Approach to Contemporary Risk Assessment, Prevention and Management of Thrombotic Complications in Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
2. Literature Review
2.1. Risk Factors for Thrombosis
2.1.1. Patient-Related Risk Factors
2.1.2. Disease-Specific Risk Factors
2.1.3. Treatment-Related Risk Factors
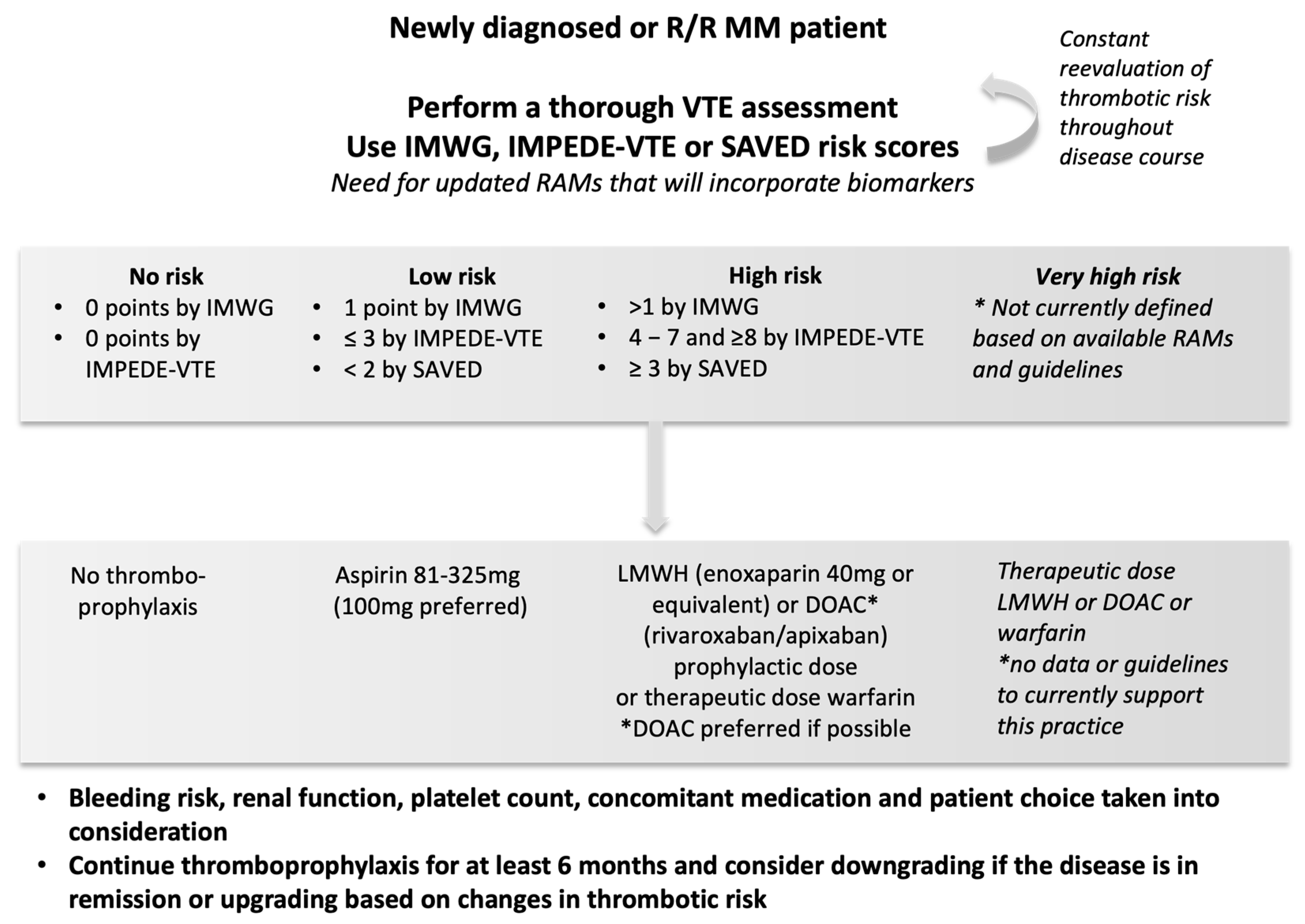
2.2. Risk Assessment Models for VTE in Multiple Myeloma
2.3. Primary VTE Prevention in Multiple Myeloma
2.4. Treatment of VTE and Secondary Prevention of VTE
3. Arterial Thromboembolic Risk in MM
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carrier, M.; Le Gal, G.; Tay, J.; Wu, C.; Lee, A.Y. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: A systematic review and meta-analysis. J. Thromb. Haemost. 2011, 9, 653–663. [Google Scholar] [CrossRef]
- De Stefano, V.; Za, T.; Rossi, E. Venous thromboembolism in multiple myeloma. Semin. Thromb. Hemost. 2014, 40, 338–347. [Google Scholar]
- Zangari, M.; Barlogie, B.; Cavallo, F.; Bolejack, V.; Fink, L.; Tricot, G. Effect on survival of treatment-associated venous thromboembolism in newly diagnosed multiple myeloma patients. Blood Coagul. Fibrinolysis 2007, 18, 595–598. [Google Scholar] [CrossRef]
- Zangari, M.; Tricot, G.; Polavaram, L.; Zhan, F.; Finlayson, A.; Knight, R.; Fu, T.; Weber, D.; Dimopoulos, M.A.; Niesvizky, R.; et al. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high-dose dexamethasone. J. Clin. Oncol. 2010, 28, 132–135. [Google Scholar] [CrossRef]
- Schoen, M.W.; Carson, K.R.; Luo, S.; Gage, B.F.; Li, A.; Afzal, A.; Sanfilippo, K.M. Venous thromboembolism in multiple myeloma is associated with increased mortality. Res. Pract. Thromb. Haemost. 2020, 4, 1203–1210. [Google Scholar] [CrossRef]
- Khorana, A.A.; Dalal, M.R.; Lin, J.; Connolly, G.C. Health care costs associated with venous thromboembolism in selected high-risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clin. Outcomes Res. 2013, 5, 101–108. [Google Scholar] [CrossRef]
- Palumbo, A.; Rajkumar, S.V.; San Miguel, J.F.; Larocca, A.; Niesvizky, R.; Morgan, G.; Landgren, O.; Hajek, R.; Einsele, H.; Anderson, K.C.; et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J. Clin. Oncol. 2014, 32, 587–600. [Google Scholar] [CrossRef]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z.; et al. Cancer-Associated Venous Thromboembolic Disease, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1181–1201. [Google Scholar]
- De Stefano, V.; Larocca, A.; Carpenedo, M.; Cavo, M.; Di Raimondo, F.; Falanga, A.; Offidani, M.; Petrucci, M.T.; Ruggeri, M.; Santi, R.M.; et al. Thrombosis in multiple myeloma: Risk stratification, antithrombotic prophylaxis, and management of acute events. A consensus-based position paper from an ad hoc expert panel. Haematologica 2022, 107, 2536–2547. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Luo, S.; Wang, T.F.; Fiala, M.; Schoen, M.; Wildes, T.M.; Mikhael, J.; Kuderer, N.M.; Calverley, D.C.; Keller, J.; et al. Predicting venous thromboembolism in multiple myeloma: Development and validation of the IMPEDE VTE score. Am. J. Hematol. 2019, 94, 1176–1184. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Pfeiffer, R.M.; Bjorkholm, M.; Goldin, L.R.; Schulman, S.; Blimark, C.; Mellqvist, U.H.; Wahlin, A.; Turesson, I.; Landgren, O. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: A population-based study. Blood 2010, 115, 4991–4998. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Fears, T.R.; Gridley, G.; Turesson, I.; Mellqvist, U.H.; Bjorkholm, M.; Landgren, O. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood 2008, 112, 3582–3586. [Google Scholar] [CrossRef]
- Sallah, S.; Husain, A.; Wan, J.; Vos, P.; Nguyen, N.P. The risk of venous thromboembolic disease in patients with monoclonal gammopathy of undetermined significance. Ann. Oncol. 2004, 15, 1490–1494. [Google Scholar] [CrossRef]
- Nielsen, T.; Kristensen, S.R.; Gregersen, H.; Teodorescu, E.M.; Pedersen, S. Prothrombotic abnormalities in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. Thromb. Res. 2021, 202, 108–118. [Google Scholar] [CrossRef]
- Palumbo, A.; Rajkumar, S.V.; Dimopoulos, M.A.; Richardson, P.G.; San Miguel, J.; Barlogie, B.; Harousseau, J.; Zonder, J.A.; Cavo, M.; Zangari, M.; et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008, 22, 414–423. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Leleu, X.; Palumbo, A.; Moreau, P.; Delforge, M.; Cavo, M.; Ludwig, H.; Morgan, G.J.; Davies, F.E.; Sonneveld, P.; et al. Expert panel consensus statement on the optimal use of pomalidomide in relapsed and refractory multiple myeloma. Leukemia 2014, 28, 1573–1585. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Palumbo, A.; Attal, M.; Beksac, M.; Davies, F.E.; Delforge, M.; Einsele, H.; Hajek, R.; Harousseau, J.L.; da Costa, F.L.; et al. Optimizing the use of lenalidomide in relapsed or refractory multiple myeloma: Consensus statement. Leukemia 2011, 25, 749–760. [Google Scholar] [CrossRef]
- Palumbo, A.; Cavo, M.; Bringhen, S.; Zamagni, E.; Romano, A.; Patriarca, F.; Rossi, D.; Gentilini, F.; Crippa, C.; Galli, M.; et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: A phase III, open-label, randomized trial. J. Clin. Oncol. 2011, 29, 986–993. [Google Scholar] [CrossRef]
- Larocca, A.; Cavallo, F.; Bringhen, S.; Di Raimondo, F.; Falanga, A.; Evangelista, A.; Cavalli, M.; Stanevsky, A.; Corradini, P.; Pezzatti, S.; et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012, 119, 933–939; quiz 1093. [Google Scholar] [CrossRef]
- Cortelezzi, A.; Moia, M.; Falanga, A.; Pogliani, E.M.; Agnelli, G.; Bonizzoni, E.; Gussoni, G.; Barbui, T.; Mannucci, P.M.; Group, C.S. Incidence of thrombotic complications in patients with haematological malignancies with central venous catheters: A prospective multicentre study. Br. J. Haematol. 2005, 129, 811–817. [Google Scholar] [CrossRef]
- Fotiou, D.; Gavriatopoulou, M.; Terpos, E. Multiple Myeloma and Thrombosis: Prophylaxis and Risk Prediction Tools. Cancers 2020, 12, 191. [Google Scholar] [CrossRef]
- Cini, M.; Zamagni, E.; Valdre, L.; Palareti, G.; Patriarca, F.; Tacchetti, P.; Legnani, C.; Catalano, L.; Masini, L.; Tosi, P.; et al. Thalidomide-dexamethasone as up-front therapy for patients with newly diagnosed multiple myeloma: Thrombophilic alterations, thrombotic complications, and thromboprophylaxis with low-dose warfarin. Eur. J. Haematol. 2010, 84, 484–492. [Google Scholar] [CrossRef]
- Bagratuni, T.; Kastritis, E.; Politou, M.; Roussou, M.; Kostouros, E.; Gavriatopoulou, M.; Eleutherakis-Papaiakovou, E.; Kanelias, N.; Terpos, E.; Dimopoulos, M.A. Clinical and genetic factors associated with venous thromboembolism in myeloma patients treated with lenalidomide-based regimens. Am. J. Hematol. 2013, 88, 765–770. [Google Scholar] [CrossRef]
- Chakraborty, R.; Bin Riaz, I.; Malik, S.U.; Marneni, N.; Mejia Garcia, A.; Anwer, F.; Khorana, A.A.; Rajkumar, S.V.; Kumar, S.; Murad, M.H.; et al. Venous thromboembolism risk with contemporary lenalidomide-based regimens despite thromboprophylaxis in multiple myeloma: A systematic review and meta-analysis. Cancer 2020, 126, 1640–1650. [Google Scholar] [CrossRef]
- Gregersen, H.; Norgaard, M.; Severinsen, M.T.; Engebjerg, M.C.; Jensen, P.; Sorensen, H.T. Monoclonal gammopathy of undetermined significance and risk of venous thromboembolism. Eur. J. Haematol. 2011, 86, 129–134. [Google Scholar] [CrossRef]
- Yasin, Z.; Quick, D.; Thiagarajan, P.; Spoor, D.; Caraveo, J.; Palascak, J. Light-chain paraproteins with lupus anticoagulant activity. Am. J. Hematol. 1999, 62, 99–102. [Google Scholar] [CrossRef]
- Carr, M.E., Jr.; Dent, R.M.; Carr, S.L. Abnormal fibrin structure and inhibition of fibrinolysis in patients with multiple myeloma. J. Lab. Clin. Med. 1996, 128, 83–88. [Google Scholar] [CrossRef]
- Lackner, H.; Hunt, V.; Zucker, M.B.; Pearson, J. Abnormal fibrin ultrastructure, polymerization, and clot retraction in multiple myeloma. Br. J. Haematol. 1970, 18, 625–636. [Google Scholar] [CrossRef]
- O’Kane, M.J.; Wisdom, G.B.; Desai, Z.R.; Archbold, G.P. Inhibition of fibrin monomer polymerisation by myeloma immunoglobulin. J. Clin. Pathol. 1994, 47, 266–268. [Google Scholar] [CrossRef]
- van Marion, A.M.; Auwerda, J.J.; Minnema, M.C.; van Oosterom, R.; Adelmeijer, J.; de Groot, P.G.; Leebeek, F.W.; Sonneveld, P.; Lokhorst, H.M.; Lisman, T. Hypofibrinolysis during induction treatment of multiple myeloma may increase the risk of venous thrombosis. Thromb. Haemost. 2005, 94, 1341–1343. [Google Scholar] [CrossRef]
- Martini, F.; Cecconi, N.; Paolicchi, A.; Galimberti, S.; Cervetti, G.; Buda, G.; Petrini, M. Interference of Monoclonal Gammopathy with Fibrinogen Assay Producing Spurious Dysfibrinogenemia. TH Open 2019, 3, e64–e66. [Google Scholar] [CrossRef]
- Robak, M.; Trelinski, J.; Chojnowski, K. Hemostatic changes after 1 month of thalidomide and dexamethasone therapy in patients with multiple myeloma. Med. Oncol. 2012, 29, 3574–3580. [Google Scholar] [CrossRef][Green Version]
- Zamagni, E.; Brioli, A.; Tacchetti, P.; Zannetti, B.; Pantani, L.; Cavo, M. Multiple myeloma, venous thromboembolism, and treatment-related risk of thrombosis. Semin. Thromb. Hemost. 2011, 37, 209–219. [Google Scholar] [CrossRef]
- Auwerda, J.J.; Yuana, Y.; Osanto, S.; de Maat, M.P.; Sonneveld, P.; Bertina, R.M.; Leebeek, F.W. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb. Haemost. 2011, 105, 14–20. [Google Scholar] [CrossRef]
- Comerford, C.; Glavey, S.; Quinn, J.; O’Sullivan, J.M. The role of VWF/FVIII in thrombosis and cancer progression in multiple myeloma and other hematological malignancies. J. Thromb. Haemost. 2022, 20, 1766–1777. [Google Scholar] [CrossRef]
- Egan, K.; Cooke, N.; Dunne, E.; Murphy, P.; Quinn, J.; Kenny, D. Platelet hyporeactivity in active myeloma. Thromb. Res. 2014, 134, 747–749. [Google Scholar] [CrossRef]
- O’Sullivan, L.R.; Meade-Murphy, G.; Gilligan, O.M.; Mykytiv, V.; Young, P.W.; Cahill, M.R. Platelet hyperactivation in multiple myeloma is also evident in patients with premalignant monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2021, 192, 322–332. [Google Scholar] [CrossRef]
- Baccouche, H.; Hadhri, M.; Aissi, W.; Chakroun, A.; Bahri, D.; Mahjoub, S.; Ben Romdhane, N. The hypercoagulable state in multiple myeloma: The contribution of thrombin generation test. Int. J. Lab. Hematol. 2019, 41, 684–690. [Google Scholar] [CrossRef]
- Chalayer, E.; Tardy-Poncet, B.; Karlin, L.; Chapelle, C.; Montmartin, A.; Piot, M.; Guyotat, D.; Collet, P.; Lecompte, T.; Tardy, B. Thrombin generation in newly diagnosed multiple myeloma during the first three cycles of treatment: An observational cohort study. Res. Pract. Thromb. Haemost. 2019, 3, 89–98. [Google Scholar] [CrossRef]
- Gracheva, M.A.; Urnova, E.S.; Sinauridze, E.I.; Tarandovskiy, I.D.; Orel, E.B.; Poletaev, A.V.; Mendeleeva, L.P.; Ataullakhanov, F.I.; Balandina, A.N. Thromboelastography, thrombin generation test and thrombodynamics reveal hypercoagulability in patients with multiple myeloma. Leuk. Lymphoma 2015, 56, 3418–3425. [Google Scholar] [CrossRef]
- Ye, R.; Ye, C.; Huang, Y.; Liu, L.; Wang, S. Circulating tissue factor positive microparticles in patients with acute recurrent deep venous thrombosis. Thromb. Res. 2012, 130, 253–258. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zhang, H.B.; Liu, Y.H.; Zhang, F.L.; Zhu, Y.Z.; Li, Y.; Bai, J.P.; Liu, L.R.; Qu, Y.C.; Qu, X.; et al. Quantitative cell-free circulating EGFR mutation concentration is correlated with tumor burden in advanced NSCLC patients. Lung Cancer 2017, 109, 124–127. [Google Scholar] [CrossRef]
- O’Connell, G.C.; Petrone, A.B.; Tennant, C.S.; Lucke-Wold, N.; Kabbani, Y.; Tarabishy, A.R.; Chantler, P.D.; Barr, T.L. Circulating extracellular DNA levels are acutely elevated in ischaemic stroke and associated with innate immune system activation. Brain Inj. 2017, 31, 1369–1375. [Google Scholar] [CrossRef]
- Li, M.; Lin, C.; Deng, H.; Strnad, J.; Bernabei, L.; Vogl, D.T.; Burke, J.J.; Nefedova, Y. A Novel Peptidylarginine Deiminase 4 (PAD4) Inhibitor BMS-P5 Blocks Formation of Neutrophil Extracellular Traps and Delays Progression of Multiple Myeloma. Mol. Cancer 2020, 19, 1530–1538. [Google Scholar] [CrossRef]
- Palumbo, A.; Palladino, C. Venous and arterial thrombotic risks with thalidomide: Evidence and practical guidance. Adv. Drug Saf. 2012, 3, 255–266. [Google Scholar] [CrossRef]
- Fouquet, G.; Tardy, S.; Demarquette, H.; Bonnet, S.; Gay, J.; Debarri, H.; Herbaux, C.; Guidez, S.; Michel, J.; Perrot, A.; et al. Efficacy and safety profile of long-term exposure to lenalidomide in patients with recurrent multiple myeloma. Cancer 2013, 119, 3680–3686. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.E.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R.; et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef]
- Zonder, J.A.; Crowley, J.; Hussein, M.A.; Bolejack, V.; Moore, D.F., Sr.; Whittenberger, B.F.; Abidi, M.H.; Durie, B.G.; Barlogie, B. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: A randomized Southwest Oncology Group trial (S0232). Blood 2010, 116, 5838–5841. [Google Scholar] [CrossRef]
- Bradbury, C.A.; Craig, Z.; Cook, G.; Pawlyn, C.; Cairns, D.A.; Hockaday, A.; Paterson, A.; Jenner, M.W.; Jones, J.R.; Drayson, M.T.; et al. Thrombosis in patients with myeloma treated in the Myeloma IX and Myeloma XI phase 3 randomized controlled trials. Blood 2020, 136, 1091–1104. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Swern, A.S.; Li, J.S.; Hussein, M.; Weiss, L.; Nagarwala, Y.; Baz, R. Efficacy and safety of long-term treatment with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Cancer J. 2014, 4, e257. [Google Scholar] [CrossRef]
- Richardson, P.G.; Siegel, D.S.; Vij, R.; Hofmeister, C.C.; Baz, R.; Jagannath, S.; Chen, C.; Lonial, S.; Jakubowiak, A.; Bahlis, N.; et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: A randomized phase 2 study. Blood 2014, 123, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Leleu, X.; Attal, M.; Arnulf, B.; Moreau, P.; Traulle, C.; Marit, G.; Mathiot, C.; Petillon, M.O.; Macro, M.; Roussel, M.; et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009–2002. Blood 2013, 121, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Pegourie, B.; Karlin, L.; Benboubker, L.; Orsini-Piocelle, F.; Tiab, M.; Auger-Quittet, S.; Rodon, P.; Royer, B.; Leleu, X.; Bareau, B.; et al. Apixaban for the prevention of thromboembolism in immunomodulatory-treated myeloma patients: Myelaxat, a phase 2 pilot study. Am. J. Hematol. 2019, 94, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, C. Thrombotic Events in Patients with Myeloma Treated with Immunomodulatory Drugs; Results of the Myeloma XI Study. Blood 2017, 130, 553. [Google Scholar]
- Storrar, N.P.F.; Mathur, A.; Johnson, P.R.E.; Roddie, P.H. Safety and efficacy of apixaban for routine thromboprophylaxis in myeloma patients treated with thalidomide- and lenalidomide-containing regimens. Br. J. Haematol. 2019, 185, 142–144. [Google Scholar] [CrossRef]
- Cornell, R.F.; Goldhaber, S.Z.; Engelhardt, B.G.; Moslehi, J.; Jagasia, M.; Harrell, S.; Rubinstein, S.M.; Hall, R.; Wyatt, H.; Piazza, G. Primary prevention of venous thromboembolism with apixaban for multiple myeloma patients receiving immunomodulatory agents. Br. J. Haematol. 2020, 190, 555–561. [Google Scholar] [CrossRef]
- Facon, T.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.V.; Belch, A.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.J.; et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood 2018, 131, 301–310. [Google Scholar] [CrossRef]
- Hou, J.; Du, X.; Jin, J.; Cai, Z.; Chen, F.; Zhou, D.B.; Yu, L.; Ke, X.; Li, X.; Wu, D.; et al. A Multicenter, Open-Label, Phase 2 Study of Lenalidomide Plus Low-Dose Dexamethasone in Chinese Patients with Relapsed/Refractory Multiple Myeloma: The Mm-021 Trial. J. Hematol. Oncol. 2013, 6, 41. [Google Scholar] [CrossRef]
- Hou, J.; Jin, J.; Xu, Y.; Wu, D.; Ke, X.; Zhou, D.; Lu, J.; Du, X.; Chen, X.; Li, J.; et al. Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China Continuation study. J. Hematol. Oncol. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Spicka, I.; Oriol, A.; Hajek, R.; Rosinol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef]
- Richardson, P.G.; Xie, W.; Jagannath, S.; Jakubowiak, A.; Lonial, S.; Raje, N.S.; Alsina, M.; Ghobrial, I.M.; Schlossman, R.L.; Munshi, N.C.; et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood 2014, 123, 1461–1469. [Google Scholar] [CrossRef]
- Durie, B.G.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Kumar, S.K.; Jacobus, S.J.; Cohen, A.D.; Weiss, M.; Callander, N.; Singh, A.K.; Parker, T.L.; Menter, A.; Yang, X.; Parsons, B.; et al. Carfilzomib or Bortezomib in Combination with Lenalidomide and Dexamethasone for Patients with Newly Diagnosed Multiple Myeloma without Intention for Immediate Autologous Stem-Cell Transplantation (Endurance): A Multicentre, Open-Label, Phase 3, Randomised, Controlled Trial. Lancet. Oncol. 2020, 21, 1317–1330. [Google Scholar]
- Piedra, K.; Peterson, T.; Tan, C.; Orozco, J.; Hultcrantz, M.; Hassoun, H.; Mailankody, S.; Lesokhin, A.; Shah, U.; Lu, S.; et al. Comparison of Venous Thromboembolism Incidence in Newly Diagnosed Multiple Myeloma Patients Receiving Bortezomib, Lenalidomide, Dexamethasone (Rvd) or Carfilzomib, Lenalidomide, Dexamethasone (Krd) with Aspirin or Rivaroxaban Thromboprophylaxis. Br. J. Haematol. 2022, 196, 105–109. [Google Scholar] [CrossRef]
- Sayar, Z.; Gates, C.; Bristogiannis, S.; Patel, A.; Ogunbiyi, M.O.; Tailor, A.; Yong, K.; Thomas, M. Safety and efficacy of apixaban as thromboprophylaxis in myeloma patients receiving chemotherapy: A prospective cohort study. Thromb. Res. 2022, 213, 27–29. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Moreau, and Pollux Investigators. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Sonneveld, and Castor Investigators. “Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma”. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Sborov, D.; Baljevic, M.; Reeves, B.; Laubach, J.; Efebera, Y.; Rodriguez, C.; Costa, l.; Chari, A.; Silbermann, R.; Holstein, S.; et al. Daratumumab (Dara) Plus Lenalidomide, Bortezomib, and Dexamethasone (Rvd) in Newly Diagnosed Multiple Myeloma (Ndmm): Analysis of Vascular Thrombotic Events (Vtes) in the Griffin Study [Abstract]. Clin. Lymphoma Myeloma 2021, 21, 135–136. [Google Scholar] [CrossRef]
- Rupa-Matysek, J.; Gil, L.; Wojtasińska, E.; Nowicki, A.; Dytfeld, D.; Kaźmierczak, M.; Komarnicki, M. Inhibitory effects of bortezomib on platelet aggregation in patients with multiple myeloma. Thromb. Res. 2014, 134, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Jilma, B.; Cvitko, T.; Winter-Fabry, A.; Petroczi, K.; Quehenberger, P.; Blann, A.D. High dose dexamethasone increases circulating P-selectin and von Willebrand factor levels in healthy men. Thromb. Haemost. 2005, 94, 797–801. [Google Scholar] [CrossRef]
- Swystun, L.L.; Shin, L.Y.Y.; Beaudin, S.; Liaw, P.C. Chemotherapeutic agents doxorubicin and epirubicin induce a procoagulant phenotype on endothelial cells and blood monocytes. J. Thromb. Haemost. 2009, 7, 619–626. [Google Scholar] [CrossRef]
- Rosenthal, A.; Luthi, J.; Belohlavek, M.; Kortum, K.M.; Mookadam, F.; Mayo, A.; Fonseca, R.; Bergsagel, P.L.; Reeder, C.B.; Mikhael, J.R.; et al. Carfilzomib and the Cardiorenal System in Myeloma: An Endothelial Effect? Blood Cancer, J. 2016, 6, e384. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Park, C.; Arroyo-Suarez, R. Venous thromboembolism in patients with multiple myeloma receiving daratumumab-based regimens: A post hoc analysis of phase 3 clinical trials. Leuk. Lymphoma 2021, 62, 2219–2226. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Carson, K.R.; Wang, T.; Luo, S.; Edwin, N.; Kuderer, N.; Keller, J.M.; Gage, B.F. Evaluation of the Khorana score for prediction of venous thromboembolism in patients with multiple myeloma. Res. Pr. Thromb. Haemost. 2022, 6, e12634. [Google Scholar] [CrossRef]
- Barrett, A.; Quinn, J.; Lavin, M.; Thornton, P.; O’Donnell, J.; Murphy, P.; Glavey, S. Validation of Risk-Adapted Venous Thromboembolism Prediction in Multiple Myeloma Patients. J. Clin. Med. 2021, 10, 3536. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wu, Q.; Luo, S.; Warnick, G.S.; Zakai, N.A.; Libby, E.N.; Gage, B.F.; Garcia, D.A.; Lyman, G.H.; Sanfilippo, K.M. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug–Associated Thrombosis Among Patients With Multiple Myeloma. J. Natl. Compr. Cancer Netw. 2019, 17, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.A.; Brown, A.R.; Mahnken, J.D.; Shireman, T.I.; Webb, C.E.; Lipe, B.C. Application of Risk Factors for Venous Thromboembolism in Patients with Multiple Myeloma Starting Chemotherapy, a Real-World Evaluation. Cancer Med. 2019, 8, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Covut, F.; Ahmed, R.; Chawla, S.; Ricaurte, F.; Samaras, C.J.; Anwer, F.; Garcia, A.V.M.; Angelini, D.E.; Mazzoni, S.; Faiman, B.; et al. Validation of the IMPEDE VTE score for prediction of venous thromboembolism in multiple myeloma: A retrospective cohort study. Br. J. Haematol. 2021, 193, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, K.M. Assessing the risk of venous thromboembolism in multiple myeloma. Thromb. Res. 2020, 191, S74–S78. [Google Scholar] [CrossRef]
- Callander, N.S.; Baljevic, M.; Adekola, K.; Anderson, L.D.; Campagnaro, E.; Castillo, J.J.; Costello, C.; Devarakonda, S.; Elsedawy, N.; Faiman, M.; et al. Nccn Guidelines(R) Insights: Multiple Myeloma, Version 3.2022. J. Natl. Compr. Canc. Netw. 2022, 20, 8–19. [Google Scholar] [CrossRef]
- Fotiou, D.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Migkou, M.; Dimopoulos, M.A.; Terpos, E. Updates on thrombotic events associated with multiple myeloma. Expert Rev. Hematol. 2019, 12, 355–365. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Fiala, M.; Tathireddy, H.; Feinberg, D.; Vij, R.; Gage, B.F. D-Dimer Improves Risk Prediction of Venous Thromboembolism in Patients with Multiple Myeloma [Abstract]. Blood 2020, 136, 26–27. [Google Scholar] [CrossRef]
- Kahale, L.A.; Matar, C.F.; Tsolakian, I.; Hakoum, M.B.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Barba, M.; Hicks, L.K.; Schunemann, H.; et al. Antithrombotic Therapy for Ambulatory Patients with Multiple Myeloma Receiving Immunomodulatory Agents. Cochrane Database Syst. Rev. 2021, 9, CD014739. [Google Scholar]
- Al-Ani, F.; Bermejo, J.M.B.; Mateos, M.-V.; Louzada, M. Thromboprophylaxis in multiple myeloma patients treated with lenalidomide – A systematic review. Thromb. Res. 2016, 141, 84–90. [Google Scholar] [CrossRef]
- Zoppellaro, G.; Veronese, N.; Granziera, S.; Gobbi, L.; Stubbs, B.; Cohen, A.T. Primary thromboembolic prevention in multiple myeloma patients: An exploratory meta-analysis on aspirin use. Semin. Hematol. 2018, 55, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, K.M.; Luo, S.; Carson, K.R.; Cage, B.F. Aspirin May Be Inadequate Thromboprophylaxis in Multiple Myeloma. Blood 2017, 130, 3419. [Google Scholar]
- Swan, D.; Rocci, A.; Bradbury, C.; Thachil, J. Venous Thromboembolism in Multiple Myeloma - Choice of Prophylaxis, Role of Direct Oral Anticoagulants and Special Considerations. Br. J. Haematol. 2018, 183, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. New Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. New Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
- Lim, M.S.; Enjeti, A. Safety of anticoagulation in the treatment of venous thromboembolism in patients with haematological malignancies and thrombocytopenia: Report of 5 cases and literature review. Crit. Rev. Oncol. 2016, 105, 92–99. [Google Scholar] [CrossRef][Green Version]
- Khanal, N.; Bociek, R.G.; Chen, B.; Vose, J.M.; Armitage, J.O.; Bierman, P.J.; Maness, L.J.; Lunning, M.A.; Gundabolu, K.; Bhatt, V.R. Venous thromboembolism in patients with hematologic malignancy and thrombocytopenia. Am. J. Hematol. 2016, 91, E468–E472. [Google Scholar] [CrossRef]
- Napolitano, M.; Saccullo, G.; Marietta, M.; Carpenedo, M.; Castaman, G.; Cerchiara, E.; Chistolini, A.; Contino, L.; De Stefano, V.; Falanga, A.; et al. Platelet cut-off for anticoagulant therapy in thrombocytopenic patients with blood cancer and venous thromboembolism: An expert consensus. Blood Transfus. 2019, 17, 171–180. [Google Scholar]
- Terpos, E.; Kleber, M.; Engelhardt, M.; Zweegman, S.; Gay, F.; Kastritis, E.; van de Donk, N.W.; Bruno, B.; Sezer, O.; Broijl, A.; et al. European Myeloma Network Guidelines for the Management of Multiple Myeloma-related Complications. Haematologica 2015, 100, 1254–1266. [Google Scholar] [CrossRef]
- Nccn Clinical Practice Guidelines in Oncology (Nccn Guidelines): Cancer-Associated Venous Thromboembolic Disease. National Comprehensive Cancer Network. Available online: http:www.nccn.org/professionals/physician_gls/pdf/vte.pdf. (accessed on 12 December 2022).
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. New Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G. Direct Oral Anticoagulants for Thromboprophylaxis in Ambulatory Patients with Cancer. New Engl. J. Med. 2019, 380, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Moik, F.; Posch, F.; Zielinski, C.; Pabinger, I.; Ay, C. Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: Updated systematic review and meta-analysis of randomized controlled trials. Res. Pr. Thromb. Haemost. 2020, 4, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Libourel, E.J.; Sonneveld, P.; van der Holt, B.; de Maat, M.P.M.; Leebeek, F.W.G. High incidence of arterial thrombosis in young patients treated for multiple myeloma: Results of a prospective cohort study. Blood 2010, 116, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Rybicki, L.; Valent, J.; Garcia, A.V.M.; Faiman, B.M.; Khouri, J.; Samaras, C.J.; Anwer, F.; Khorana, A.A. Arterial thromboembolism in multiple myeloma in the context of modern anti-myeloma therapy. Blood Cancer J. 2021, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, S.; Chang, S.; Seegobin, K.; Serrano-Santiago, I.; Zuberi, L. Increased risk of arterial thromboembolic events with combination lenalidomide/dexamethasone therapy for multiple myeloma. Expert Rev. Anticancer Ther. 2017, 17, 585–591. [Google Scholar] [CrossRef]
- Agnelli, G.; Gussoni, G.; Bianchini, C.; Verso, M.; Mandala, M.; Cavanna, L.; Barni, S.; Labianca, R.; Buzzi, F.; Scambia, G.; et al. Nadroparin for the Prevention of Thromboembolic Events in Ambulatory Patients with Metastatic or Locally Advanced Solid Cancer Receiving Chemotherapy: A Randomised, Placebo-Controlled, Double-Blind Study. Lancet. Oncol. 2009, 10, 943–949. [Google Scholar] [CrossRef]
- Khorana, A.A.; McNamara, M.G.; Kakkar, A.K.; Streiff, M.B.; Riess, H.; Vijapurkar, U.; Kaul, S.; Wildgoose, P.; Soff, G.A. on behalf of the CASSINI Investigators Assessing Full Benefit of Rivaroxaban Prophylaxis in High-Risk Ambulatory Patients with Cancer: Thromboembolic Events in the Randomized CASSINI Trial. TH Open 2020, 04, e107–e112. [Google Scholar]

| n | Regimen | Thromboprophylaxis | FU | VTE | ||
|---|---|---|---|---|---|---|
| Larocca et al., 2012 [19] | 176 166 | ND ND | Rd Rd | ASA 100 mg Enoxaparin 40 mg | 6 m 6 m | 8.2% 2.2% |
| Pegourie et al., 2019 [54] | 104 | RR/ND | Thal or Len | Apixaban 2.5 mg BID | 6 m | 1.9% |
| Fouquet et al., 2013 [46] | 50 | RR | Rd | All patients with event except 1 received aspirin, or LMWH or VKA | 4 years | 20% |
| Bradbury et al., 2017 [55] | 3838 | ND | CTD or CRD | 87.7% of patients with event were on aspirin, LMWH, VKA | 6 m | 11.8% |
| Dimopoulos et al., 2014 [50] | 353 | RR | Rd | Not required per protocol | 49 m | 12.7% |
| Rajkumar et al., 2010 [47] | 223 220 | ND | R-D R-d | Thromboprophylaxis recommended and then mandatory | 12.5 m | 20% 9% |
| Storrar et al., 2019 [56] | 70 | ND | Len 21.5% Thal 78.5% | Apixaban 2.5 mg BID | 6 m | 0% |
| Cornell et al., 2020 [57] | 50 | All | Len 58% Pom 42% | Apixaban 2.5 mg BID | 6 m | 0% |
| Facon et al., 2018 [58] | 1070 | ND | Rdc/Rd18 | NR | 67 m | 1.5% |
| Hou et al., 2013 [59] | 199 | RR | Rd | LMWH/warfarin for high-risk ASA for low-risk | 15 m | 0.5% |
| Hou et al., 2017 [60] | 58 57 | RR | Rd IRd | 98% on thromboprophylaxis | 20 m | 0% |
| Lonial et al., 2015 [61] | 318 317 | RR | EloRd Rd | NR applied per institutional practice | 24.5 m | 6.6% 3.8% |
| Moreau et al., 2016 [62] | 351 361 | RR | Rd IRd | NR but almost all pts received | 15 m | 11% 8% |
| Stewart et al., 2015 [63] | 389 392 | RR | Rd KRd | NR | 31.5 m | 10.2% 6.2% |
| Richardson et al., 2014 [51] | 108 221 | RR | Pom PomDex | Aspirin unless contraindicated | 14 m | 3% 2% |
| Richardson et al., 2010 [64] | 66 | ND | VRD | At least aspirin in all patients | 21 m | 10% |
| Richardson 2014 [51,65] | 64 | RR | VRD | Aspirin, LMWH or VKA | 44 m | 1.6% |
| Durie et al., 2017 [66] | 241 226 | VRD Rd | Aspirin 325 mg | 55 m | ||
| San Miguel et al., 2013 [52] | 300 150 | RR | Pomd PomD | physicians’ discretion | 10 m | 2% 1% |
| Leleu et al., 2013 [53] | 92 | RR | Pd | 70% aspirin, 40.5% LMWH, 14% VKA | 23 m | 4% |
| Kumar et al., 2020 [67] | 527 526 | ND | VRd KRd | NR | 5 years | 2.5% 5.7% |
| Piedra et al., 2022 [68] | 124 99 82 | ND | VRd KRd KRd | ASA 81 mg ASA 81 mg Rivaroxaban 10 mg OD | 3 m | 4.8% 16.1% 4.8% |
| Sayar et al., 2022 [69] | 98 82 | RR/ND | IMIDs | ASA 75 mg Apixaban 2.5 mg od | NR | 4% 0% |
| Dimopoulos et al., 2016 [70] | 285 281 | RR | DRd Rd | ASA 77.7%, LMWH 24%, VKA 2.3%, DOAC 3% | 13 m | All 5.5% * |
| Palumbo et al., 2016 [71] | 243 237 | RR | DVd Vd | ASA 21.5%, LMWH 10.6%, VKA 1.5% DOAC 2.4% | 7 m | All 2.1% * |
| Facon et al., 2019 [72] | 364 265 | ND | DRd Rd | N/A data but LMWH or VKA required by protocol | 28 m | All 12.4% * |
| Sborov et al., 2022 [73] | 104 103 | ND | D-VRd VRd | Per IMWG guidelines | 38.6 m | 10% 15.7% |
| (a) | ||
|---|---|---|
| IWMG Score and Algorithm for MM Patient Risk Stratification | ||
| Patient-Related Risk Factors 1 Point for Each | Disease-Related Risk Factors: 1 Point for Each | Treatment-Related Risk Factors: Points as Seen Below: |
| Body mass index >25, age > 75, Personal or family history of VTE, Central venous catheter, Acute infection or Hospitalization, Blood clotting disorders or Thrombophilia, Immobility with a performance status of >1, Comorbidities (liver, renal impairment, Chronic obstructive pulmonary disorder, diabetes mellitus, chronic inflammatory bowel disease), Race (Caucasian) |
|
|
| Risk stratification and recommended thromboprophylaxis: 0 points: Low risk—None 1 point: Intermediate risk—Aspirin at 100 mg >1 point: High risk—Low molecular weight heparin at prophylactic dose or therapeutic dose of warfarin | ||
| (b) | ||
| CLINICAL RAMs for VTE in MM | ||
| IMPEDE VTE Score | SAVED * Score | |
| Immunomodulatory drug (+4) BMI ≥ 25 kg/m2 (+1) Pathologic fracture pelvis/femur (+4) Erythropoiesis-stimulating agent (+1) Dexamethasone (High-dose, ≥1600 mg/cycle) (+4) Dexamethasone Low-Dose (<160 mg/cycle) (+2) Doxorubicin (+3) Ethnicity/Race = Asian (−3) VTE history (+5) Tunnelled line/CVC (+2) Existing use of therapeutic warfarin or low molecular weight heparin (LWMH) (−5) Existing use of prophylactic LMWH or aspirin (−3) | Surgery (within the last 90 days) (+2) Asian Race (−3) VTE history (+3) Eighty (age ≥ 80 y) (+1) Dexamethasone dose Standard (120–160 mg/cycle) (+1) High (>160 mg/cycle) (+2) | |
| Stratified risk groups based on a weighted scoring system | ||
| Low risk (score of ≤3) Intermediate-risk (score of 4–7) High risk (score of ≥8) | High risk (score of ≥2) Low risk (≤1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotiou, D.; Dimopoulos, M.A.; Kastritis, E. Approach to Contemporary Risk Assessment, Prevention and Management of Thrombotic Complications in Multiple Myeloma. Cancers 2022, 14, 6216. https://doi.org/10.3390/cancers14246216
Fotiou D, Dimopoulos MA, Kastritis E. Approach to Contemporary Risk Assessment, Prevention and Management of Thrombotic Complications in Multiple Myeloma. Cancers. 2022; 14(24):6216. https://doi.org/10.3390/cancers14246216
Chicago/Turabian StyleFotiou, Despina, Meletios Athanasios Dimopoulos, and Efstathios Kastritis. 2022. "Approach to Contemporary Risk Assessment, Prevention and Management of Thrombotic Complications in Multiple Myeloma" Cancers 14, no. 24: 6216. https://doi.org/10.3390/cancers14246216
APA StyleFotiou, D., Dimopoulos, M. A., & Kastritis, E. (2022). Approach to Contemporary Risk Assessment, Prevention and Management of Thrombotic Complications in Multiple Myeloma. Cancers, 14(24), 6216. https://doi.org/10.3390/cancers14246216






