Modeled Early Longitudinal PSA Kinetics Prognostic Value in Rising PSA Prostate Cancer Patients after Local Therapy Treated with ADT +/− Docetaxel
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
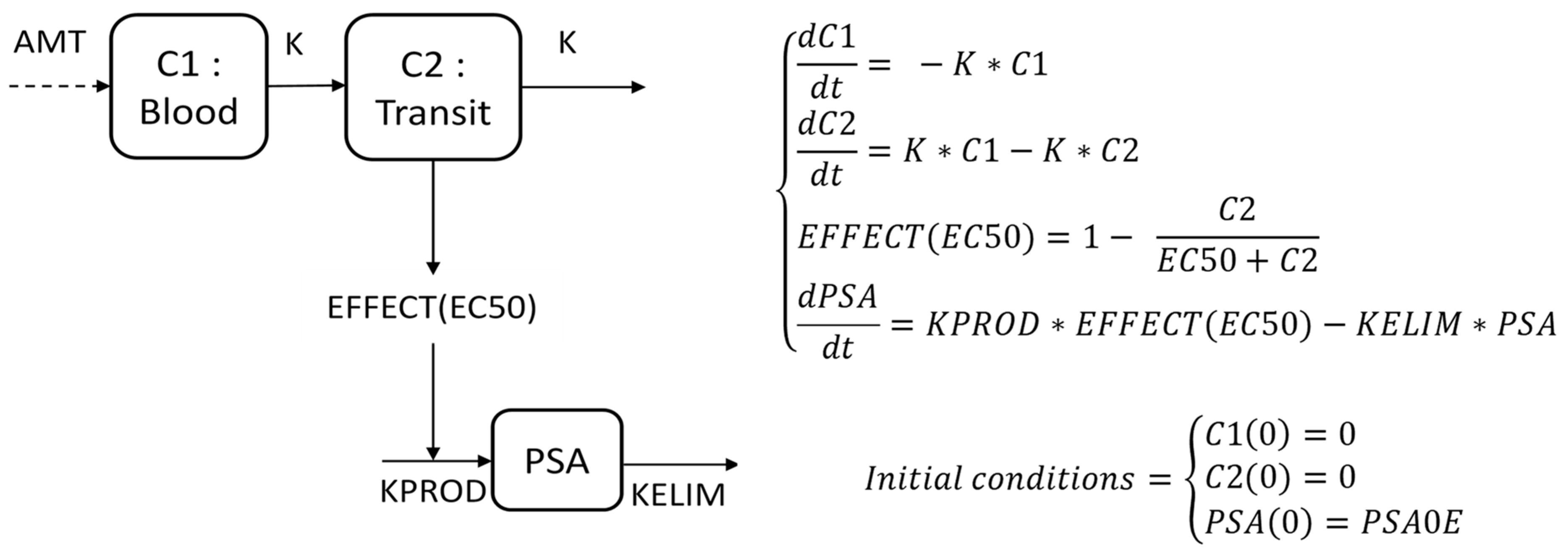
2.2. Mathematical Modeling of PSA Kinetics
2.3. Model Qualification
2.4. Prognostic Value of Modeled PSA Kinetics during the First 100 Days
2.5. Statistical Analyses and Computing Process
3. Results
3.1. Patient Selection
3.2. Model Qualification
3.3. KELIM and KPROD Prognostic Values on PSA-PFS and OS in Univariate Analyses
3.4. KELIM and KPROD Prognostic Value on PSA-PFS and OS in Multivariate Analyses
3.4.1. Multivariate Survival Models Integrating KELIM as a Modeled PSA Kinetic Parameter
3.4.2. Multivariate Survival Models Integrating KPROD as a Modeled PSA Kinetic Parameter
3.4.3. C-Index Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djavan, B.; Moul, J.W.; Zlotta, A.; Remzi, M.; Ravery, V. PSA Progression Following Radical Prostatectomy and Radiation Therapy: New Standards in the New Millenium. Eur. Urol. 2003, 43, 12–27. [Google Scholar] [CrossRef]
- Van den Broeck, T.; van den Bergh, R.C.N.; Briers, E.; Cornford, P.; Cumberbatch, M.; Tilki, D.; De Santis, M.; Fanti, S.; Fossati, N.; Gillessen, S.; et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur. Urol. Focus 2020, 6, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet Lond. Engl. 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Scher, H.I.; Eisenberger, M.; D’Amico, A.V.; Halabi, S.; Small, E.J.; Morris, M.; Kattan, M.W.; Roach, M.; Kantoff, P.; Pienta, K.J.; et al. Eligibility and Outcomes Reporting Guidelines for Clinical Trials for Patients in the State of a Rising Prostate-Specific Antigen: Recommendations From the Prostate-Specific Antigen Working Group. J. Clin. Oncol. 2004, 22, 537–556. [Google Scholar] [CrossRef]
- van den Bergh, R.C.N.; van Casteren, N.J.; van den Broeck, T.; Fordyce, E.R.; Gietzmann, W.K.M.; Stewart, F.; MacLennan, S.; Dabestani, S.; Bellmunt, J.; Bolla, M.; et al. Role of Hormonal Treatment in Prostate Cancer Patients with Nonmetastatic Disease Recurrence After Local Curative Treatment: A Systematic Review. Eur. Urol. 2016, 69, 802–820. [Google Scholar] [CrossRef]
- Yu, E.Y.; Lin, D.W. Avoiding Undertreatment of Aggressive Prostate Cancer by Early Use of Chemotherapy. JAMA Oncol. 2017, 3, 13–14. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet Lond. Engl. 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [Green Version]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef]
- Oudard, S.; Latorzeff, I.; Caty, A.; Miglianico, L.; Sevin, E.; Hardy-Bessard, A.C.; Delva, R.; Rolland, F.; Mouret, L.; Priou, F.; et al. Effect of Adding Docetaxel to Androgen-Deprivation Therapy in Patients With High-Risk Prostate Cancer With Rising Prostate-Specific Antigen Levels After Primary Local Therapy. JAMA Oncol. 2019, 5, 623–632. [Google Scholar] [CrossRef]
- You, B.; Colomban, O.; Heywood, M.; Lee, C.; Davy, M.; Reed, N.; Pignata, S.; Varsellona, N.; Emons, G.; Rehman, K.; et al. The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: Data from CALYPSO trial (a GINECO-GCIG study). Gynecol. Oncol. 2013, 130, 289–294. [Google Scholar] [CrossRef]
- You, B.; Robelin, P.; Tod, M.; Louvet, C.; Lotz, J.-P.; Abadie-Lacourtoisie, S.; Fabbro, M.; Desauw, C.; Bonichon-Lamichhane, N.; Kurtz, J.-E.; et al. CA-125 ELIMination Rate Constant K (KELIM) Is a Marker of Chemosensitivity in Patients with Ovarian Cancer: Results from the Phase II CHIVA Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4625–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colomban, O.; Tod, M.; Leary, A.; Ray-Coquard, I.; Lortholary, A.; Hardy-Bessard, A.C.; Pfisterer, J.; Du Bois, A.; Kurzeder, C.; Burges, A.; et al. Early Modeled Longitudinal CA-125 Kinetics and Survival of Ovarian Cancer Patients: A GINECO AGO MRC CTU Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5342–5350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubley, G.J.; Carducci, M.; Dahut, W.; Dawson, N.; Daliani, D.; Eisenberger, M.; Figg, W.D.; Freidlin, B.; Halabi, S.; Hudes, G.; et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 3461–3467. [Google Scholar] [CrossRef] [PubMed]
- Almufti, R.; Wilbaux, M.; Oza, A.; Henin, E.; Freyer, G.; Tod, M.; Colomban, O.; You, B. A critical review of the analytical approaches for circulating tumor biomarker kinetics during treatment. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 41–56. [Google Scholar] [CrossRef]
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, M.-H.; McLeod, D.; Carroll, P.R.; Moul, J.W.; D’Amico, A.V. Predictors of Prostate Cancer–Specific Mortality After Radical Prostatectomy or Radiation Therapy. J. Clin. Oncol. 2005, 23, 6992–6998. [Google Scholar] [CrossRef]
- Jacqmin, P.; Snoeck, E.; van Schaick, E.A.; Gieschke, R.; Pillai, P.; Steimer, J.-L.; Girard, P. Modelling response time profiles in the absence of drug concentrations: Definition and performance evaluation of the K-PD model. J. Pharmacokinet. Pharmacodyn. 2007, 34, 57–85. [Google Scholar] [CrossRef]
- Bauer, R.J.; Boeckmann, L. NONMEM User’s Guides (1989–2009) (Ellicott City, MD, USA: Icon Development Solutions). 2009. Available online: https://nonlin-model.org/ (accessed on 7 April 2021).
- Bauer, R.J. NONMEM Tutorial Part II: Estimation Methods and Advanced Examples. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 538–556. [Google Scholar]
- Karlsson, M.O.; Savic, R.M. Diagnosing Model Diagnostics. Clin. Pharmacol. Ther. 2007. Available online: https://www.meta.org/papers/diagnosing-model-diagnostics/17571070 (accessed on 18 May 2021).
- You, B.; Perrin, P.; Freyer, G.; Ruffion, A.; Tranchand, B.; Hénin, E.; Paparel, P.; Ribba, B.; Devonec, M.; Falandry, C.; et al. Advantages of prostate-specific antigen (PSA) clearance model over simple PSA half-life computation to describe PSA decrease after prostate adenomectomy. Clin. Biochem. 2008, 41, 785–795. [Google Scholar] [CrossRef]




| Univariate Analyses (Kaplan–Meier and Log-Rank Test) | |||||
| Median [95% CI] | p-Value | ||||
| PSA-PFS | |||||
| KELIM | |||||
| Unfavorable | 10.1 [9.3; 12.4] | <0.001 | |||
| Favorable | 15.1 [12.6; 22.2] | ||||
| KPROD | |||||
| Unfavorable | 9.8 [8.8; 12.1] | <0.001 | |||
| Favorable | 15.1 [12.6; 21.2] | ||||
| OS | |||||
| KELIM | |||||
| Unfavorable | 157 [115; NR] | 0.035 | |||
| Favorable | NR [NR; NR] | ||||
| KPROD | |||||
| Unfavorable | 157 [NR; NR] | 0.037 | |||
| Favorable | NR [115; NR] | ||||
| Multivariate Analyses (Cox Regression) | |||||
| HR [95% CI] | p-Value | C-Index [95% CI] | |||
| Analyses with KELIM | |||||
| PSA-PFS | |||||
| KELIM | 0.66 [0.62; 0.70] | ||||
| Unfavorable | Reference | 0.015 | |||
| Favorable | 0.63 [0.43; 0.92] | ||||
| Primary therapy | |||||
| Radiotherapy | Reference | <0.001 | |||
| Prostatectomy | 0.42 [0.29; 0.62] | ||||
| OS | |||||
| KELIM | 0.6 [0.53; 0.67] | ||||
| Unfavorable | Reference | 0.02 | |||
| Favorable | 0.55 [0.33; 0.91] | ||||
| PSA-DT | |||||
| ≥6 months | Reference | 0.029 | |||
| <6 months | 0.57 [0.34; 0.95] | ||||
| Analyses with KPROD | |||||
| PSA-PFS | |||||
| KELIM | 0.66 [0.61; 0.71] | ||||
| Unfavorable | Reference | 0.028 | |||
| Favorable | 0.65 [0.45; 0.96] | ||||
| Primary therapy | |||||
| Radiotherapy | Reference | <0.001 | |||
| Prostatectomy | 0.42 [0.29; 0.62] | ||||
| OS | |||||
| KELIM | 0.61 [0.54; 0.68] | ||||
| Unfavorable | Reference | 0.015 | |||
| Favorable | 0.53 [0.32; 0.89] | ||||
| PSA-DT | |||||
| ≥6 months | Reference | 0.021 | |||
| <6 months | 0.55 [0.33; 0.91] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrot, A.; Elaidi, R.-T.; Colomban, O.; Maillet, D.; Tod, M.; You, B.; Oudard, S. Modeled Early Longitudinal PSA Kinetics Prognostic Value in Rising PSA Prostate Cancer Patients after Local Therapy Treated with ADT +/− Docetaxel. Cancers 2022, 14, 815. https://doi.org/10.3390/cancers14030815
Carrot A, Elaidi R-T, Colomban O, Maillet D, Tod M, You B, Oudard S. Modeled Early Longitudinal PSA Kinetics Prognostic Value in Rising PSA Prostate Cancer Patients after Local Therapy Treated with ADT +/− Docetaxel. Cancers. 2022; 14(3):815. https://doi.org/10.3390/cancers14030815
Chicago/Turabian StyleCarrot, Aurore, Reza-Thierry Elaidi, Olivier Colomban, Denis Maillet, Michel Tod, Benoit You, and Stéphane Oudard. 2022. "Modeled Early Longitudinal PSA Kinetics Prognostic Value in Rising PSA Prostate Cancer Patients after Local Therapy Treated with ADT +/− Docetaxel" Cancers 14, no. 3: 815. https://doi.org/10.3390/cancers14030815
APA StyleCarrot, A., Elaidi, R.-T., Colomban, O., Maillet, D., Tod, M., You, B., & Oudard, S. (2022). Modeled Early Longitudinal PSA Kinetics Prognostic Value in Rising PSA Prostate Cancer Patients after Local Therapy Treated with ADT +/− Docetaxel. Cancers, 14(3), 815. https://doi.org/10.3390/cancers14030815








