Best Supportive Care of the Patient with Oesophageal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
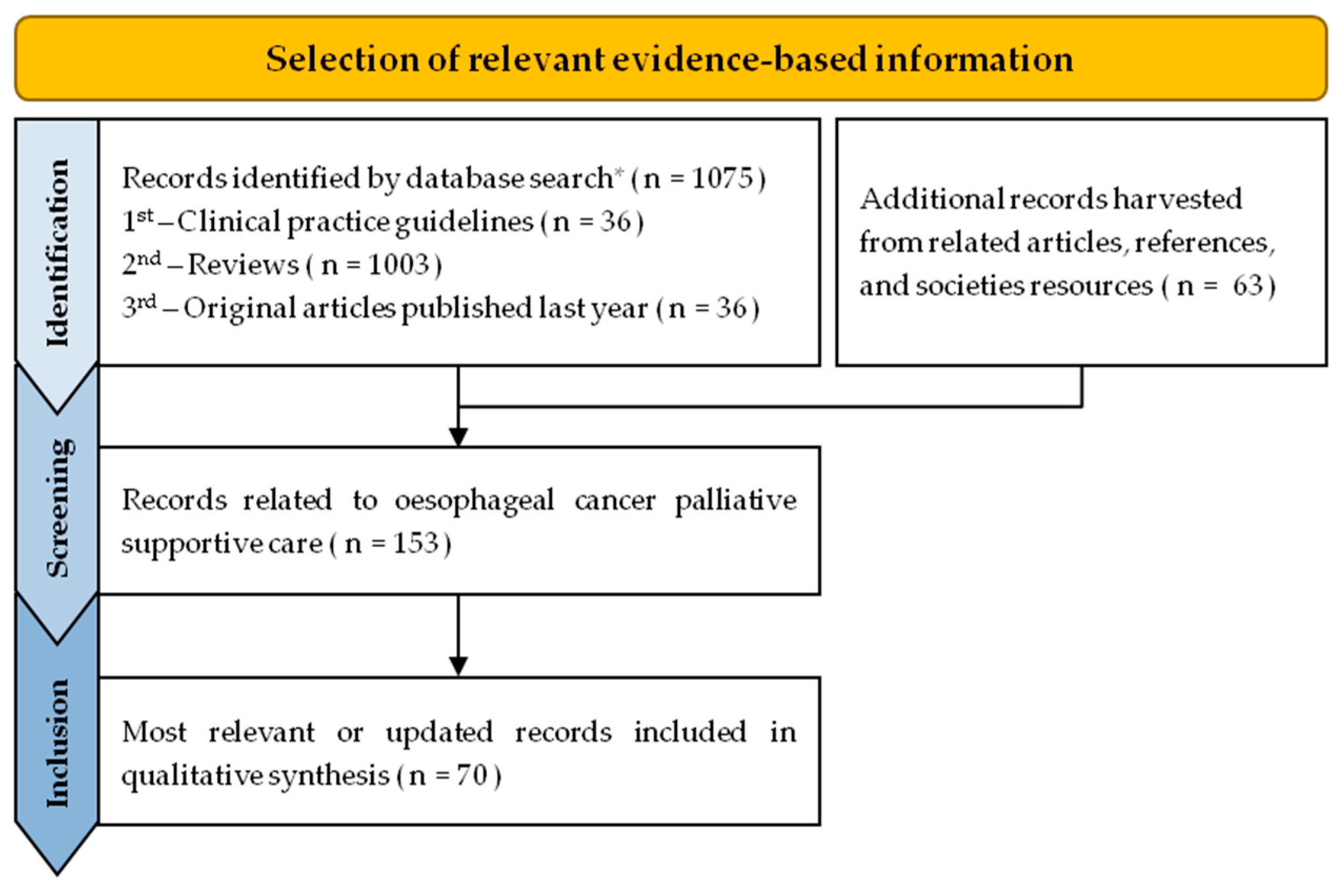
2. Methods
3. Results and Discussion
3.1. Management of Oesophageal Cancer Symptoms or Complications
3.1.1. Dysphagia or Obstruction
3.1.2. Malnutrition
3.1.3. Pain
3.1.4. Nausea and Vomiting
3.1.5. Fistula
3.1.6. Bleeding and Anaemia
3.2. Special Considerations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO); International Agency for Research on Cancer (IARC). Global Cancer Observatory (GLOBOCAN): Cancer Today. 2020. Available online: http://gco.iarc.fr/today (accessed on 25 August 2022).
- Kamangar, F.; Nasrollahzadeh, D.; Safiri, S.; Sepanlou, S.G.; Fitzmaurice, C.; Ikuta, K.S.; Bisignano, C.; Islami, F.; Roshandel, G.; Lim, S.S.; et al. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Ana da Costa Miranda, A.M.-d.-S.; Glória, L.; Brito, C. Registo Oncológico Nacional de Todos os Tumores na População Residente em Portugal, em 2018; Reprografia do IPO de Lisboa de Francisco Gentil; E.P.E.: Lisboa, Portugal, 2021. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Dandara, C.; Robertson, B.; Dzobo, K.; Moodley, L.; Parker, M.I. Patient and tumour characteristics as prognostic markers for oesophageal cancer: A retrospective analysis of a cohort of patients at Groote Schuur Hospital. Eur. J. Cardio-Thorac. Surg. 2015, 49, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.; Vogel, A.; Smyth, E. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 855–883. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Uno, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawamura, O.; Kusano, M.; Kuwano, H.; Takeuchi, H.; et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: Part 2. Esophagus Off. J. Jpn. Esophageal Soc. 2019, 16, 25–43. [Google Scholar] [CrossRef]
- Opstelten, J.L.; De Wijkerslooth, L.R.H.; Leenders, M.; Bac, D.J.; Brink, M.A.; Loffeld, B.C.A.J.; Meijnen-Bult, M.J.F.; Minderhoud, I.M.; Verhagen, M.A.M.T.; Van Oijen, M.G.H.; et al. Variation in palliative care of esophageal cancer in clinical practice: Factors associated with treatment decisions. Dis. Esophagus 2016, 30, 1–7. [Google Scholar] [CrossRef]
- Brierley, J.D.; Oza, A. Radiation and Chemotherapy in the Management of Malignant Esophageal Strictures. Gastrointest. Endosc. Clin. N. Am. 1998, 8, 451–463. [Google Scholar] [CrossRef]
- Levy, A.; Wagner, A.D.; Chargari, C.; Moehler, M.; Verheij, M.; Durand-Labrunie, J.; Kissel, M.; Chirat, E.; Burtin, P.; Ducreux, M.; et al. Palliation of dysphagia in metastatic oesogastric cancers: An international multidisciplinary position. Eur. J. Cancer 2020, 135, 103–112. [Google Scholar] [CrossRef]
- Spaander, M.C.W.; van der Bogt, R.D.; Baron, T.H.; Albers, D.; Blero, D.; de Ceglie, A.; Conio, M.; Czakó, L.; Everett, S.; Garcia-Pagán, J.-C.; et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 2021, 53, 751–762. [Google Scholar] [CrossRef]
- Ahmed, O.; Lee, J.H.; Thompson, C.C.; Faulx, A. AGA Clinical Practice Update on the Optimal Management of the Malignant Alimentary Tract Obstruction: Expert Review. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, C.; Fu, W.; Xu, W.; Yang, S. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst. Rev. 2014, 10, CD005048. [Google Scholar] [CrossRef]
- Reijm, A.N.; Didden, P.; Schelling, S.J.C.; Siersema, P.D.; Bruno, M.J.; Spaander, M.C.W. Self-expandable metal stent placement for malignant esophageal strictures—Changes in clinical outcomes over time. Endoscopy 2018, 51, 18–29. [Google Scholar] [CrossRef]
- Van Rossum, P.S.N.; Mohammad, N.H.; Vleggaar, F.P.; Van Hillegersberg, R. Treatment for unresectable or metastatic oesophageal cancer: Current evidence and trends. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Homs, M.Y.; Steyerberg, E.W.; Eijkenboom, W.M.; Tilanus, H.W.; Stalpers, L.J.; Bartelsman, J.F.; van Lanschot, J.J.; Wijrdeman, H.K.; Mulder, C.J.; Reinders, J.G.; et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: Multicentre randomised trial. Lancet 2004, 364, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.J.; Bruggeman, A.R.; Nalawade, V.V.; Sarkar, R.R.; Qiao, E.M.; Rose, B.S.; Murphy, J.D. Palliative Radiotherapy Versus Esophageal Stent Placement in the Management of Patients With Metastatic Esophageal Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 569–574. [Google Scholar] [CrossRef]
- Sinha, S.; Varagunam, M.; Park, M.; Maynard, N.; Trudgill, N.; Crosby, T.; Cromwell, D. Brachytherapy in the Palliation of Oesophageal Cancer: Effective but Impractical? Clin. Oncol. 2019, 31, e87–e93. [Google Scholar] [CrossRef]
- Merchant, S.J.; Kong, W.; Mahmud, A.; Booth, C.M.; Hanna, T.P. Palliative Radiotherapy for Esophageal and Gastric Cancer: Population-Based Patterns of Utilization and Outcomes in Ontario, Canada. J. Palliat. Care 2022. [Google Scholar] [CrossRef]
- Jeene, P.M.; Vermeulen, B.D.; Rozema, T.; Braam, P.M.; Lips, I.; Muller, K.; van Kampen, D.; Homs, M.Y.; Oppedijk, V.; Berbée, M.; et al. Short-Course External Beam Radiotherapy Versus Brachytherapy for Palliation of Dysphagia in Esophageal Cancer: A Matched Comparison of Two Prospective Trials. J. Thorac. Oncol. 2020, 15, 1361–1368. [Google Scholar] [CrossRef]
- van Rossum, P.S.; Jeene, P.M.; Rozema, T.; Braam, P.M.; Lips, I.M.; Muller, K.; van Kampen, D.; Vermeulen, B.D.; Homs, M.Y.; Oppedijk, V.; et al. Patient-reported outcomes after external beam radiotherapy versus brachytherapy for palliation of dysphagia in esophageal cancer: A matched comparison of two prospective trials. Radiother. Oncol. 2020, 155, 73–79. [Google Scholar] [CrossRef]
- Ólafsdóttir, H.S.; Klevebro, F.; Ndegwa, N.; von Döbeln, G.A. Short-course compared to long-course palliative radiotherapy for oesophageal cancer: A single centre observational cohort study. Radiat. Oncol. 2021, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Adamson, D.; Byrne, A.; Porter, C.; Blazeby, J.; Griffiths, G.; Nelson, A.; Sewell, B.; Jones, M.; Svobodova, M.; Fitzsimmons, D.; et al. Palliative radiotherapy after oesophageal cancer stenting (ROCS): A multicentre, open-label, phase 3 randomised controlled trial. Lancet Gastroenterol. Hepatol. 2021, 6, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; De Montreuil, C.B.; Schneider, S.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients With Cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ottery, F.D. Supportive nutrition to prevent cachexia and improve quality of life. Semin. Oncol. 1995, 22, 98–111. [Google Scholar]
- Nitenberg, G.; Raynard, B. Nutritional support of the cancer patient: Issues and dilemmas. Crit. Rev. Oncol. Hematol. 2000, 34, 137–168. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Silvers, M.A.; Savva, J.; Huggins, C.E.; Truby, H.; Haines, T. Potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: A pilot randomised trial. Support. Care Cancer 2014, 22, 3035–3044. [Google Scholar] [CrossRef]
- Huhmann, M.B.; August, D. Review of American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Clinical Guidelines for Nutrition Support in Cancer Patients: Nutrition Screening and Assessment. Nutr. Clin. Pract. 2008, 23, 182–188. [Google Scholar] [CrossRef]
- Alderman, B.; Allan, L.; Amano, K.; Bouleuc, C.; Davis, M.; Lister-Flynn, S.; Mukhopadhyay, S.; Davies, A. Multinational Association of Supportive Care in Cancer (MASCC) expert opinion/guidance on the use of clinically assisted nutrition in patients with advanced cancer. Support. Care Cancer 2021, 30, 2983–2992. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines☆. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Furness, K.; Silvers, M.A.; Savva, J.; Huggins, C.E.; Truby, H.; Haines, T. Long-term follow-up of the potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: A pilot randomised trial. Support. Care Cancer 2017, 25, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Crawford, G.; Dzierżanowski, T.; Hauser, K.; Larkin, P.; Luque-Blanco, A.; Murphy, I.; Puchalski, C.; Ripamonti, C. Care of the adult cancer patient at the end of life: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100225. [Google Scholar] [CrossRef]
- Cereda, E.; Pedrolli, C.A.S.P.E.N. Recommendations for Enteral Nutrition: Practice Is the Result of Potential Benefits, Harms, Clinical Judgment, and Ethical Issues. J. Parenter. Enter. Nutr. 2009, 34, 103. [Google Scholar] [CrossRef] [PubMed]
- Nunes, G.; Fonseca, J.; Barata, A.T.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Nutritional Support of Cancer Patients without Oral Feeding: How to Select the Most Effective Technique? GE-Port. J. Gastroenterol. 2019, 27, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ukleja, A.; Freeman, K.L.; Gilbert, K.; Kochevar, M.; Kraft, M.D.; Russell, M.K.; Shuster, M.H. Standards for Nutrition Support. Nutr. Clin. Pract. 2010, 25, 403–414. [Google Scholar] [CrossRef]
- Bozzetti, F.; Cozzaglio, L.; Biganzoli, E.; Chiavenna, G.; DE Cicco, M.; Donati, D.; Gilli, G.; Percolla, S.; Pironi, L. Quality of life and length of survival in advanced cancer patients on home parenteral nutrition. Clin. Nutr. 2002, 21, 281–288. [Google Scholar] [CrossRef]
- Guyer, D.L.; Almhanna, K.; McKee, K.Y. Palliative care for patients with esophageal cancer: A narrative review. Ann. Transl. Med. 2020, 8, 1103. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; McNicol, E.D.; Bell, R.F.; Carr, D.B.; McIntyre, M.; Wee, B. Oral paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst. Rev. 2017, 2020, CD012637. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Derry, S.; Wiffen, P.J.; Moore, R.A.; McNicol, E.D.; Bell, R.F.; Carr, D.B.; McIntyre, M.; Wee, B. Oral nonsteroidal anti-inflammatory drugs (NSAIDs) for cancer pain in adults. Cochrane Database Syst. Rev. 2017, 2020, CD012638. [Google Scholar] [CrossRef]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, J.; Stamer, U.M.; Remi, C.; Voltz, R.; Bausewein, C.; Sabatowski, R.; Wirz, S.; Müller-Mundt, G.; Simon, S.T.; Pralong, A.; et al. Metamizole/dipyrone for the relief of cancer pain: A systematic review and evidence-based recommendations for clinical practice. Palliat. Med. 2016, 31, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Wee, B.; Moore, R.A. Oral morphine for cancer pain. Cochrane Database Syst. Rev. 2016, 2021, CD003868. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Wee, B.; Derry, S.; Bell, R.F.; Moore, R.A. Opioids for cancer pain—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2017, 2020, CD012592. [Google Scholar] [CrossRef]
- Larkin, P.; Cherny, N.; La Carpia, D.; Guglielmo, M.; Ostgathe, C.; Scotté, F.; Ripamonti, C. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv111–iv125. [Google Scholar] [CrossRef]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2016, 7, 4–12. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; May, B.H.; Zhang, A.L.; Liu, Y.; Lu, C.; Mao, J.J.; Xue, C.C.; Zhang, H. Clinical Evidence for Association of Acupuncture and Acupressure With Improved Cancer Pain: A Systematic Review and Meta-Analysis. JAMA Oncol. 2020, 6, 271. [Google Scholar] [CrossRef]
- Bruera, E.; Belzile, M.; Neumann, C.; Harsanyi, Z.; Babul, N.; Darke, A. A Double-Blind, Crossover Study of Controlled-Release Metoclopramide and Placebo for the Chronic Nausea and Dyspepsia of Advanced Cancer. J. Pain Symptom Manag. 2000, 19, 427–435. [Google Scholar] [CrossRef]
- Davis, M.; Hui, D.; Davies, A.; Ripamonti, C.; Capela, A.; DeFeo, G.; Del Fabbro, E.; Bruera, E. MASCC antiemetics in advanced cancer updated guideline. Support. Care Cancer 2021, 29, 8097–8107. [Google Scholar] [CrossRef]
- Siersema, P.D.; Vleggaar, F.P. Esophageal strictures, tumors, and fistulae: Alternative techniques for palliating primary esophageal cancer. Tech. Gastrointest. Endosc. 2010, 12, 203–209. [Google Scholar] [CrossRef][Green Version]
- Di Mauro, P.; Ferrari, V. Bronchoesophageal Fistula. New Engl. J. Med. 2020, 383, e137. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.F.; Mathisen, D.J. Tracheoesophageal fistula. Chest Surg. Clin. N. Am. 2003, 13, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Ell, C. Palliative Treatment of Malignant Esophagorespiratory Fistulas With Gianturco-Z Stents: A Prospective Clinical Trial and Review of the Literature on Covered Metal Stents. Am. J. Gastroenterol. 1998, 93, 532–535. [Google Scholar] [CrossRef]
- Shin, J.H.; Song, H.-Y.; Ko, G.-Y.; Lim, J.-O.; Yoon, H.-K.; Sung, K.-B. Esophagorespiratory Fistula: Long-term Results of Palliative Treatment with Covered Expandable Metallic Stents in 61 Patients. Radiology 2004, 232, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Vleggaar, F.; Siersema, P. Expandable Stents for Malignant Esophageal Disease. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 377–388. [Google Scholar] [CrossRef]
- Heller, S.J.; Tokar, J.L.; Nguyen, M.T.; Haluszka, O.; Weinberg, D.S. Management of bleeding GI tumors. Gastrointest. Endosc. 2010, 72, 817–824. [Google Scholar] [CrossRef]
- Schwartz, S.I.; Brunicardi, F.C. Schwartz’s Principles of Surgery; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Imbesi, J.J.; Kurtz, R.C. A multidisciplinary approach to gastrointestinal bleeding in cancer patients. J. Support. Oncol. 2005, 3, 101–110. [Google Scholar]
- Akhtar, K.; Byrne, J.; Bancewicz, J.; Attwood, S.E.A. Argon beam plasma coagulation in the management of cancers of the esophagus and stomach. Surg. Endosc. 2000, 14, 1127–1130. [Google Scholar] [CrossRef]
- de Rezende, D.T.; Brunaldi, V.O.; Bernardo, W.M.; Ribeiro, I.B.; Mota, R.C.L.; Baracat, F.I.; de Moura, D.T.H.; Baracat, R.; Matuguma, S.E.; de Moura, E.G.H. Use of hemostatic powder in treatment of upper gastrointestinal bleeding: A systematic review and meta-analysis. Endosc. Int. Open 2019, 07, E1704–E1713. [Google Scholar] [CrossRef]
- Hughes, C.; Radhakrishna, G. Haemostatic radiotherapy for bleeding cancers of the upper gastrointestinal tract. Br. J. Hosp. Med. 2019, 80, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Rana, V.; Janjan, N.A.; Das, P.; Phan, A.T.; Delclos, M.E.; Mansfield, P.F.; Ajani, J.A.; Crane, C.H.; Krishnan, S. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008, 47, 421–427. [Google Scholar] [CrossRef]
- Chin-Yee, N.; Taylor, J.; Downar, J.; Tanuseputro, P.; Saidenberg, E. Red Blood Cell Transfusion in Palliative Care: A Survey of Palliative Care Physicians. J. Palliat. Med. 2019, 22, 1139–1142. [Google Scholar] [CrossRef]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv96–iv110. [Google Scholar] [CrossRef] [PubMed]
- Bracken-Clarke, D.; Farooq, A.R.; Horgan, A.M. Management of Locally Advanced and Metastatic Esophageal Cancer in the Older Population. Curr. Oncol. Rep. 2018, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Allum, W.H.; Blazeby, J.M.; Griffin, S.M.; Cunningham, D.; Jankowski, J.A.; Wong, R. Guidelines for the management of oesophageal and gastric cancer. Gut 2011, 60, 1449–1472. [Google Scholar] [CrossRef] [PubMed]
| Dysphagia | Malnutrition | Pain | Nausea and Vomiting | Fistula | Bleeding and Anaemia | |
|---|---|---|---|---|---|---|
| Do | Hypofractioned EBRT Brachytherapy Covered SEMS placement Pain control Dietary changes Adequate drug formulations (ex: liquid, powder, td, transmucosal, sc) | Malnutrition screening Early nutrition intervention Dysphagia treatment As-needed enteral tube feeding and parenteral nutrition (if expected survival of months) Assessment of end-of-life concerns on nutrition and hydration | (Re-)Assessment of the pain cause and its characteristics Stepwise approach Paracetamol a NSAID a,b Opioids (preferred oral morphine, or td fentanyl; or iv route for rapid pain control) Neuropathic-pain agents Corticosteroids Palliative antalgic RT Acupuncture and acupressure | Metoclopramide Sedative antiemetic drugs (ex: haloperidol, olanzapine) 5-HT3 agonists Anxiolytics Treat mechanical obstruction, if feasible Dexametasone, as part of the treatment of brain metastases or malignant bowel obstruction | Immediate treatment SEMS placement | Hemospray Palliative haemostatic RT Advanced care planning for the scenario of massive bleeding Assessment and treatment of causes of anaemia Red blood cell transfusion for symptomatic anaemia |
| Don’t | Rigid plastic tube insertion Dilatation Laser ablation Photodynamic therapy | End-of-life invasive clinical assisted nutrition | Forget about side effects | Surgery in the palliative setting | RT for massive bleeding (due to great vessels fistula) Erythropoiesis stimulating agents | |
| Don’t know | RT and SEMS combination | Add dexametasone c | Argon plasma coagulation Arterial embolization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pichel, R.C.; Araújo, A.; Domingues, V.D.S.; Santos, J.N.; Freire, E.; Mendes, A.S.; Romão, R.; Araújo, A. Best Supportive Care of the Patient with Oesophageal Cancer. Cancers 2022, 14, 6268. https://doi.org/10.3390/cancers14246268
Pichel RC, Araújo A, Domingues VDS, Santos JN, Freire E, Mendes AS, Romão R, Araújo A. Best Supportive Care of the Patient with Oesophageal Cancer. Cancers. 2022; 14(24):6268. https://doi.org/10.3390/cancers14246268
Chicago/Turabian StylePichel, Rita Carrilho, Alexandra Araújo, Vital Da Silva Domingues, Jorge Nunes Santos, Elga Freire, Ana Sofia Mendes, Raquel Romão, and António Araújo. 2022. "Best Supportive Care of the Patient with Oesophageal Cancer" Cancers 14, no. 24: 6268. https://doi.org/10.3390/cancers14246268
APA StylePichel, R. C., Araújo, A., Domingues, V. D. S., Santos, J. N., Freire, E., Mendes, A. S., Romão, R., & Araújo, A. (2022). Best Supportive Care of the Patient with Oesophageal Cancer. Cancers, 14(24), 6268. https://doi.org/10.3390/cancers14246268





