Senolysis-Based Elimination of Chemotherapy-Induced Senescent Breast Cancer Cells by Quercetin Derivative with Blocked Hydroxy Groups
Abstract
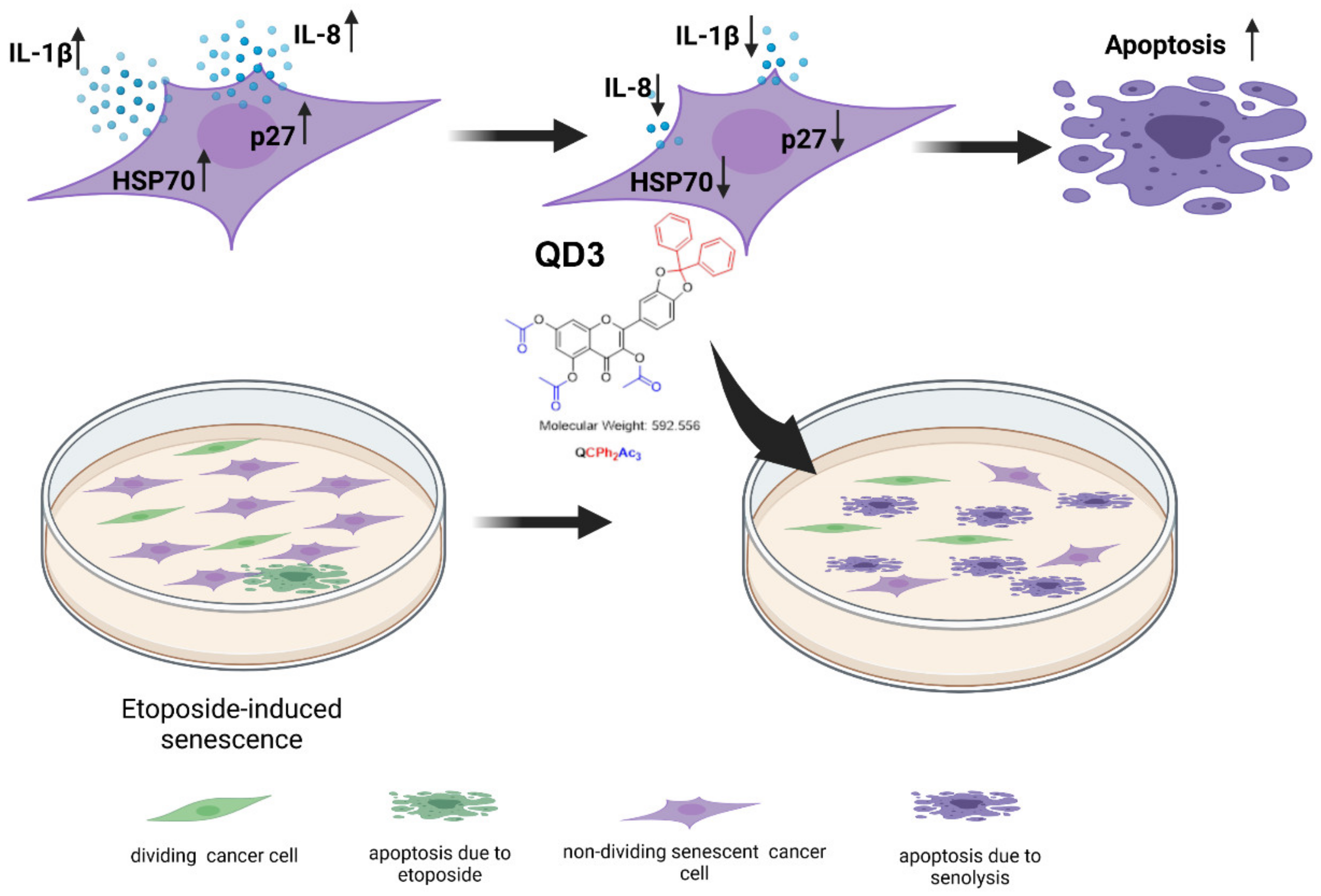
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Drug-Induced Senescence Protocol
2.2. Synthesis of Quercetin Derivatives
2.3. Apoptosis and Necrosis
2.4. Senescence-Associated β-Galactosidase Activity
2.5. Glutathione Redox Potential
2.6. Intracellular pH
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results and Discussion
3.1. Short-Term Effects of Quercetin Derivatives against HMEC and MDA-MB-231 Cells
3.2. Quercetin Derivative QD3 Exerts Senolytic Activity against Etoposide-Induced Senescent Breast Cancer Cells
3.3. Quercetin Derivative QD3-Mediated Decrease in the Levels of p27, IL-1β, IL-8 and HSP70 in Etoposide-Induced Senescent Breast Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lozano-Torres, B.; Estepa-Fernández, A.; Rovira, M.; Orzáez, M.; Serrano, M.; Martínez-Máñez, R.; Sancenón, F. The Chemistry of Senescence. Nat. Rev. Chem. 2019, 3, 426–441. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the P53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Campisi, J. Four Faces of Cellular Senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Naylor, R.M.; Baker, D.J.; van Deursen, J.M. Senescent Cells: A Novel Therapeutic Target for Aging and Age-Related Diseases. Clin. Pharmacol. Ther. 2013, 93, 105–116. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent Cells: An Emerging Target for Diseases of Ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Recent Advances in the Discovery of Senolytics. Mech. Ageing Dev. 2021, 200, 111587. [Google Scholar] [CrossRef]
- Li, W.; Qin, L.; Feng, R.; Hu, G.; Sun, H.; He, Y.; Zhang, R. Emerging Senolytic Agents Derived from Natural Products. Mech. Ageing Dev. 2019, 181, 1–6. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New Agents That Target Senescent Cells: The Flavone, Fisetin, and the BCL-XL Inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin Is a Senotherapeutic That Extends Health and Lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chang, J.; Liu, X.; Zhang, X.; Zhang, S.; Zhang, X.; Zhou, D.; Zheng, G. Discovery of Piperlongumine as a Potential Novel Lead for the Development of Senolytic Agents. Aging 2016, 8, 2915–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, Y.; Zhang, X.; Gao, Z.; Zhang, S.; Shi, P.; Zhang, X.; Song, L.; Hendrickson, H.; Zhou, D.; et al. Senolytic Activity of Piperlongumine Analogues: Synthesis and Biological Evaluation. Bioorg. Med. Chem. 2018, 26, 3925–3938. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, Y.; Zhang, R.; Zheng, G.; Zhou, D. The Curcumin Analog EF24 Is a Novel Senolytic Agent. Aging 2019, 11, 771–782. [Google Scholar] [CrossRef]
- Yang, D.; Tian, X.; Ye, Y.; Liang, Y.; Zhao, J.; Wu, T.; Lu, N. Identification of GL-V9 as a Novel Senolytic Agent against Senescent Breast Cancer Cells. Life Sci. 2021, 272, 119196. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The Biological Activities, Chemical Stability, Metabolism and Delivery Systems of Quercetin: A Review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Olszowka, J.; Kulpa-Greszta, M.; Litwinienko, G.; Tomaszewska, A.; Wnuk, M.; Pazik, R. AMPK-Mediated Senolytic and Senostatic Activity of Quercetin Surface Functionalized Fe3O4 Nanoparticles during Oxidant-Induced Senescence in Human Fibroblasts. Redox Biol. 2020, 28, 101337. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential Toxicity of Flavonoids and Other Dietary Phenolics: Significance for Their Chemopreventive and Anticancer Properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Bloniarz, D.; Adamczyk-Grochala, J.; Lewinska, A.; Wnuk, M. The Lack of Functional DNMT2/TRDMT1 Gene Modulates Cancer Cell Responses during Drug-Induced Senescence. Aging 2021, 13, 15833–15874. [Google Scholar] [CrossRef]
- Kajjout, M.; Rolando, C. Regiospecific Synthesis of Quercetin O-β-d-Glucosylated and O-β-d-Glucuronidated Isomers. Tetrahedron 2011, 67, 4731–4741. [Google Scholar] [CrossRef]
- Moine, E.; Boukhallat, M.; Cia, D.; Jacquemot, N.; Guillou, L.; Durand, T.; Vercauteren, J.; Brabet, P.; Crauste, C. New Lipophenols Prevent Carbonyl and Oxidative Stresses Involved in Macular Degeneration. Free Radic. Biol. Med. 2021, 162, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-H.; Li, N.-G.; Tang, Y.-P.; Wei-Li; Lian-Yin; Yang, J.-P.; Hao-Tang; Duan, J.-A. Metabolism-Based Synthesis, Biologic Evaluation and SARs Analysis of O-Methylated Analogs of Quercetin as Thrombin Inhibitors. Eur. J. Med. Chem. 2012, 54, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kim, M.K.; Park, K.; Choo, H.; Chong, Y. Quercetin–POC Conjugates: Differential Stability and Bioactivity Profiles between Breast Cancer (MCF-7) and Colorectal Carcinoma (HCT116) Cell Lines. Bioorg. Med. Chem. 2013, 21, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Bednarz, D.; Adamczyk-Grochala, J.; Wnuk, M. Phytochemical-Induced Nucleolar Stress Results in the Inhibition of Breast Cancer Cell Proliferation. Redox Biol. 2017, 12, 469–482. [Google Scholar] [CrossRef]
- Antoniak, M.A.; Pązik, R.; Bazylińska, U.; Wiwatowski, K.; Tomaszewska, A.; Kulpa-Greszta, M.; Adamczyk-Grochala, J.; Wnuk, M.; Maćkowski, S.; Lewińska, A.; et al. Multimodal Polymer Encapsulated CdSe/Fe3O4 Nanoplatform with Improved Biocompatibility for Two-Photon and Temperature Stimulated Bioapplications. Mater. Sci. Eng. C 2021, 127, 112224. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; Ribeiro de Oliveira, M.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S.; et al. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef] [Green Version]
- Samuni, Y.; Ishii, H.; Hyodo, F.; Samuni, U.; Krishna, M.C.; Goldstein, S.; Mitchell, J.B. Reactive Oxygen Species Mediate Hepatotoxicity Induced by the Hsp90 Inhibitor Geldanamycin and Its Analogs. Free Radic. Biol. Med. 2010, 48, 1559–1563. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Park, K.-S.; Chong, Y. Remarkable Stability and Cytostatic Effect of a Quercetin Conjugate, 3,7-Bis-O-Pivaloxymethyl (POM) Quercetin. ChemMedChem 2012, 7, 229–232. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, K.-S.; Lee, C.; Park, H.R.; Choo, H.; Chong, Y. Enhanced Stability and Intracellular Accumulation of Quercetin by Protection of the Chemically or Metabolically Susceptible Hydroxyl Groups with a Pivaloxymethyl (POM) Promoiety. J. Med. Chem. 2010, 53, 8597–8607. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Rayman, M.P.; Zhang, J. Prospective Selective Mechanism of Emerging Senolytic Agents Derived from Flavonoids. J. Agric. Food Chem. 2021, 69, 12418–12423. [Google Scholar] [CrossRef] [PubMed]
- Cherif, H.; Bisson, D.; Jarzem, P.; Weber, M.; Ouellet, J.; Haglund, L. Curcumin and O-Vanillin Exhibit Evidence of Senolytic Activity in Human IVD Cells In Vitro. J. Clin. Med. 2019, 8, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 Inhibitors as a Novel Class of Senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagadish, N.; Agarwal, S.; Gupta, N.; Fatima, R.; Devi, S.; Kumar, V.; Suri, V.; Kumar, R.; Suri, V.; Sadasukhi, T.C.; et al. Heat Shock Protein 70-2 (HSP70-2) Overexpression in Breast Cancer. J. Exp. Clin. Cancer Res. 2016, 35, 150. [Google Scholar] [CrossRef] [Green Version]
- Sverchinsky, D.; Nikotina, A.; Komarova, E.; Mikhaylova, E.; Aksenov, N.; Lazarev, V.; Mitkevich, V.; Suezov, R.; Druzhilovskiy, D.; Poroikov, V.; et al. Etoposide-Induced Apoptosis in Cancer Cells Can Be Reinforced by an Uncoupled Link between Hsp70 and Caspase-3. Int. J. Mol. Sci. 2018, 19, 2519. [Google Scholar] [CrossRef] [Green Version]
- Önay Uçar, E.; Şengelen, A.; Mertoğlu, E.; Pekmez, M.; Arda, N. Suppression of HSP70 Expression by Quercetin and Its Therapeutic Potential against Cancer. In HSP70 in Human Diseases and Disorders; Asea, A.A.A., Kaur, P., Eds.; Heat Shock Proteins; Springer International Publishing: Cham, Switzerland, 2018; Volume 14, pp. 361–379. ISBN 978-3-319-89550-5. [Google Scholar]
- Yang, W.; Cui, M.; Lee, J.; Gong, W.; Wang, S.; Fu, J.; Wu, G.; Yan, K. Heat Shock Protein Inhibitor, Quercetin, as a Novel Adjuvant Agent to Improve Radiofrequency Ablation-Induced Tumor Destruction and Its Molecular Mechanism. Chin. J. Cancer Res. Chung-Kuo Yen Cheng Yen Chiu 2016, 28, 19–28. [Google Scholar] [CrossRef]
- Kıyga, E.; Şengelen, A.; Adıgüzel, Z.; Önay Uçar, E. Investigation of the Role of Quercetin as a Heat Shock Protein Inhibitor on Apoptosis in Human Breast Cancer Cells. Mol. Biol. Rep. 2020, 47, 4957–4967. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Liu, X.; Wang, Y.; Chang, J.; Zhang, X.; Mackintosh, S.G.; Tackett, A.J.; He, Y.; Lv, D.; et al. Oxidation Resistance 1 Is a Novel Senolytic Target. Aging Cell 2018, 17, e12780. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewińska, A.; Przybylski, P.; Adamczyk-Grochala, J.; Błoniarz, D.; Litwinienko, G.; Wnuk, M. Senolysis-Based Elimination of Chemotherapy-Induced Senescent Breast Cancer Cells by Quercetin Derivative with Blocked Hydroxy Groups. Cancers 2022, 14, 605. https://doi.org/10.3390/cancers14030605
Lewińska A, Przybylski P, Adamczyk-Grochala J, Błoniarz D, Litwinienko G, Wnuk M. Senolysis-Based Elimination of Chemotherapy-Induced Senescent Breast Cancer Cells by Quercetin Derivative with Blocked Hydroxy Groups. Cancers. 2022; 14(3):605. https://doi.org/10.3390/cancers14030605
Chicago/Turabian StyleLewińska, Anna, Paweł Przybylski, Jagoda Adamczyk-Grochala, Dominika Błoniarz, Grzegorz Litwinienko, and Maciej Wnuk. 2022. "Senolysis-Based Elimination of Chemotherapy-Induced Senescent Breast Cancer Cells by Quercetin Derivative with Blocked Hydroxy Groups" Cancers 14, no. 3: 605. https://doi.org/10.3390/cancers14030605
APA StyleLewińska, A., Przybylski, P., Adamczyk-Grochala, J., Błoniarz, D., Litwinienko, G., & Wnuk, M. (2022). Senolysis-Based Elimination of Chemotherapy-Induced Senescent Breast Cancer Cells by Quercetin Derivative with Blocked Hydroxy Groups. Cancers, 14(3), 605. https://doi.org/10.3390/cancers14030605






