Chemotherapy Side-Effects: Not All DNA Damage Is Equal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Chemotherapy Side-Effects Observed in Cancer Survivors
2.1. Nephrotoxicity
2.1.1. Alkylating Agents
2.1.2. Antimetabolites
2.1.3. Anti-Cancer Antibiotics
2.2. Hepatotoxicity
2.2.1. Alkylating Agents
2.2.2. Antimetabolites
2.2.3. Topoisomerase Inhibitors
2.2.4. Other Chemotherapeutics
2.2.5. Radiotherapy
2.3. Neurotoxicity
2.3.1. Alkylating Agents
2.3.2. Antimetabolites
2.3.3. Mitotic Inhibitors
2.3.4. Proteasome Inhibitors
2.4. Cardiotoxicity
2.4.1. Anthracyclines/Cytostatic Antibiotics
2.4.2. Other Chemotherapeutic Agents
2.5. Hematological Toxicities
2.6. Other Toxicities
2.6.1. Ototoxicity
2.6.2. Gastro-Intestinal Toxicity
2.6.3. Gonadal Toxicity
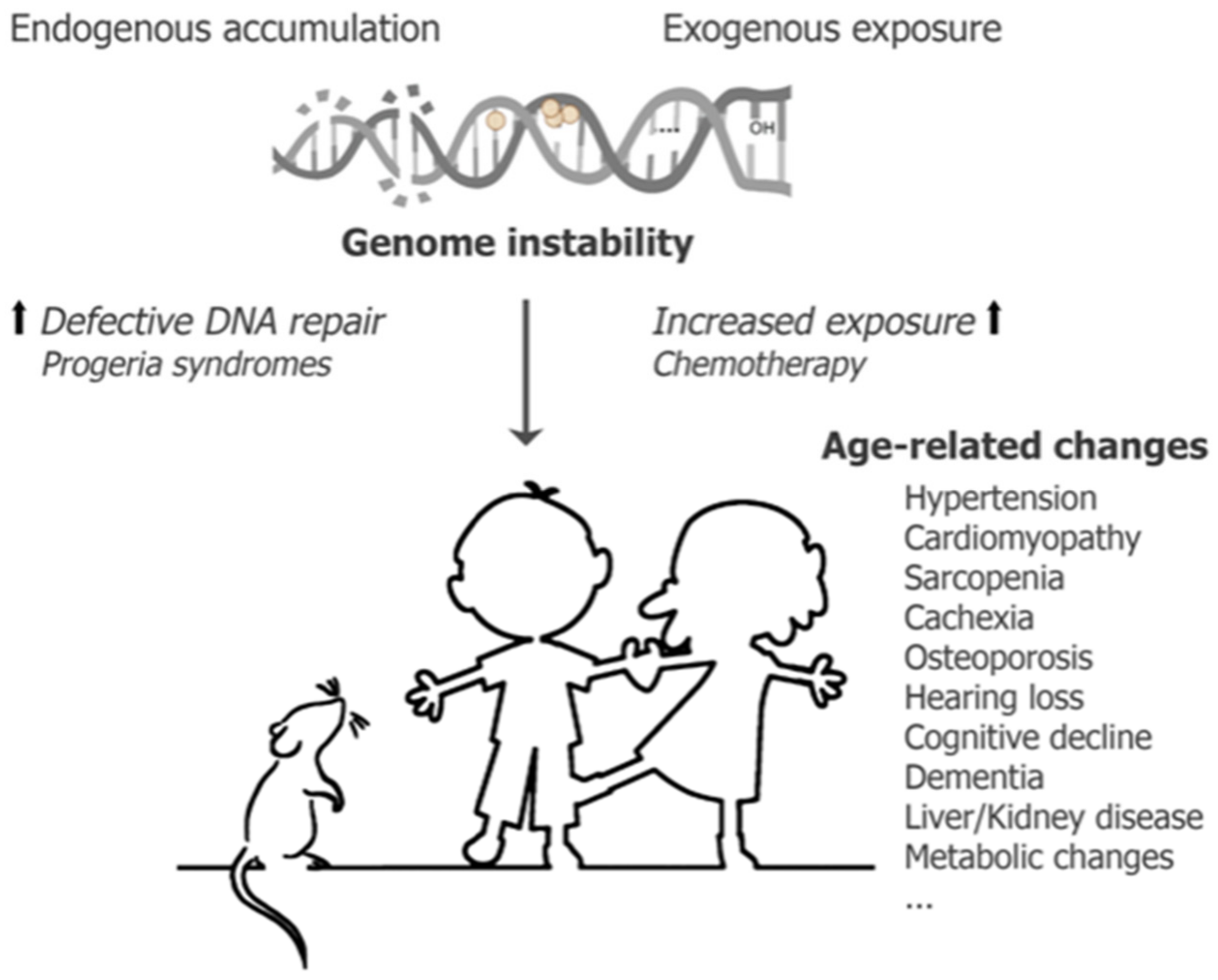
3. Conceptualization of Chemotherapeutics Driving Segmental Aging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaatsch, P. Epidemiology of childhood cancer. Cancer Treat. Rev. 2010, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Schulpen, M.; Visser, O.; Reedijk, A.M.; Kremer, L.C.; Zwaan, C.M.; Eggermont, A.M.; Coebergh, J.W.; Pieters, R.; Karim-Kos, H.E. Significant improvement in survival of advanced stage childhood and young adolescent cancer in the Netherlands since the 1990s. Eur. J. Cancer 2021, 157, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; De Santis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Goodman, L.S.; Wintrobe, M.M.; Dameshek, W.; Goodman, M.J.; Gilman, A.; McLennan, M.T. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946, 132, 126–132. [Google Scholar] [CrossRef]
- Farber, S.; Diamond, L.K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J. Med. 1948, 238, 787–793. [Google Scholar] [CrossRef]
- Frei, E.; Holland, J.F.; Schneiderman, M.A.; Pinkel, D.; Selkirk, G.; Freireich, E.J.; Silver, R.T.; Gold, G.L.; Regelson, W. A comparative study of two regimens of combination chemotherapy in acute leukemia. Blood 1958, 13, 1126–1148. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Soares, J.; Greninger, P.; Edelman, E.J.; Lightfoot, H.; Forbes, S.; Bindal, N.; Beare, D.; Smith, J.A.; Thompson, I.R.; et al. Genomics of Drug Sensitivity in Cancer (GDSC): A resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2012, 41, D955–D961. [Google Scholar] [CrossRef] [Green Version]
- Armenian, S.H.; Gibson, C.J.; Rockne, R.C.; Ness, K.K. Premature Aging in Young Cancer Survivors. J. Natl. Cancer Inst. 2019, 111, 226–232. [Google Scholar] [CrossRef]
- Cupit-Link, M.C.; Kirkland, J.L.; Ness, K.K.; Armstrong, G.T.; Tchkonia, T.; LeBrasseur, N.K.; Armenian, S.H.; Ruddy, K.J.; Hashmi, S.K. Biology of premature ageing in survivors of cancer. ESMO Open 2017, 2, e000250. [Google Scholar] [CrossRef] [Green Version]
- Guida, J.L.; Ahles, T.A.; Belsky, D.; Campisi, J.; Cohen, H.J.; DeGregori, J.; Fuldner, R.; Ferrucci, L.; Gallicchio, L.; Gavrilov, L.; et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J. Natl. Cancer Inst. 2019, 111, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccormick, R.E. Possible acceleration of aging by adjuvant chemotherapy: A cause of early onset frailty? Med. Hypotheses 2006, 67, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Wang, S.; Prizment, A.; Thyagarajan, B.; Blaes, A. Cancer Treatment-Induced Accelerated Aging in Cancer Survivors: Biology and Assessment. Cancers 2021, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Nonnekens, J.; Hoeijmakers, J.H. After surviving cancer, what about late life effects of the cure? EMBO Mol. Med. 2017, 9, 4–6. [Google Scholar] [CrossRef]
- Crom, W.R. Pharmacokinetics in the child. Environ. Health Perspect. 1994, 102 (Suppl. 11), 111–117. [Google Scholar] [CrossRef] [Green Version]
- Adamson, P.C. Improving the outcome for children with cancer: Development of targeted new agents. CA Cancer J. Clin. 2015, 65, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Hoeijmakers, J.H. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411, 366–374. [Google Scholar] [CrossRef]
- Kitao, H.; Iimori, M.; Kataoka, Y.; Wakasa, T.; Tokunaga, E.; Saeki, H.; Oki, E.; Maehara, Y. DNA replication stress and cancer chemotherapy. Cancer Sci. 2018, 109, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Caglar, K.; Kinalp, C.; Arpaci, F.; Turan, M.; Saglam, K.; Ozturk, B.; Komurcu, Ş.; Yavuz, İ.; Yenicesu, M.; Ozet, A. Cumulative prior dose of cisplatin as a cause of the nephrotoxicity of high-dose chemotherapy followed by autologous stem-cell transplantation. Nephrol. Dial. Transplant. 2002, 17, 1931–1935. [Google Scholar] [CrossRef] [Green Version]
- Fukasawa, H.; Furuya, R.; Yasuda, H.; Yamamoto, T.; Hishida, A.; Kitagawa, M. Anti-cancer agent-induced nephrotoxicity. Anticancer Agents Med. Chem. 2014, 14, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Geenen, M.M.; Cardous-Ubbink, M.C.; Kremer, L.C.; van den Bos, C.; van der Pal, H.J.; Heinen, R.C.; Jaspers, M.W.; Koning, C.C.; Oldenburger, F.; Langeveld, N.E.; et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. Jama 2007, 297, 2705–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidera, Y.; Kawakami, H.; Sakiyama, T.; Okamoto, K.; Tanaka, K.; Takeda, M.; Kaneda, H.; Nishina, S.-I.; Tsurutani, J.; Fujiwara, K. Risk factors for cisplatin-induced nephrotoxicity and potential of magnesium supplementation for renal protection. PLoS ONE 2014, 9, e101902. [Google Scholar]
- Małyszko, J.; Kozłowska, K.; Kozłowski, L.; Małyszko, J. Nephrotoxicity of anticancer treatment. Nephrol. Dial. Transplant. 2016, 32, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [Green Version]
- Nicolaysen, A. Nephrotoxic Chemotherapy Agents: Old and New. Adv. Chronic Kidney Dis. 2020, 27, 38–49. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Perazella, M.A.; Moeckel, G.W. Nephrotoxicity from chemotherapeutic agents: Clinical manifestations, pathobiology, and prevention/therapy. Semin. Nephrol. 2010, 30, 570–581. [Google Scholar] [CrossRef]
- Sharbaf, F.G.; Farhangi, H.; Assadi, F. Prevention of chemotherapy-induced nephrotoxicity in children with cancer. Int. J. Prev. Med. 2017, 8, 76. [Google Scholar] [CrossRef]
- Hoekman, K.; van der Vijgh, W.J.; Vermorken, J.B. Clinical and preclinical modulation of chemotherapy-induced toxicity in patients with cancer. Drugs 1999, 57, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.S. An overview of chemical processes that damage cellular DNA: Spontaneous hydrolysis, alkylation, and reactions with radicals. Chem. Res. Toxicol. 2009, 22, 1747–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.L.C.; de Brito, B.B.; da Silva, F.A.F.; dos Santos Botelho, A.C.; de Melo, F.F. Nephrotoxicity in cancer treatment: An overview. World J. Clin. Oncol. 2020, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Lameire, N. Nephrotoxicity of recent anti-cancer agents. Clin. Kidney J. 2013, 7, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Jungsuwadee, P.; Vore, M.; Butterfield, D.A.; St Clair, D.K. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol. Interv. 2007, 7, 147–156. [Google Scholar] [CrossRef]
- Cai, Z.; Yang, J.; Shu, X.; Xiong, X. Chemotherapy-associated hepatotoxicity in colorectal cancer. Vascular 2014, 14, 15. [Google Scholar]
- Grigorian, A.; O’Brien, C.B. Hepatotoxicity secondary to chemotherapy. J. Clin. Transl. Hepatol. 2014, 2, 95. [Google Scholar]
- Thatishetty, A.V.; Agresti, N.; O’Brien, C.B. Chemotherapy-Induced Hepatotoxicity. Clin. Liver Dis. 2013, 17, 671–686. [Google Scholar] [CrossRef]
- Torrisi, J.M.; Schwartz, L.H.; Gollub, M.J.; Ginsberg, M.S.; Bosl, G.J.; Hricak, H. CT findings of chemotherapy-induced toxicity: What radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology 2011, 258, 41–56. [Google Scholar] [CrossRef]
- Zorzi, D.; Laurent, A.; Pawlik, T.; Lauwers, G.; Vauthey, J.; Abdalla, E. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. J. Br. Surg. 2007, 94, 274–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floyd, J.; Mirza, I.; Sachs, B.; Perry, M.C. Hepatotoxicity of Chemotherapy. Semin. Oncol. 2006, 33, 50–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, P.D.; Perry, M.C. Hepatotoxicity of chemotherapy. Oncologist 2001, 6, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Guha, C.; Kavanagh, B.D. Hepatic radiation toxicity: Avoidance and amelioration. In Seminars in Radiation Oncology; WB Saunders: Philadelphia, PA, USA, 2011; pp. 256–263. [Google Scholar]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015, 28, 500–507. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol./Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Magge, R.S.; DeAngelis, L.M. The double-edged sword: Neurotoxicity of chemotherapy. Blood Rev. 2015, 29, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Taillibert, S.; Le Rhun, E.; Chamberlain, M.C. Chemotherapy-related neurotoxicity. Curr. Neurol. Neurosci. Rep. 2016, 16, 1–14. [Google Scholar] [CrossRef]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.-Y.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef]
- Pai, V.B.; Nahata, M.C. Cardiotoxicity of Chemotherapeutic Agents. Drug Saf. 2000, 22, 263–302. [Google Scholar] [CrossRef]
- Yeh, E.T. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu. Rev. Med. 2006, 57, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Simbre, V.C.; Duffy, S.A.; Dadlani, G.H.; Miller, T.L.; Lipshultz, S.E. Cardiotoxicity of Cancer Chemotherapy. Pediatr. Drugs 2005, 7, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, C.; Deidda, M.; Piras, A.; Cadeddu, C.; Demurtas, L.; Puzzoni, M.; Piscopo, G.; Scartozzi, M.; Mercuro, G. Pathophysiology of cardiotoxicity induced by nonanthracycline chemotherapy. J. Cardiovasc. Med. 2016, 17, S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.T.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albini, A.; Pennesi, G.; Donatelli, F.; Cammarota, R.; De Flora, S.; Noonan, D.M. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J. Natl. Cancer Inst. 2010, 102, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Florescu, M.; Cinteza, M.; Vinereanu, D. Chemotherapy-induced Cardiotoxicity. Maedica 2013, 8, 59–67. [Google Scholar]
- Elliott, P. Pathogenesis of cardiotoxicity induced by anthracyclines. Semin. Oncol. 2006, 33, 2–7. [Google Scholar] [CrossRef]
- Tamamyan, G.; Danielyan, S.; Lambert, M.P. Chemotherapy induced thrombocytopenia in pediatric oncology. Crit. Rev. Oncol. Hematol. 2016, 99, 299–307. [Google Scholar] [CrossRef]
- Ten Berg, M.J.; van den Bemt, P.M.; Shantakumar, S.; Bennett, D.; Voest, E.E.; Huisman, A.; van Solinge, W.W.; Egberts, T.C. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: Results from a retrospective hospital-based cohort study. Drug Saf. 2011, 34, 1151–1160. [Google Scholar] [CrossRef]
- Lokich, J.J. Managing chemotherapy-induced bone marrow suppression in cancer. Hosp. Pract. 1976, 11, 61–67. [Google Scholar] [CrossRef]
- Hoagland, H.C. Hematologic complications of cancer chemotherapy. Semin. Oncol. 1982, 91, 95–102. [Google Scholar]
- Germanas, J.; Pandya, A.G. Alkylating agents. Dermatol. Ther. 2002, 15, 317–324. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Stober, C.; Vandermeer, L.; Dudani, S.; Ibrahim, M.F.K.; Majeed, H.; Perdrizet, K.; Shorr, R.; Hutton, B.; et al. Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel–cyclophosphamide chemotherapy for breast cancer: A systematic review. Breast Cancer Res. Treat. 2017, 161, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Othieno-Abinya, N.; Waweru, A.; Nyabola, L. Chemotherapy induced myelosuppression. East Afr. Med. J. 2007, 84, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chrischilles, E.A.; Link, B.K.; Scott, S.D.; Delgado, D.J.; Fridman, M. Factors associated with early termination of CHOP therapy and the impact on survival among patients with chemosensitive intermediate-grade non-Hodgkin’s lymphoma. Cancer Control 2003, 10, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Crivellari, D.; Bonetti, M.; Castiglione-Gertsch, M.; Gelber, R.D.; Rudenstam, C.M.; Thürlimann, B.; Price, K.N.; Coates, A.S.; Hürny, C.; Bernhard, J.; et al. Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: The International Breast Cancer Study Group Trial VII. J. Clin. Oncol. 2000, 18, 1412–1422. [Google Scholar] [CrossRef]
- de la Maza Cantero, S.S.; Martín, A.J.; de Felipe Mimbrera, A.; Corral, Í.C. Cerebellar toxicity due to cytarabine: A series of 4 cases. Neurol. A 2016, 7, 491–492. [Google Scholar] [CrossRef]
- dos Santos, N.A.; Carvalho Rodrigues, M.A.; Martins, N.M.; dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef]
- Ciarimboli, G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014, 34, 547–550. [Google Scholar] [CrossRef]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Ciarimboli, G. Membrane transporters as mediators of Cisplatin effects and side effects. Scientifica 2012, 2012, 473829. [Google Scholar] [CrossRef] [PubMed]
- Harrach, S.; Ciarimboli, G. Role of transporters in the distribution of platinum-based drugs. Front. Pharmacol. 2015, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Noll, D.M.; Mason, T.M.; Miller, P.S. Formation and repair of interstrand cross-links in DNA. Chem. Rev. 2006, 106, 277–301. [Google Scholar] [CrossRef]
- Hayati, F.; Hossainzadeh, M.; Shayanpour, S.; Abedi-Gheshlaghi, Z.; Beladi Mousavi, S.S. Prevention of cisplatin nephrotoxicity. J. Nephropharmacol. 2016, 5, 57–60. [Google Scholar]
- Martins, I.; Kepp, O.; Schlemmer, F.; Adjemian, S.; Tailler, M.; Shen, S.; Michaud, M.; Menger, L.; Gdoura, A.; Tajeddine, N.; et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 2011, 30, 1147–1158. [Google Scholar] [CrossRef] [Green Version]
- Kooijmans, E.C.; Bökenkamp, A.; Tjahjadi, N.S.; Tettero, J.M.; van Dulmen-den Broeder, E.; van der Pal, H.J.; Veening, M.A. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst. Rev. 2019, 3, Cd008944. [Google Scholar] [CrossRef]
- Garofeanu, C.G.; Weir, M.; Rosas-Arellano, M.P.; Henson, G.; Garg, A.X.; Clark, W.F. Causes of reversible nephrogenic diabetes insipidus: A systematic review. Am. J. Kidney Dis. 2005, 45, 626–637. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Perazella, M.A. Drug-induced crystal nephropathy: An update. Expert Opin. Drug Saf. 2008, 7, 147–158. [Google Scholar] [CrossRef]
- Mini, E.; Nobili, S.; Caciagli, B.; Landini, I.; Mazzei, T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006, 17, v7–v12. [Google Scholar] [CrossRef]
- Johnson-Arbor, K.; Dubey, R. Doxorubicin. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ferrazzi, E.; Woynarowski, J.M.; Arakali, A.; Brenner, D.E.; Beerman, T.A. DNA damage and cytotoxicity induced by metabolites of anthracycline antibiotics, doxorubicin and idarubicin. Cancer Commun. 1991, 3, 173–180. [Google Scholar] [CrossRef] [PubMed]
- L’Ecuyer, T.; Sanjeev, S.; Thomas, R.; Novak, R.; Das, L.; Campbell, W.; Heide, R.V. DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1273–H1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E. Clinical ascertainment of health outcomes among adults treated for childhood cancer. Jama 2013, 309, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Yoshioka, A.; Tanaka, S.; Hiraoka, O.; Koyama, Y.; Hirota, Y.; Ayusawa, D.; Seno, T.; Garrett, C.; Wataya, Y. Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J. Biol. Chem. 1987, 262, 8235–8241. [Google Scholar] [CrossRef]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Xu, Y.; Her, C. Inhibition of Topoisomerase (DNA) I (TOP1): DNA Damage Repair and Anticancer Therapy. Biomolecules 2015, 5, 1652–1670. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, J.T.; Lipp, H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar]
- Andreyev, J. Gastrointestinal complications of pelvic radiotherapy: Are they of any importance? Gut 2005, 54, 1051–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauer-Jensen, M.; Wang, J.; Denham, J.W. Bowel injury: Current and evolving management strategies. Semin. Radiat. Oncol. 2003, 13, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef]
- Mollman, J.E.; Glover, D.J.; Hogan, W.M.; Furman, R.E. Cisplatin neuropathy. Risk factors, prognosis, and protection by WR-2721. Cancer 1988, 61, 2192–2195. [Google Scholar] [CrossRef]
- Fricker, L.D. Proteasome Inhibitor Drugs. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 457–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brana, I.; Zamora, E.; Oristrell, G.; Tabernero, J. Cardiotoxicity. In Side Effects of Medical Cancer Therapy; Springer: Berlin/Heidelberg, Germany, 2018; pp. 367–406. [Google Scholar]
- Armstrong, G.T.; Liu, Q.; Yasui, Y.; Neglia, J.P.; Leisenring, W.; Robison, L.L.; Mertens, A.C. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2328–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okwuosa, T.M.; Anzevino, S.; Rao, R. Cardiovascular disease in cancer survivors. Postgrad. Med. J. 2017, 93, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, X.; van der Zanden, S.Y.; Wander, D.P.A.; Borràs, D.M.; Song, J.Y.; Li, X.; van Duikeren, S.; van Gils, N.; Rutten, A.; van Herwaarden, T.; et al. Uncoupling DNA damage from chromatin damage to detoxify doxorubicin. Proc. Natl. Acad. Sci. USA 2020, 117, 15182–15192. [Google Scholar] [CrossRef]
- Sawyer, D.B. Anthracyclines and Heart Failure. N. Engl. J. Med. 2013, 368, 1154–1156. [Google Scholar] [CrossRef] [Green Version]
- Doroshow, J.H. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 1983, 43, 460–472. [Google Scholar]
- Muindi, J.R.; Sinha, B.K.; Gianni, L.; Myers, C.E. Hydroxyl radical production and DNA damage induced by anthracycline-iron complex. FEBS Lett. 1984, 172, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Myers, C.E.; Gianni, L.; Simone, C.B.; Klecker, R.; Greene, R. Oxidative destruction of erythrocyte ghost membranes catalyzed by the doxorubicin-iron complex. Biochemistry 1982, 21, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Bachur, N.R.; Gee, M.V.; Friedman, R.D. Nuclear catalyzed antibiotic free radical formation. Cancer Res. 1982, 42, 1078–1081. [Google Scholar] [PubMed]
- Zhi, L.; Yuzhang, Z.; Tianliang, H.; Hisatome, I.; Yamamoto, T.; Jidong, C. High uric acid induces insulin resistance in cardiomyocytes in vitro and in vivo. PLoS ONE 2016, 11, e0147737. [Google Scholar] [CrossRef] [PubMed]
- Vander Heide, R.S.; L’Ecuyer, T.J. Molecular basis of anthracycline-induced cardiotoxicity. Heart Metab. 2007, 35, 1–4. [Google Scholar]
- Tewey, K.M.; Rowe, T.C.; Yang, L.; Halligan, B.D.; Liu, L.F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 1984, 226, 466–468. [Google Scholar] [CrossRef]
- Capranico, G.; Tinelli, S.; Austin, C.A.; Fisher, M.L.; Zunino, F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim. Biophys. Acta 1992, 1132, 43–48. [Google Scholar] [CrossRef]
- Lowe, M.C.; Smallwood, J.I. Adriamycin and daunomycin dose-dependent effects upon contractions of isolated rat myocytes. Cancer Chemother. Pharmacol. 1980, 5, 61–65. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Pang, B.; Qiao, X.; Janssen, L.; Velds, A.; Groothuis, T.; Kerkhoven, R.; Nieuwland, M.; Ovaa, H.; Rottenberg, S.; van Tellingen, O.; et al. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat. Commun. 2013, 4, 1908. [Google Scholar] [CrossRef] [Green Version]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H. Mitomycin C-enhanced superoxide and hydrogen peroxide formation in rat heart. J. Pharmacol. Exp. Ther. 1981, 218, 206–211. [Google Scholar]
- Ilinskaya, A.N.; Clogston, J.D.; McNeil, S.E.; Dobrovolskaia, M.A. Induction of oxidative stress by Taxol® vehicle Cremophor-EL triggers production of interleukin-8 by peripheral blood mononuclear cells through the mechanism not requiring de novo synthesis of mRNA. Nanomedicine 2015, 11, 1925–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparreboom, A.; van Tellingen, O.; Nooijen, W.J.; Beijnen, J.H. Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res. 1996, 56, 2112–2115. [Google Scholar] [PubMed]
- Szebeni, J.; Muggia, F.M.; Alving, C.R. Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: An in vitro study. J. Natl. Cancer Inst. 1998, 90, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Bow, E.J. Infection risk and cancer chemotherapy: The impact of the chemotherapeutic regimen in patients with lymphoma and solid tissue malignancies. J. Antimicrob. Chemother. 1998, 41 (Suppl. D), 1–5. [Google Scholar] [CrossRef] [Green Version]
- Talcott, J.A.; Finberg, R.; Mayer, R.J.; Goldman, L. The medical course of cancer patients with fever and neutropenia. Clinical identification of a low-risk subgroup at presentation. Arch. Intern. Med. 1988, 148, 2561–2568. [Google Scholar] [CrossRef]
- Okunaka, M.; Kano, D.; Matsui, R.; Kawasaki, T.; Uesawa, Y. Comprehensive Analysis of Chemotherapeutic Agents That Induce Infectious Neutropenia. Pharmaceuticals 2021, 14, 681. [Google Scholar] [CrossRef]
- Shayne, M.; Culakova, E.; Poniewierski, M.S.; Wolff, D.; Dale, D.C.; Crawford, J.; Lyman, G.H. Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer 2007, 110, 1611–1620. [Google Scholar] [CrossRef]
- Smith, T.J.; Khatcheressian, J.; Lyman, G.H.; Ozer, H.; Armitage, J.O.; Balducci, L.; Bennett, C.L.; Cantor, S.B.; Crawford, J.; Cross, S.J.; et al. 2006 Update of Recommendations for the Use of White Blood Cell Growth Factors: An Evidence-Based Clinical Practice Guideline. J. Clin. Oncol. 2006, 24, 3187–3205. [Google Scholar] [CrossRef] [Green Version]
- Lyman, G.H.; Abella, E.; Pettengell, R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit. Rev. Oncol. Hematol. 2014, 90, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.K.; Chandra, J.; Narayan, S.; Sharma, S.; Singh, V.; Dutta, A.K. Pancytopenia in children: Etiological profile. J. Trop. Pediatr. 2005, 51, 236–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landier, W. Ototoxicity and cancer therapy. Cancer 2016, 122, 1647–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokemeyer, C.; Berger, C.C.; Hartmann, J.T.; Kollmannsberger, C.; Schmoll, H.J.; Kuczyk, M.A.; Kanz, L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br. J. Cancer 1998, 77, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Coradini, P.P.; Cigana, L.; Selistre, S.G.; Rosito, L.S.; Brunetto, A.L. Ototoxicity from cisplatin therapy in childhood cancer. J. Pediatr. Hematol. Oncol. 2007, 29, 355–360. [Google Scholar] [CrossRef]
- Knight, K.R.; Kraemer, D.F.; Neuwelt, E.A. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J. Clin. Oncol. 2005, 23, 8588–8596. [Google Scholar] [CrossRef] [Green Version]
- Meijer, A.J.M.; Diepstraten, F.A.; Langer, T.; Broer, L.; Domingo, I.K.; Clemens, E.; Uitterlinden, A.G.; de Vries, A.C.H.; van Grotel, M.; Vermeij, W.P.; et al. TCERG1L allelic variation is associated with cisplatin-induced hearing loss in childhood cancer, a PanCareLIFE study. NPJ Precis. Oncol. 2021, 5, 64. [Google Scholar] [CrossRef]
- Dehne, N.; Lautermann, J.; Petrat, F.; Rauen, U.; de Groot, H. Cisplatin ototoxicity: Involvement of iron and enhanced formation of superoxide anion radicals. Toxicol. Appl. Pharmacol. 2001, 174, 27–34. [Google Scholar] [CrossRef]
- Cappaert, N.L.M.; Klis, S.F.L.; Wijbenga, J.; Smoorenburg, G.F. Acceleration of cisplatin ototoxicity by perilymphatic application of 4-methylthiobenzoic acid. Hear. Res. 2005, 203, 80–87. [Google Scholar] [CrossRef]
- Clemens, E.; de Vries, A.C.; Pluijm, S.F.; Am Zehnhoff-Dinnesen, A.; Tissing, W.J.; Loonen, J.J.; van Dulmen-den Broeder, E.; Bresters, D.; Versluys, B.; Kremer, L.C.; et al. Determinants of ototoxicity in 451 platinum-treated Dutch survivors of childhood cancer: A DCOG late-effects study. Eur. J. Cancer 2016, 69, 77–85. [Google Scholar] [CrossRef]
- Olgun, Y.; Aktaş, S.; Altun, Z.; Kırkım, G.; Kızmazoğlu, D.; Erçetin, A.P.; Demir, B.; İnce, D.; Mutafoğlu, K.; Demirağ, B.; et al. Analysis of genetic and non genetic risk factors for cisplatin ototoxicity in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2016, 90, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Secombe, K.R.; Coller, J.K.; Gibson, R.J.; Wardill, H.R.; Bowen, J.M. The bidirectional interaction of the gut microbiome and the innate immune system: Implications for chemotherapy-induced gastrointestinal toxicity. Int. J. Cancer 2019, 144, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. WJG 2014, 20, 3751. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.P. Gastrointestinal toxicity of chemotherapeutic agents. Semin. Oncol. 2006, 33, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Shahid, F.; Farooqui, Z.; Khan, F. Cisplatin-induced gastrointestinal toxicity: An update on possible mechanisms and on available gastroprotective strategies. Eur. J. Pharmacol. 2018, 827, 49–57. [Google Scholar] [CrossRef]
- Oosterom, N.; Griffioen, P.H.; den Hoed, M.A.H.; Pieters, R.; de Jonge, R.; Tissing, W.J.E.; van den Heuvel-Eibrink, M.M.; Heil, S.G. Global methylation in relation to methotrexate-induced oral mucositis in children with acute lymphoblastic leukemia. PLoS ONE 2018, 13, e0199574. [Google Scholar] [CrossRef]
- Wardill, H.R.; da Silva Ferreira, A.R.; Lichtenberg Cloo, S.; Havinga, R.; Harmsen, H.J.M.; Vermeij, W.P.; Tissing, W.J.E. Pre-therapy fasting slows epithelial turnover and modulates the microbiota but fails to mitigate methotrexate-induced gastrointestinal mucositis. Gut Microbes 2020, 12, 1–9. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Henriksson, R.; Bergström, P.; Franzén, L.; Lewin, F.; Wagenius, G. Aspects on reducing gastrointestinal adverse effects associated with radiotherapy. Acta Oncol. 1999, 38, 159–164. [Google Scholar] [CrossRef]
- Boekelheide, K. Mechanisms of Toxic Damage to Spermatogenesis. JNCI Monogr. 2005, 2005, 6–8. [Google Scholar] [CrossRef] [Green Version]
- Levi, M.; Hasky, N.; Stemmer, S.M.; Shalgi, R.; Ben-Aharon, I. Anti-Müllerian Hormone Is a Marker for Chemotherapy-Induced Testicular Toxicity. Endocrinology 2015, 156, 3818–3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Aharon, I.; Shalgi, R. What lies behind chemotherapy-induced ovarian toxicity? Reproduction 2012, 144, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roness, H.; Kashi, O.; Meirow, D. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 2016, 105, 20–29. [Google Scholar] [CrossRef]
- Takemura, N.; Kawasaki, T.; Kunisawa, J.; Sato, S.; Lamichhane, A.; Kobiyama, K.; Aoshi, T.; Ito, J.; Mizuguchi, K.; Karuppuchamy, T.; et al. Blockade of TLR3 protects mice from lethal radiation-induced gastrointestinal syndrome. Nat. Commun. 2014, 5, 3492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merritt, A.J.; Potten, C.S.; Kemp, C.J.; Hickman, J.A.; Balmain, A.; Lane, D.P.; Hall, P.A. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994, 54, 614–617. [Google Scholar] [PubMed]
- Wang, Y.; Schulte, B.A.; LaRue, A.C.; Ogawa, M.; Zhou, D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006, 107, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeij, W.P.; Hoeijmakers, J.H.; Pothof, J. Aging: Not all DNA damage is equal. Curr. Opin. Genet. Dev. 2014, 26, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef]
- Lindahl, T.; Barnes, D.E. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 127–133. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Lu, K.; Moeller, B.C.; Gao, L.; Upton, P.B.; Nakamura, J.; Starr, T.B. Endogenous versus exogenous DNA adducts: Their role in carcinogenesis, epidemiology, and risk assessment. Toxicol Sci. 2011, 120 (Suppl. 1), S130–S145. [Google Scholar] [CrossRef]
- Vijg, J. From DNA damage to mutations: All roads lead to aging. Ageing Res. Rev. 2021, 68, 101316. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, W.M.; van den Heuvel-Eibrink, M.M.; Hoeijmakers, J.H.; Vermeij, W.P. Nutritional Preconditioning in Cancer Treatment in Relation to DNA Damage and Aging. Annu. Rev. Cancer Biol. 2021, 5, 161–179. [Google Scholar] [CrossRef]
- Petr, M.A.; Tulika, T.; Carmona-Marin, L.M.; Scheibye-Knudsen, M. Protecting the Aging Genome. Trends Cell Biol. 2020, 30, 117–132. [Google Scholar] [CrossRef] [Green Version]
- van de Kamp, G.; Heemskerk, T.; Kanaar, R.; Essers, J. DNA Double Strand Break Repair Pathways in Response to Different Types of Ionizing Radiation. Front. Genet. 2021, 12, 738230. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. Mammalian DNA base excision repair: Dancing in the moonlight. DNA Repair 2020, 93, 102921. [Google Scholar] [CrossRef] [PubMed]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Waters, L.S.; Minesinger, B.K.; Wiltrout, M.E.; D’Souza, S.; Woodruff, R.V.; Walker, G.C. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol. Mol. Biol. Rev. 2009, 73, 134–154. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, T.B. Understanding the odd science of aging. Cell 2005, 120, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.M.; Oshima, J. Lessons from human progeroid syndromes. Nature 2000, 408, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, W.P.; Hoeijmakers, J.H.; Pothof, J. Genome Integrity in Aging: Human Syndromes, Mouse Models, and Therapeutic Options. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 427–445. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Jones, L.; Muss, H.B. Cancer Treatment as an Accelerated Aging Process: Assessment, Biomarkers, and Interventions. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e516–e522. [Google Scholar] [CrossRef]
- Meijer, A.J.M.; van den Heuvel-Eibrink, M.M.; Brooks, B.; Am Zehnhoff-Dinnesen, A.G.; Knight, K.R.; Freyer, D.R.; Chang, K.W.; Hero, B.; Papadakis, V.; Frazier, A.L.; et al. Recommendations for Age-Appropriate Testing, Timing, and Frequency of Audiologic Monitoring During Childhood Cancer Treatment: An International Society of Paediatric Oncology Supportive Care Consensus Report. JAMA Oncol. 2021, 7, 1550–1558. [Google Scholar] [CrossRef]
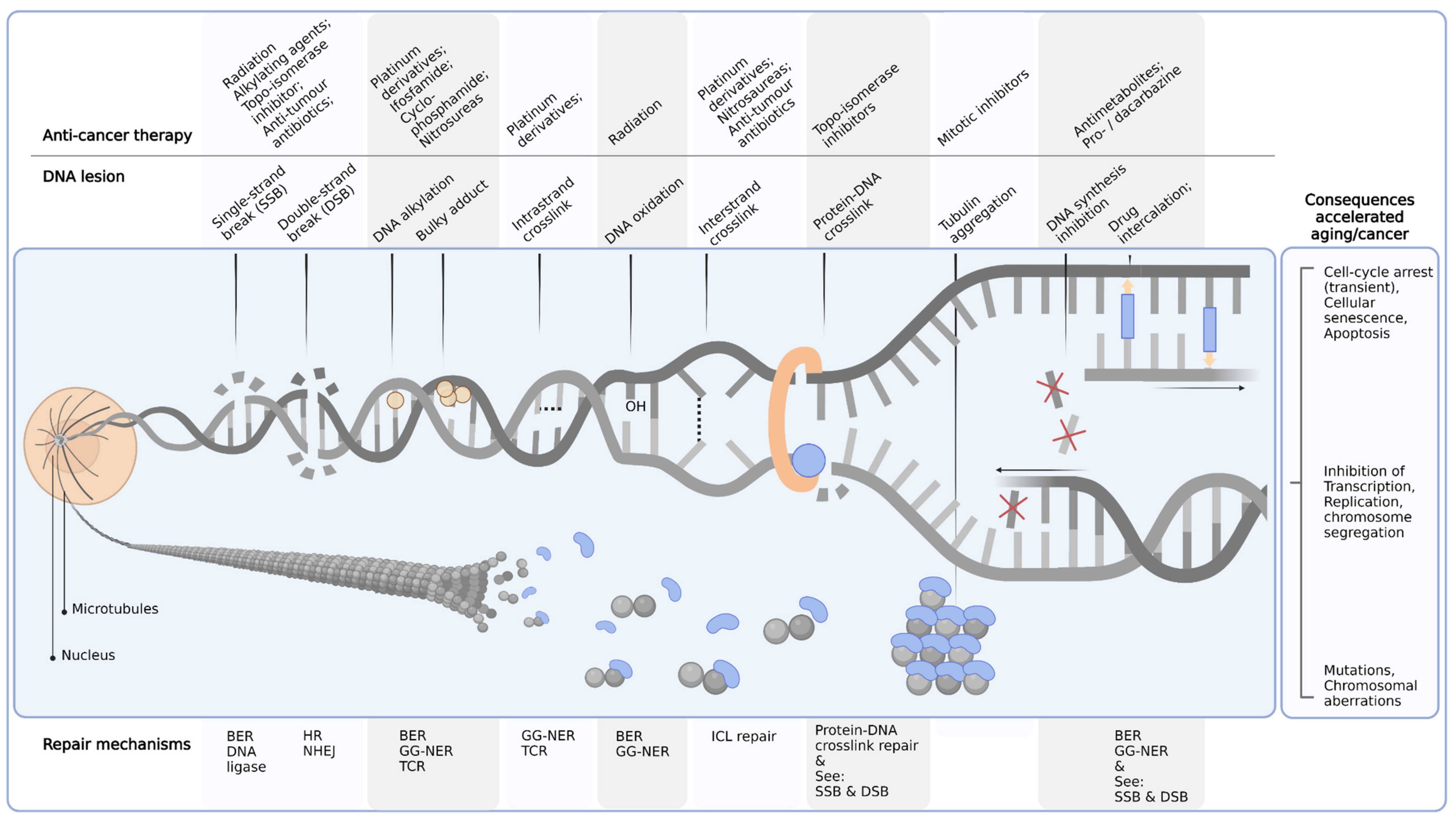

| Toxicity | Chemotherapy | Toxicity Details | |
|---|---|---|---|
| Nephrotoxicity | Alkylating agents | Platinum derivatives | |
| Cisplatin [20,21,22,23,24,25,26,27,28,29,30] | FS [21,25,26,27,28,29], salt wasting [21,24,26,27,28,29,30], hypomagnesemia [21,23,24,25,26,27,28,29,30,31], NDI [22,26,28,29,32], AKI [21,24,25,26,27,28,29,30,31], CKD [21,26,28,29], distal renal tubular acidosis [21,24,25,26,28], TMA [21,25,26,28], erythropoietin deficiency [21,26], renal concentrating defect [25], transient proteinuria [21], glomerular disease [30], tubulointerstitial disease [30], HUS [30], chronic renal failure [21] | ||
| Carboplatin [24,30,31] | Hypomagnesemia [24], Glomerular disease [30], Tubulointerstitial disease [30] | ||
| Ifosfamide [21,24,27,29,30,31] | FS [21,24,27,29,30], NDI [24,27,29], salt wasting [21], hyponatremia-SIADH [24], hemorrhagic cystitis [24,27,31], AKI (ATN) [21,27,29], CKD [27], renal tubular acidosis [24], glomerular disease [21,30], tubulointerstitial disease [30] | ||
| Cyclophosphamide [24,29,31] | Hemorrhagic cystitis [24,31], NDI [29]/hyponatremia-SIADH [24] | ||
| Melphalan [24,30] | Hyponatremia-SIADH [24], glomerular dysfunction [30] | ||
| Antimetabolites | Methotrexate [21,24,27,29,30,31,33,34] | Crystal nephropathy [24,27,29,31,33,34], AKI [21,24,27,30], renal tubular obstruction [30,33], CKD [29], acute tubular necrosis [21], hyponatremia-SIADH [24], hypomagnesemia [30], hypokalemia [30], hypocalcemia [30], glomerular disease [30,33], tubulointerstitial disease [30] | |
| Gemcitabine [21,24,27,29,30,33,35] | TMA [21,27,29,33,35], HUS [27,29,30,33,35], AKI [24,27,29] | ||
| Pemetrexed [24,27,30,33] | AKI (ATN) [24,27,33], AIN [27], renal tubular acidosis [24,27,33], NDI [24,27,33], glomerular disease [30] | ||
| Nitrosoureas [24,29,30,31] | CKD [24,29] (chronic interstitial nephritis, glomerulosclerosis) | ||
| Streptozocin [30] | FS [30] | ||
| Anthracyclines | |||
| Doxorubicin [33,36] | Focal segmental glomerular sclerosis [33], TMA [33], AKI [33] | ||
| Anti-cancer antibiotics | Mitomycin [30,33,35] | HUS [30,33,35], prerenal azotemia [30], glomerular disease [30], TMA [35] | |
| Plicamycin [30] | Glomerular disease [30] | ||
| Hepatotoxicity | Alkylating agents | Platinum derivatives | |
| Oxaliplatin [37,38,39,40,41] | Fatty liver/steatosis [38,39,40], VOD [38,39,40], SOS [37,40,41], nodular hyperplasia [38], fibrosis [37,38] | ||
| Cisplatin [28,39,42,43] | Sinusoidal dilation and obstruction [28,42], necrosis [42], infiltration of inflammatory cells [42], steatosis [39], cholestasis [39] | ||
| Carboplatin [39] | Cholestasis [39], VOD [39] | ||
| Cyclophosphamide [38,39,43] | VOD [38], hepatitis [39] | ||
| Melphalan [38,39,43] | Hepatitis [38,39], VOD [38] | ||
| Busulfan [31,38,39,43] | Hepatitis [38], cholestasis [38,39], VOD [38,40] | ||
| Nitrosoureas [38,40,43] | Hepatitis [38] | ||
| Carmustine [39] | Hepatitis [39] | ||
| Lomustine [39] | Hepatitis [39] | ||
| Chlorambucil [38,39,43] | Cholestasis [38,39] | ||
| Dacarbazine [39] | VOD [39] | ||
| Procarbazine [39] | Hepatitis [39] | ||
| Topoisomerase inhibitors | Irinotecan [37,38,39,40,43] | Fatty liver/steatosis [37,38,39,40,43], hepatitis [37,43], VOD [39] | |
| Etoposide [39,44] | Hepatitis [39], cholestasis [39], hepatocellular injury [44], hyperbilirubinemia [44] | ||
| Enzyme | Asparaginase [38,39] | Steatosis [38,39], hepatitis [38,39] | |
| Antimetabolites | Cytarabine [38,39,40,43] | Biliary stricture [38,39,43], cholestasis [38,39,43,44], fibrosis [43] | |
| 5-Fluorouracil [37,38,39,40,41] | Steatosis [37,38], hepatitis [38,39], VOD [38], hyperbilirubinemia [37], cirrhosis [41] | ||
| Methotrexate [38,39,40,43,44] | Hepatitis [38,39], cirrhosis [38,39,43,44], nodular hyperplasia [38], fibrosis [38,39,43,44] | ||
| Anthracyclines [38,40] | Hepatitis [38], cholestasis [38], VOD [38,40] | ||
| Doxorubicin [39] | Hepatitis [39], cholestasis [39], VOD [39] | ||
| Capecitabine [37,39] | Cholestasis [39], steatosis [37] | ||
| Floxuridine [39,41] | Hepatitis [39], cholestasis [39], bile duct sclerosis [41] | ||
| Gemcitabine [39] | Hepatitis [39], cholestasis [39] | ||
| Mercaptopurine [38,39,43,44] | VOD [39,43], cholestasis [38,43,44], jaundice [38], hepatocellular injury [43,44], hepatitis [38,44] | ||
| Anti-cancer antibiotics | Dactinomycin [39] | Hepatitis [39], VOD [39] | |
| Mitomycin [39] | Hepatitis [39], VOD [39] | ||
| Mitoxantrone [39] | Hepatitis [39] | ||
| Mitotic inhibitors | Vinca alkaloids | ||
| Vincristine [39] | Hepatitis [39] | ||
| Vinblastine [39,43] | Hepatitis [43], VOD [39] | ||
| Radiotherapy [43,44,45] | RILD [45], VOD [43,44,45], combined modality-induced liver damage (CMILD) [45], SOS [45] | ||
| Neurotoxicity | Alkylating agents | Platinum derivatives [46,47] | Distal symmetrical sensory impairment [46,47], ataxia [46,47], (peripheral) neurotoxicity [46,47], loss of deep tendon reflexes [47] |
| Cisplatin [48,49,50] | Acute encephalopathy [49], chronic leukoencephalopathy (if combined with radiotherapy) [49], posterior reversible (leuko)encephalopathy [48,49], stroke/arterial ischemia [48,49], seizures [48,49], SIADH [48,49], transient cortical blindness [48,49], myelopathy [48,49], peripheral neuropathy [48], sensory impairment [48,50], sensory ataxia [48], myasthenic syndrome [48], muscle cramps [48], Lhermitte symptom [48] | ||
| Carboplatin [48,49] | Posterior reversible (leuko)encephalopathy [49], peripheral neuropathy [47,48], acute (cold-induced) transient syndrome [47], paresthesiasis in distal extremities and perioral region [47], chronic cumulative sensory neuropathy [47] | ||
| Oxaliplatin [46,47,48,49,50] | Posterior reversible (leuko)encephalopathy [49], acute (peripheral) neurotoxicity [46,48,50], chronic sensory neuropathy [48,50], acute cramps and fasciculations [50] | ||
| Cyclophosphamide [49] | Posterior reversible (leuko)encephalopathy [49], stroke/arterial ischemia [49], SIADH [49] | ||
| Nitrosoureas | |||
| Carmustine [49] | Acute encephalopathy [49] | ||
| Busulfan [49] | Seizures [49] | ||
| Chlorambucil [49] | Acute encephalopathy [49], seizures [49] | ||
| Procarbazine [48,49] | Acute encephalopathy [48,49], paresthesiasis [48], myalgia [48] | ||
| Ifosfamide [49] | Acute encephalopathy [49], posterior reversible (leuko)encephalopathy [49], seizures [49], extrapyramidal syndrome [49] | ||
| Thiotepa [49] | Acute encephalopathy [49], myelopathy [49] | ||
| Topoisomerase inhibitors | Etoposide [49] | Acute encephalopathy [49], chronic leukoencephalopathy [49], seizures [49], transient cortical blindness [49] | |
| Antimetabolite | Methotrexate [48,49] | Acute encephalopathy [48,49], chronic leukoencephalopathy [48,49], posterior reversible (leuko)encephalopathy [49], stroke/arterial ischemia [48,49], focal neurologic deficits [49], seizures [49], myelopathy [49] | |
| Cytarabine [48,49] | Acute encephalopathy [48,49], posterior reversible (leuko)encephalopathy [49], acute pancerebellar syndrome * [48,49], extrapyramidal syndrome [48,49], peripheral neuropathies [48], brachial plexopathy [48], myelopathy (liposomal cytarabine) [49], Horner syndrome [48], lateral bulbar palsy [48], nystagmus [48], lethargy [48], aseptic meningitis [48], locked-in syndrome [48] | ||
| Gemcitabine [49] | Acute encephalopathy [49], posterior reversible (leuko)encephalopathy [49], thrombotic microangiopathy [49], seizures [49] | ||
| 5-Fluorouracil [49] | Acute encephalopathy [49], acute pancerebellar syndrome [49], stroke/arterial ischemia [49], extrapyramidal syndrome [49] | ||
| Capecitabine [49] | Acute encephalopathy [49], acute pancerebellar syndrome [49] | ||
| Fludarabine [48,49] | Acute encephalopathy [49], chronic leukoencephalopathy [48,49], transient cortical blindness [48,49], seizures [48], paresthesiasis [48], ataxia [48], paralysis [48] | ||
| Pentostatin [49] | Acute encephalopathy [49], chronic leukoencephalopathy [49] | ||
| Nelarabine [48,49] | Acute encephalopathy [49], seizures [48,49], distal sensory impairment [48], distal motor impairment [48], myelopathy [49], tremor [48], muscle weakness [48] | ||
| Hydroxyurea [49] | Chronic leukoencephalopathy [49], seizures [49] | ||
| Anthracyclines | |||
| Doxorubicin [49] | Extrapyramidal syndrome [49] | ||
| Anti-cancer antibiotics | Mitomycin [49] | Acute encephalopathy [49] | |
| Bleomycin [48] | Cerebral and myocardial infarcts [48] | ||
| Enzyme | Asparaginase [49] | Acute encephalopathy [49], intracranial hemorrhage [49], sinus/cortical vein thrombosis [49] | |
| Mitotic inhibitors | Vinca alkaloids | ||
| Vincristine [46,48,49,50] (and Vinblastine, Vindesine, Vinorelbine) [48] | Acute encephalopathy [48,49], posterior reversible (leuko)encephalopathy [49], acute pancerebellar syndrome [49], seizures [48,49], SIADH [48,49], transient cortical blindness [48,49], sensory impairment [46,48,50], distal motor impairment [46,48], ataxia [46,48], autonomic neuropathy [46,48,50], muscle cramps [48,50], mild distal weakness [48,50], parkinsonism [48], athetosis [48] | ||
| Vinflunine [49] | Posterior reversible (leuko)encephalopathy [49] | ||
| Taxanes | |||
| Paclitaxel [46,47,49,50] | Distal sensory impairment [46,50], AMS [46,50], myopathy [50], distal motor impairment [46] | ||
| Docetaxel [47,50] | Sensory impairment [50], myalgia [50], myopathy [50] | ||
| Epothilones [46] | Spinal cord injury [46], distal sensory impairment [46], distal motor impairment [46] | ||
| Eribulin [46] | Sensory impairment [46], motor impairment [46] | ||
| Proteasome inhibitors | Bortezomib [46,48,50] | Severe neuropathic pain [46,48,50], autonomic neuropathy [50], dizziness [48], aphasia [48] | |
| Radiotherapy [49] (combined with chemotherapy) | Chronic leukoencephalopathy [49], myelopathy [49] | ||
| Cardiotoxicity | Alkylating agents | Cisplatin [42,51,52,53,54,55] | Degeneration and necrosis of cardiac muscle [42], fibrosis [42], vacuolated cytoplasm [42], blood infiltration [42], palpitations [51,52], left-sided chest pain [51,52]/angina pectoris [54], hypotension [51,54], arrhythmias [51,53,54], interventricular block [51], MI [51,52,53,54], atrial fibrillation [53], left bundle branch block [53], ischemia [52], hypertension [52,54], thromboembolism [54,55], myocarditis [54], CHF [54], cardiomyopathy [54] |
| Mitomycin [51,52,54] | CHF [51,54], HF [52], cardiomyopathy [52], | ||
| Carmustine [51] | Chest pain [51], hypotension [51], arrhythmia [51] | ||
| Busulfan [51] | CHF [51], palpitations [51], cardiac tamponade [51], pulmonary congestion [51], cardiomegaly [51], pericardial effusion [51] | ||
| Chlormethine [51] | Persistent tachycardia [51], pulse irregularity [51], junctional or atrial ectopic beats [51] | ||
| Amsacrine [53] | Arrhythmia [53], CHF [53], cardiomyopathy [53] | ||
| Capecitabine [55] | Ischemia [55] | ||
| Cyclophosphamide [51,53,54,55,56,57] | Neurohumoral activation [56,57], mitral regurgitation [56,57] CHF [51,53], chest pain [51], pleural and pericardial effusions [51], pericardial friction rub [51], cardiomegaly [51] myo(peri)carditis [53,54], HF [52,54,57], cardiomyopathy [52], left ventricular dysfunction [54,55], arrhythmia [54] | ||
| Ifosfamide [51,54,55] | CHF [51,54], pleural effusion [51], arrhythmia [51,54], left ventricular dysfunction [55] | ||
| Antimetabolites | Anthracyclines [51,52,53,54,56,57] | CHF [53,56], left ventricular dysfunction [53,56,57], (acute) myocarditis [56,57], arrhythmia [53,56,57] | |
| Doxorubicin [52,53,57,58] | Reversible acute myopericarditis [53], HF [52,57], CHF [58], left ventricular dysfunction [55] | ||
| Doxorubicin, Daunorubicin [51] | Reversible acute myopericarditis [51], HF [51] | ||
| Epirubicin [51,58] | Arrhythmia [51], pericarditis [51], myocarditis [51], CHF [51,58], left ventricular dysfunction [55] | ||
| Idarubicin [51,55] | CHF [51], left ventricular dysfunction [55], arrhythmias [51], angina pectoris [51], MI [51] | ||
| Mitoxantrone [51,54] | Arrhythmias [51,54], CHF [51,54], MI [51,54], HF [52] | ||
| Capecitabine, 5-fluorouracil, cytarabine [56] | Ischemia [56], pericarditis [56], CHF [56], cardiogenic shock [56] | ||
| Capecitabine, 5-fluorouracil [54] | Angina-like chest pain [54], MI [54], arrhythmia [54], HF [54] | ||
| 5-Fluorouracil [51,52,53,55,57] | Angina pectoris [51,52,53], MI [51,52,55], hypotension [51,53], cardiogenic shock [51,53,55,57], left ventricular dysfunction [53], HF (with global hypokinesis [53]) [55,57], ischemia [52,55,57], pericarditis [57], arrhythmia [55] | ||
| Cytarabine [53] | Arrhythmia [53], pericarditis [53], acute respiratory distress [53], CHF [53] | ||
| Clofarabine [55] | Left ventricular dysfunction [55] | ||
| Topoisomerase inhibitors | Etoposide [51,52] | Hypotension [51,52], MI [51] | |
| Teniposide [51] | Arrhythmia [51], hypotension [51] | ||
| Mitotic inhibitors | Paclitaxel, vinca alkaloids [56,57] | Sinus bradycardia [56,57], ventricular tachycardia [56,57], atrioventricular block [56,57], hypotension [56], CHF [56], ischemia [56,57], HF [57] | |
| Paclitaxel [51,52,53,54,55] | Arrhythmia [51,52,53,54,55], MI [51,53,55], atrioventricular or left bundle branch block [51,54], second- or third-degree heart block [53], cardiac ischemia [53,54,55], heart block [52] | ||
| Vinca alkaloids [51,52] | MI [51,52], dyspnea [51], pulmonary oedema [51], atrial fibrillation [51], angina pectoris [52] | ||
| Docetaxel [54,55] | Left ventricular dysfunction [55], ischemia [54,55], MI [55] | ||
| Other | Tretinoin [51] | Retinoic acid syndrome ** [51], arrhythmia [51], hypotension [51], hypertension [51], HF [51] | |
| Pentostatin [51] | Angina pectoris [51], MI [51], CHF [51], arrhythmia [51] | ||
| Hematological Toxicity | Alkylating agents | Platinum derivatives | |
| Carboplatin [59,60] | Thrombocytopenia [59,60] | ||
| Oxaliplatin [60] | Thrombocytopenia [60] | ||
| Nitrogen mustard [61,62] | Bone marrow suppression [61], myelosuppression [62] | ||
| Melphalan [61] | Bone marrow suppression [61] | ||
| Cyclophosphamide [34,61] | Myelosuppression (leukopenia)/neutropenia [34], anemia [34], bone marrow suppression [34,61] | ||
| Chlorambucil [61,63] | Bone marrow suppression [61,63] | ||
| Nitrosoureas [61,62] | Bone marrow suppression [61], myelosuppression [62] | ||
| Dacarbazine [62] | Myelosuppression [62] | ||
| Busulfan [62] | Myelosuppression [62] | ||
| Antimetabolites | Methotrexate [61] | Bone marrow suppression [61] | |
| Antipyrimidines [62] | Myelosuppression [62] | ||
| 5-Fluorouracil [61] | Bone marrow suppression [61] | ||
| Cytarabine [61] | Bone marrow suppression [61] | ||
| 6-Mercaptopurine [61] | Bone marrow suppression [61] | ||
| Anthracyclines [62] | Myelosuppression [62] | ||
| Doxorubicin [61] | Bone marrow suppression [61] | ||
| Anti-cancer antibiotics | Dactinomycin [61] | Bone marrow suppression [61] | |
| Combination therapies | Docetaxel-cyclophosphamide [64] | Febrile neutropenia [64] | |
| Cyclophosphamide-doxorubicin—vincristine-prednisone [65] | Neutropenia [65], febrile neutropenia [66] | ||
| Cyclophosphamide—methotrexate—5-fluorouracil [67] | Leukopenia [67], anemia [67], neutropenia [67], thrombocytopenia [67] | ||
| Doxorubicin-cyclophosphamide [65] | Neutropenia [65] | ||
| Cyclophosphamide-doxorubicin-5-fluorouracil [65] | Neutropenia [65] | ||
| 5-Fluorouracil-cisplatin [65] | Neutropenia [65] | ||
| Etoposide-doxorubicin-5-fluorouracil [65] | Neutropenia [65] | ||
| Docetaxel-doxorubicin [65] | Neutropenia [65] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. https://doi.org/10.3390/cancers14030627
van den Boogaard WMC, Komninos DSJ, Vermeij WP. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers. 2022; 14(3):627. https://doi.org/10.3390/cancers14030627
Chicago/Turabian Stylevan den Boogaard, Winnie M. C., Daphne S. J. Komninos, and Wilbert P. Vermeij. 2022. "Chemotherapy Side-Effects: Not All DNA Damage Is Equal" Cancers 14, no. 3: 627. https://doi.org/10.3390/cancers14030627






