancer Core Needle Biopsy Samples: An Analysis of 5610 Consecutive Patients. Cancers. 2022; 14(24):6200. https://doi.org/10.3390/cancers14246200
Chicago/Turabian StyleLu, Yujie, Siji Zhu, Yiwei Tong, Xiaochun Fei, Wu Jiang, Kunwei Shen, and Xiaosong Chen. 2022. "HER2-Low Status Is Not Accurate in Breast Cancer Core Needle Biopsy Samples: An Analysis of 5610 Consecutive Patients" Cancers 14, no. 24: 6200. https://doi.org/10.3390/cancers14246200






