Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice
Abstract
:Simple Summary
Abstract
1. Introduction
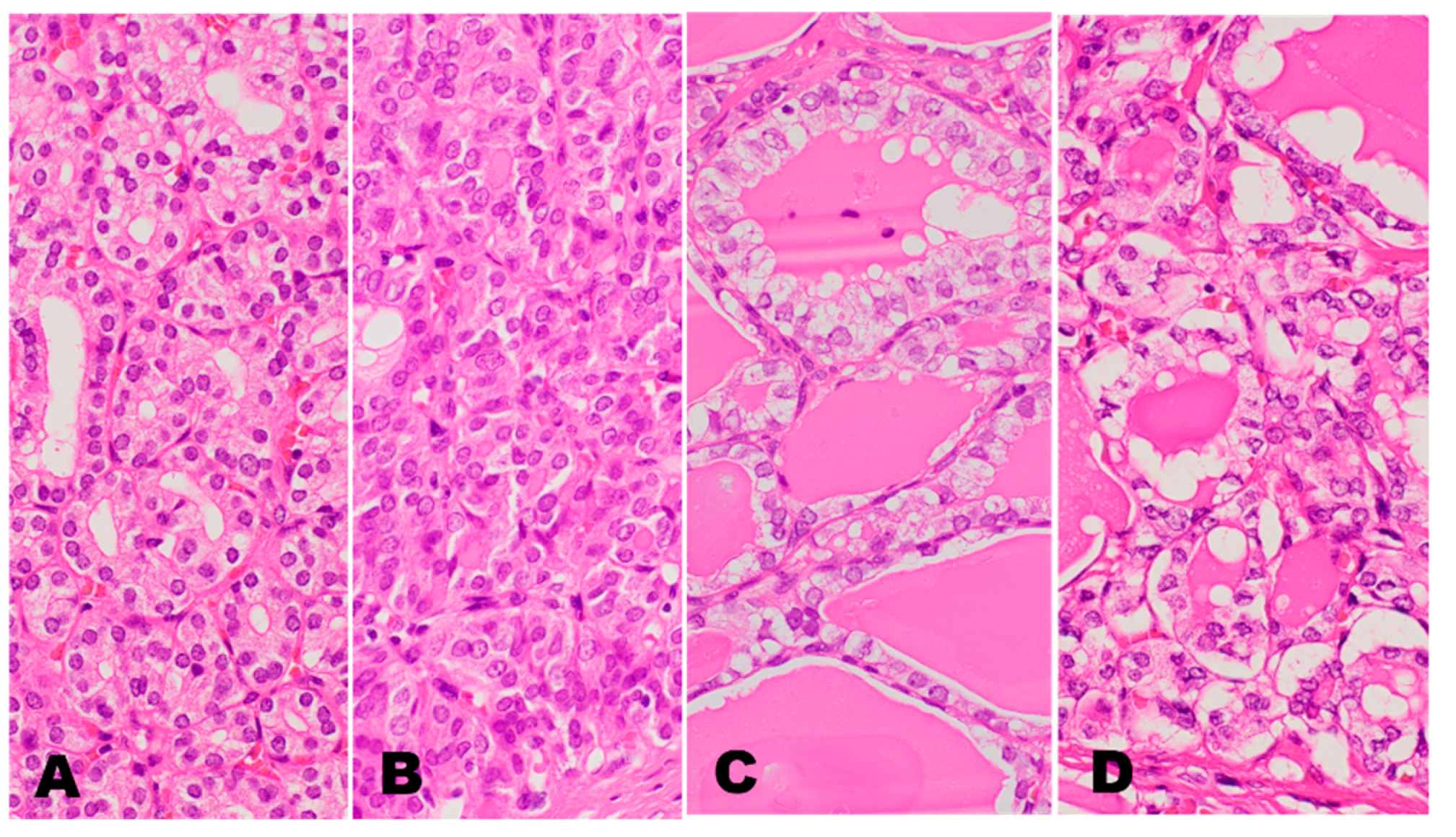
2. RAS Mutated Follicular Pattern Thyroid Tumors
3. RAS-like PTC
4. PTC in Asian Practice and Western Practice


5. Why the Prevalence of BRAFV600E Mutation Was High in Asian PTC Cohorts but Low in Most Western PTC Cohorts
6. Why the BRAFV600E Gene Test Identifies High-Risk PTCs in Western Patients, but It Was Not Reproducible in Most Asian Patient Cohorts
7. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Kakudo, K. Asian and Western practice in thyroid pathology: Similarities and differences. Gland Surg. 2020, 9, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.A.; Munkhdelger, J.; Fukuoka, J.; Bychkov, A. Prevalence of BRAFV600E mutation in Asian series of papillary thyroid carcinoma-a contemporary systematic review. Gland Surg. 2020, 9, 1878–1900. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, A.; Tallini, G.; et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2005, 90, 6373–6379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeniran, A.J.; Zhu, Z.; Gandhi, M.; Steward, D.L.; Fidler, J.P.; Giordano, T.J.; Biddinger, P.W.; Nikiforov, Y.E. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am. J. Surg. Pathol. 2006, 30, 216–222. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lee, K.C.; Schneider, E.B.; Zeiger, M.A. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: A meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 4559–4570. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.T.; Chen, Y.J.; Chou, F.F.; Li, C.L.; Wu, W.L.; Tsai, P.C.; Huang, C.C.; Cheng, J.T. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin. Endocrinol. 2005, 63, 461–466. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, W.B.; Song, J.Y.; Rhee, Y.S.; Gong, G.; Cho, Y.M.; Kim, S.Y.; Kim, S.C.; Hong, S.J.; Shong, Y.K. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin. Endocrinol. 2005, 63, 588–593. [Google Scholar] [CrossRef]
- Ito, Y.; Yoshida, H.; Maruo, R.; Morita, S.; Takano, T.; Hirokawa, M.; Yabuta, T.; Fukushima, M.; Inoue, H.; Tomoda, C.; et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: Its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr. J. 2009, 56, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.; Park, J.S.; Sohn, J.H.; Kim, J.H.; Park, S.K.; Seo, A.N.; Park, J.Y. BRAFV600E mutation does not serve as a prognostic factor in Korean patients with papillary thyroid carcinoma. Auris Nasus Larynx. 2012, 39, 198–203. [Google Scholar] [CrossRef]
- Nam, J.K.; Jung, C.K.; Song, B.J.; Lim, D.J.; Chae, B.J.; Lee, N.S.; Park, W.C.; Kim, J.S.; Jung, S.S.; Bae, J.S. Is the BRAFV600E mutation useful as a predictor of preoperative risk in papillary thyroid cancer? Am. J. Surg. 2012, 203, 436–441. [Google Scholar] [CrossRef]
- Ji, W.; Xie, H.; Wei, B.; Shen, H.; Liu, A.; Gao, Y.; Wang, L. Relationship between BRAF V600E gene mutation and the clinical and pathologic characteristics of papillary thyroid microcarcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 3492–3499. [Google Scholar]
- Yan, C.; Huang, M.; Li, X.; Wang, T.; Ling, R. Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocr. Connect. 2019, 8, 988–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfi, G.; Sancisi, V.; Piana, S.; Ciarrocchi, A. Time to re-consider the meaning of BRAF V600E mutation in papillary thyroid carcinoma. Int. J. Cancer 2015, 137, 1001–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Toraldo, G.; Cerda, S.; Godley, F.A.; Rao, S.R.; McAneny, D.; Doherty, G.; Braverman, L.; Lee, S.L. Utilities of RAS Mutations in Preoperative Fine Needle Biopsies for Decision Making for Thyroid Nodule Management: Results from a Single-Center Prospective Cohort. Thyroid 2020, 30, 536–547. [Google Scholar] [CrossRef]
- Soares, P.; Póvoa, A.A.; Melo, M.; Vinagre, J.; Máximo, V.; Eloy, C.; Cameselle-Teijeiro, J.M.; Sobrinho-Simões, M. Molecular Pathology of Non-familial Follicular Epithelial-Derived Thyroid Cancer in Adults: From RAS/BRAF-like Tumor Designations to Molecular Risk Stratification. Endocr. Pathol. 2021, 32, 44–62. [Google Scholar] [CrossRef]
- Kakudo, K.; Katoh, R.; Sakamoto, A.; Asa, S.; DeLellis, R.A.; Carney, J.A.; Naganuma, H.; Kameyama, K.; Takami, H. Thyroid gland: International case conference. Endocr. Pathol. 2002, 13, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, M.; Carney, J.A.; Goellner, J.R.; DeLellis, R.A.; Heffess, C.S.; Katoh, R.; Tsujimoto, M.; Kakudo, K. Observer variation of encapsulated follicular lesions of the thyroid gland. Am. J. Surg. Pathol. 2002, 26, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- De Lellis, R.A.; Lloyd, R.V.; Heitz, P.U.; Eng, C.; World Health Organization. Pathology and Genetics of Tumours of Endocrine Organs, 3rd ed.; IARC Press: Lyon, France, 2004. [Google Scholar]
- Liu, J.; Singh, B.; Tallini, G.; Carlson, D.L.; Katabi, N.; Shaha, A.; Tuttle, R.M.; Ghossein, R.A. Follicular variant of papillary thyroid carcinoma: A clinicopathologic study of a problematic entity. Cancer 2006, 107, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.; Ricarte-Filho, J.; Knauf, J.; Shaha, A.; Tuttle, M.; Fagin, J.A.; Ghossein, R.A. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod. Pathol. 2010, 23, 1191–1200. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakudo, K.; Higuchi, M.; Hirokawa, M.; Satoh, S.; Jung, C.K.; Bychkov, A. Thyroid FNA cytology in Asian practice-Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017, 28, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A.; Keelawat, S.; Agarwal, S.; Jain, D.; Jung, C.K.; Hong, S.; Lai, C.R.; Satoh, S.; Kakudo, K. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: A multi-institutional study in five Asian countries. Pathology 2018, 50, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, C.; Vuong, H.G.; Nguyen, T.Q.; Nguyen, H.C.; Jung, C.K.; Kakudo, K.; Bychkov, A. The Incidence of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: A Meta-Analysis Assessing Worldwide Impact of the Reclassification. Thyroid 2021, 31, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gandhi, M.; Nikiforova, M.N.; Fischer, A.H.; Nikiforov, Y.E. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am. J. Clin. Pathol. 2003, 120, 71–77. [Google Scholar] [CrossRef]
- Sobrinho-Simões, M.; Preto, A.; Rocha, A.S.; Castro, P.; Máximo, V.; Fonseca, E.; Soares, P. Molecular pathology of well-differentiated thyroid carcinomas. Virchows Arch. 2005, 447, 787–793. [Google Scholar] [CrossRef]
- Castro, P.; Rebocho, A.P.; Soares, R.J.; Magalhães, J.; Roque, L.; Trovisco, V.; Vieira de Castro, I.; Cardoso-de-Oliveira, M.; Fonseca, E.; Soares, P.; et al. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T.; Ezzat, S.; Asa, S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Rev. Cancer 2006, 6, 292–306. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, R.; Osamura, R.Y.; Kloppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Johnson, D.N.; Furtado, L.V.; Long, B.C.; Zhen, C.J.; Wurst, M.; Mujacic, I.; Kadri, S.; Segal, J.P.; Antic, T.; Cipriani, N.A. Noninvasive Follicular Thyroid Neoplasms with Papillary-like Nuclear Features Are Genetically and Biologically Similar to Adenomatous Nodules and Distinct from Papillary Thyroid Carcinomas with Extensive Follicular Growth. Arch. Pathol. Lab. Med. 2018, 142, 838–850. [Google Scholar] [CrossRef]
- Acquaviva, G.; Visani, M.; Repaci, A.; Rhoden, K.J.; de Biase, D.; Pession, A.; Giovanni, T. Molecular pathology of thyroid tumours of follicular cells: A review of genetic alterations and their clinicopathological relevance. Histopathology 2018, 72, 6–31. [Google Scholar] [CrossRef] [PubMed]
- The International Agency for Research on Cancer, World Health Organization Classification of Tumors of Endocrine Organs, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2022; (in press).
- Tallini, G.; Tuttle, R.M.; Ghossein, R.A. The History of the Follicular Variant of Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakudo, K.; Bai, Y.; Liu, Z.; Ozaki, T. Encapsulated papillary thyroid carcinoma, follicular variant: A misnomer. Pathol. Int. 2012, 62, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, K.; Bai, Y.; Liu, Z.; Li, Y.; Ito, Y.; Ozaki, T. Classification of thyroid follicular cell tumors: With special reference to borderline lesions. Endocr. J. 2012, 59, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakudo, K. How to handle borderline/precursor thyroid tumors in management of patients with thyroid nodules. Gland Surg. 2018, 7 (Suppl. S1), S8–S18. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, L.D. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: A name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod. Pathol. 2016, 29, 698–707. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, G.; Nakamura, M.; Koike, E.; Li, Y.; Ozaki, T.; Mori, I.; Taniguchi, E.; Kakudo, K. Encapsulated follicular thyroid tumor with equivocal nuclear changes, so-called well-differentiated tumor of uncertain malignant potential: A morphological, immunohistochemical, and molecular appraisal. Cancer Sci. 2011, 102, 288–294. [Google Scholar] [CrossRef]
- Bychkov, A.; Hirokawa, M.; Jung, C.K.; Liu, Z.; Zhu, Y.; Hong, S.W.; Satoh, S.; Lai, C.R.; Huynh, L.; Kakudo, K. Low Rate of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice. Thyroid 2017, 27, 983–984. [Google Scholar] [CrossRef]
- Liu, Z.; Bychkov, A.; Jung, C.K.; Hirokawa, M.; Sui, S.; Hong, S.; Lai, C.R.; Jain, D.; Canberk, S.; Kakudo, K. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol. Int. 2019, 69, 202–210. [Google Scholar] [CrossRef]
- Seo, J.Y.; Park, J.H.; Pyo, J.Y.; Cha, Y.J.; Jung, C.K.; Song, D.E.; Kwak, J.J.; Park, S.Y.; Na, H.Y.; Kim, J.H.; et al. A Multi-institutional Study of Prevalence and Clinicopathologic Features of Non-invasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features (NIFTP) in Korea. J. Pathol. Transl. Med. 2019, 53, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Erickson, L.A.; Casey, M.B.; Lam, K.Y.; Lohse, C.M.; Asa, S.L.; Chan, J.K.; DeLellis, R.A.; Harach, H.R.; Kakudo, K.; et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am. J. Surg. Pathol. 2004, 28, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, T.M.; Asa, S.L.; Chan, J.K.; DeLellis, R.A.; Heffess, C.S.; LiVolsi, V.A.; Wenig, B.M. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am. J. Clin. Pathol. 2008, 130, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Poller, D.N.; Johnson, S.J.; Bongiovanni, M. Measures to reduce diagnostic error and improve clinical decision making in thyroid FNA aspiration cytology: A proposed framework. Cancer Cytopathol. 2020, 128, 917–927. [Google Scholar] [CrossRef]
- Jung, C.K.; Bychkov, A.; Song, D.E.; Kim, J.H.; Zhu, Y.; Liu, Z.; Keelawat, S.; Lai, C.R.; Hirokawa, M.; Kameyama, K.; et al. Molecular Correlates and Nuclear Features of Encapsulated Follicular-Patterned Thyroid Neoplasms. Endocrinol. Metab. 2021, 36, 123–133. [Google Scholar] [CrossRef]
- Labarge, B.; Walter, V.; Lengerich, E.J.; Crist, H.; Karamchandani, D.; Williams, N.; Goldenberg, D.; Bann, D.V.; Warrick, J.I. Evidence of a positive association between malpractice climate and thyroid cancer incidence in the United States. PLoS ONE 2018, 13, e0199862. [Google Scholar] [CrossRef]
- Warrick, J.; Lengerich, E. Thyroid Cancer Overdiagnosis and Malpractice Climate. Arch. Pathol. Lab. Med. 2019, 143, 414–415. [Google Scholar] [CrossRef] [Green Version]
- Renshaw, A.A.; Gould, E.W. Why there is the tendency to "overdiagnose" the follicular variant of papillary thyroid carcinoma. Am. J. Clin. Pathol. 2002, 117, 19–21. [Google Scholar] [CrossRef]
- Strickland, K.C.; Vivero, M.; Jo, V.Y.; Lowe, A.C.; Hollowell, M.; Qian, X.; Wieczorek, T.J.; French, C.A.; Teot, L.A.; Sadow, P.M.; et al. Preoperative Cytologic Diagnosis of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: A Prospective Analysis. Thyroid 2016, 26, 1466–1471. [Google Scholar] [CrossRef] [Green Version]
- Faquin, W.C.; Wong, L.Q.; Afrogheh, A.H.; Ali, S.Z.; Bishop, J.A.; Bongiovanni, M.; Pusztaszeri, M.P.; VandenBussche, C.J.; Gourmaud, J.; Vaickus, L.J.; et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol. 2016, 124, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Reinke, R.H.; Larsen, S.R.; Mathiesen, J.S.; Godballe, C.; Londero, S.C. Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features is Rare: A Population Based Study of Incidence. Head Neck Pathol. 2020, 14, 144–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paja, M.; Zafón, C.; Iglesias, C.; Ugalde, A.; Cameselle-Teijeiro, J.M.; Rodríguez-Carnero, G.; Fernández-Seara, P.; Anda, E.; Povoa, A.; Quiceno, H.; et al. Rate of non-invasive follicular thyroid neoplasms with papillary-like nuclear features depends on pathologist’s criteria: A multicentre retrospective Southern European study with prolonged follow-up. Endocrine 2021, 73, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Turan, G.; Özkara, S.K. Pathological findings of the retrospective diagnosis of NIFTP (non-invasive follicular thyroid neoplasm with papillary-like nuclear features) in 84 cases from Turkey and systematic review. Ann. Diagn. Pathol. 2021, 53, 151764. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.; Guan, H.; Ponchiardi, C.; Cerda, S.; Marwaha, N.; Yilmaz, O.H.; Pinjic, E.; McAneny, D.; Lee, S.L.; Drake, F.T. Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: Epidemiology and Long-Term Outcomes in a Strictly Defined Cohort. Thyroid 2021, 31, 68–75. [Google Scholar] [CrossRef]
- Hodak, S.; Tuttle, R.M.; Maytal, G.; Nikiforov, Y.E.; Randolph, G. Changing the Cancer Diagnosis: The Case of Follicular Variant of Papillary Thyroid Cancer-Primum Non Nocere and NIFTP. Thyroid 2016, 26, 869–871. [Google Scholar] [CrossRef]
- Chan, J. Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am. J. Clin. Pathol. 2002, 117, 16–18. [Google Scholar] [CrossRef]
- Cipriani, N.A.; Nagar, S.; Kaplan, S.P.; White, M.G.; Antic, T.; Sadow, P.M.; Aschebrook-Kilfoy, B.; Angelos, P.; Kaplan, E.L.; Grogan, R.H. Follicular Thyroid Carcinoma: How Have Histologic Diagnoses Changed in the Last Half-Century and What Are the Prognostic Implications? Thyroid 2015, 25, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Mehrzad, R.; Nishino, M.; Connolly, J.; Wang, H.; Mowschenson, P.; Hasselgren, P.O. The relationship between the follicular variant of papillary thyroid cancer and follicular adenomas. Surgery 2016, 159, 1396–1406. [Google Scholar] [CrossRef]
- Widder, S.; Guggisberg, K.; Khalil, M.; Pasieka, J.L. A pathologic re-review of follicular thyroid neoplasms: The impact of changing the threshold for the diagnosis of the follicular variant of papillary thyroid carcinoma. Surgery 2008, 144, 80–85. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; Feng, D.; Sheng, J.; Yang, W.; Liu, B. Targeted next generation sequencing identifies somatic mutations and gene fusions in papillary thyroid carcinoma. Oncotarget 2017, 8, 45784–45792. [Google Scholar] [CrossRef]
- Song, Y.S.; Kang, B.H.; Lee, S.; Yoo, S.K.; Choi, Y.S.; Park, J.; Park, D.Y.; Lee, K.E.; Seo, J.S.; Park, Y.J. Genomic and Transcriptomic Characteristics According to Size of Papillary Thyroid Microcarcinoma. Cancers 2020, 12, 1345. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Jiang, B.; Chen, Y.; Du, X.; Peng, Y.; Wang, W.; Wang, Z.; Li, X. Prediction of novel target genes and pathways involved in tall cell variant papillary thyroid carcinoma. Medicine 2018, 97, e13802. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.J.; Piccin, O.; Sciascia, S.; Cavicchi, O.; Repaci, A.; Vicennati, V.; Fiorentino, M. Clinical significance of BRAF mutation in thyroid papillary cancer. Otolaryngol. Head Neck Surg. 2013, 148, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Villar-Taibo, R.; Peteiro-González, D.; Cabezas-Agrícola, J.M.; Aliyev, E.; Barreiro-Morandeira, F.; Ruiz-Ponte, C.; Cameselle-Teijeiro, J.M. Aggressiveness of the tall cell variant of papillary thyroid carcinoma is independent of the tumor size and patient age. Oncol. Lett. 2017, 13, 3501–3507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virk, R.K.; Van Dyke, A.L.; Finkelstein, A.; Prasad, A.; Gibson, J.; Hui, P.; Theoharis, C.G.; Carling, T.; Roman, S.A.; Sosa, J.A.; et al. BRAFV600E mutation in papillary thyroid microcarcinoma: A genotype-phenotype correlation. Mod. Pathol. 2013, 26, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Song, D.E.; Ahn, J.; Song, E.; Lee, Y.M.; Sung, T.Y.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Jeon, M.J.; et al. Genetic Profiles of Aggressive Variants of Papillary Thyroid Carcinomas. Cancers 2021, 13, 892. [Google Scholar] [CrossRef]
- Gandolfi, G.; Sancisi, V.; Torricelli, F.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A. Allele percentage of the BRAF V600E mutation in papillary thyroid carcinomas and corresponding lymph node metastases: No evidence for a role in tumor progression. J. Clin. Endocrinol. Metab. 2013, 98, 934–942. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Song, Y.S.; Kim, Y.A.; Lim, J.A.; Cho, S.W.; Moon, J.H.; Hahn, S.; Park, D.J.; Park, Y.J. Effects of Coexistent BRAFV600E and TERT Promoter Mutations on Poor Clinical Outcomes in Papillary Thyroid Cancer: A Meta-Analysis. Thyroid 2017, 27, 651–660. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, S.; Han, Y.; Li, Y.; Yu, Y.; Yun, X.; Ren, X.; Gao, M. Papillary microcarcinoma of the thyroid: Clinical characteristics and BRAFV600E mutational status of 977 cases. Ann. Surg. Oncol. 2013, 20, 2266–2273. [Google Scholar] [CrossRef]
- Lee, J.; Ha, E.J.; Roh, J.; Kim, H.K. Presence of TERT ± BRAF V600E mutation is not a risk factor for the clinical management of patients with papillary thyroid microcarcinoma. Surgery 2021, 170, 743–747. [Google Scholar] [CrossRef]
- Ugolini, C.; Giannini, R.; Lupi, C.; Salvatore, G.; Miccoli, P.; Proietti, A.; Elisei, R.; Santoro, M.; Basolo, F. Presence of BRAF V600E in very early stages of papillary thyroid carcinoma. Thyroid 2007, 17, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, E.; Ladra-González, M.J.; Sánchez-Ares, M.; Abdulkader-Nallib, I.; Piso-Neira, M.; Rodríguez-Carnero, G.; Vieiro-Balo, P.; Pérez-Becerra, R.; Gude-Sampedro, F.; Barreiro-Morandeira, F.; et al. Thyroid Papillary Microtumor: Validation of the (Updated) Porto Proposal Assessing Sex Hormone Receptor Expression and Mutational BRAF Gene Status. Am. J. Surg. Pathol. 2020, 44, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Samà, M.T.; Grosso, E.; Mele, C.; Laurora, S.; Monzeglio, O.; Marzullo, P.; Boldorini, R.; Aluffi Valletti, P.; Aimaretti, G.; Scatolini, M.; et al. Molecular characterisation and clinical correlation of papillary thyroid microcarcinoma. Endocrine 2021, 71, 149–157. [Google Scholar] [CrossRef]
- Sancisi, V.; Nicoli, D.; Ragazzi, M.; Piana, S.; Ciarrocchi, A. BRAFV600E mutation does not mean distant metastasis in thyroid papillary carcinomas. J. Clin. Endocrinol. Metab. 2012, 97, E1745–E1749. [Google Scholar] [CrossRef] [Green Version]
- Guerra, A.; Sapio, M.R.; Marotta, V.; Campanile, E.; Rossi, S.; Forno, I.; Fugazzola, L.; Budillon, A.; Moccia, T.; Fenzi, G.; et al. The primary occurrence of BRAF(V600E) is a rare clonal event in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2012, 97, 517–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piana, S.; Ragazzi, M.; Tallini, G.; de Biase, D.; Ciarrocchi, A.; Frasoldati, A.; Rosai, J. Papillary thyroid microcarcinoma with fatal outcome: Evidence of tumor progression in lymph node metastases: Report of 3 cases, with morphological and molecular analysis. Hum. Pathol. 2013, 44, 556–565. [Google Scholar] [CrossRef]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.Y.; Shibru, D.; Bastian, B.; Griffin, A. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann. Surg. 2007, 246, 466–470. [Google Scholar] [CrossRef]
- Lupi, C.; Giannini, R.; Ugolini, C.; Proietti, A.; Berti, P.; Minuto, M.; Materazzi, G.; Elisei, R.; Santoro, M.; Miccoli, P.; et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2007, 92, 4085–4090. [Google Scholar] [CrossRef] [Green Version]
- Elisei, R.; Ugolini, C.; Viola, D.; Lupi, C.; Biagini, A.; Giannini, R.; Romei, C.; Miccoli, P.; Pinchera, A.; Basolo, F. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 3943–4949. [Google Scholar] [CrossRef] [Green Version]
- Oler, G.; Cerutti, J.M. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: Correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer 2009, 115, 972–980. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.Z.; Zhang, Q.; Guan, Y.X.; Chen, Q.J.; Zhu, Q.Y. Meta-Analyses of Association Between BRAF(V600E) Mutation and Clinicopathological Features of Papillary Thyroid Carcinoma. Cell. Physiol. Biochem. 2016, 38, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Shen, F.; Deng, X.; Feng, J.; Lu, J.; Cai, W.; Peng, L.; Zheng, W.; Wang, W.; Huang, P.; et al. Prognostic implications of the BRAF-V600E mutation in papillary thyroid carcinoma based on a new cut-off age stratification. Oncol. Lett. 2020, 19, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puxeddu, E.; Moretti, S.; Elisei, R.; Romei, C.; Pascucci, R.; Martinelli, M.; Marino, C.; Avenia, N.; Rossi, E.D.; Fadda, G.; et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2004, 89, 2414–2420. [Google Scholar] [CrossRef] [Green Version]
- Trovisco, V.; Soares, P.; Preto, A.; de Castro, I.V.; Lima, J.; Castro, P.; Máximo, V.; Botelho, T.; Moreira, S.; Meireles, A.M.; et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch. 2005, 446, 589–595. [Google Scholar] [CrossRef]
- Fugazzola, L.; Puxeddu, E.; Avenia, N.; Romei, C.; Cirello, V.; Cavaliere, A.; Faviana, P.; Mannavola, D.; Moretti, S.; Rossi, S.; et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: Data from a multicentric Italian study and review of the literature. Endocr. Relat. Cancer 2006, 13, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Brzeziańska, E.; Pastuszak-Lewandoska, D.; Wojciechowska, K.; Migdalska-Sek, M.; Cyniak-Magierska, A.; Nawrot, E.; Lewiński, A. Investigation of V600E BRAF mutation in papillary thyroid carcinoma in the Polish population. Neuro Endocrinol. Lett. 2007, 28, 351–359. [Google Scholar]
- Xing, M.; Tufano, R.P.; Tufaro, A.P.; Basaria, S.; Ewertz, M.; Rosenbaum, E.; Byrne, P.J.; Wang, J.; Sidransky, D.; Ladenson, P.W. Detection of BRAF mutation on fine needle aspiration biopsy specimens: A new diagnostic tool for papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2004, 89, 2867–2872. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, J.I.; Kim, J.W.; Ki, C.S.; Oh, Y.L.; Choi, Y.L.; Shin, J.H.; Kim, H.K.; Jang, H.W.; Chung, J.H. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: A large series in a BRAFV600E-prevalent population. J. Clin. Endocrinol. Metab. 2010, 95, 3693–3700. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.T.; Kim, S.W.; Ki, C.S.; Jang, J.H.; Shin, J.H.; Oh, Y.L.; Kim, J.W.; Chung, J.H. Clinical implication of highly sensitive detection of the BRAF V600E mutation in fine-needle aspirations of thyroid nodules: A comparative analysis of three molecular assays in 4585 consecutive cases in a BRAF V600E mutation-prevalent area. J. Clin. Endocrinol. Metab. 2012, 97, 2299–2306. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, I.; Iervasi, G.; Mazzanti, C.M.; Lessi, F.; Tomei, S.; Naccarato, A.G.; Aretini, P.; Alberti, B.; Di Coscio, G.; Bevilacqua, G. Detection of the BRAF(V600E) mutation in fine needle aspiration cytology of thyroid papillary microcarcinoma cells selected by manual macrodissection: An easy tool to improve the preoperative diagnosis. Thyroid 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Rosario, P.W.; Ward, L.S.; Graf, H.; Vaisman, F.; Mourão, G.F.; Vaisman, M. Thyroid nodules ≤ 1 cm and papillary thyroid microcarcinomas: Brazilian experts opinion. Arch. Endocrinol. Metab. 2019, 63, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakudo, K.; Bychkov, A.; Abelardo, A.; Keelawat, S.; Kumarasinghe, P. Malpractice Climate Is a Key Difference in Thyroid Pathology Practice Between North America and the Rest of the World. Arch Pathol. Lab Med. 2019, 143, 1171. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakudo, K. Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice. Cancers 2022, 14, 812. https://doi.org/10.3390/cancers14030812
Kakudo K. Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice. Cancers. 2022; 14(3):812. https://doi.org/10.3390/cancers14030812
Chicago/Turabian StyleKakudo, Kennichi. 2022. "Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice" Cancers 14, no. 3: 812. https://doi.org/10.3390/cancers14030812
APA StyleKakudo, K. (2022). Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice. Cancers, 14(3), 812. https://doi.org/10.3390/cancers14030812





