Collagen Biomarkers Quantify Fibroblast Activity In Vitro and Predict Survival in Patients with Pancreatic Ductal Adenocarcinoma
Abstract
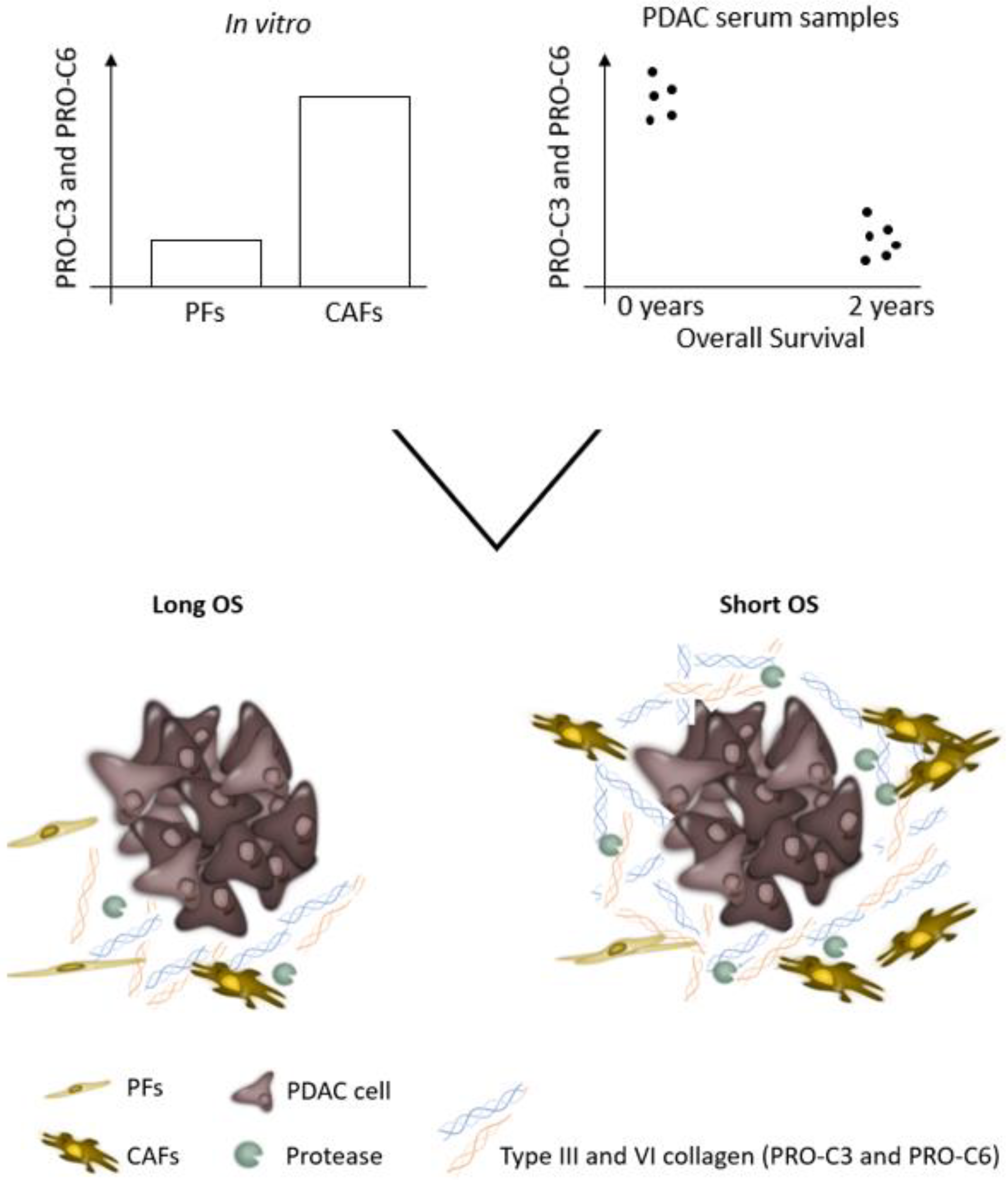
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fibroblast Cell Cultures—Scar-In-A-Jar
2.2. Assessment of Metabolic Cell Viability by Alamar Blue
2.3. Decellularization of Matrix, Sirius Red Staining, and Fibril Orientation Quantification
2.4. Assessment of Collagen Formation in Cell Cultures and Human Serum Samples
2.5. Patients
2.6. Statistical Analysis
3. Results
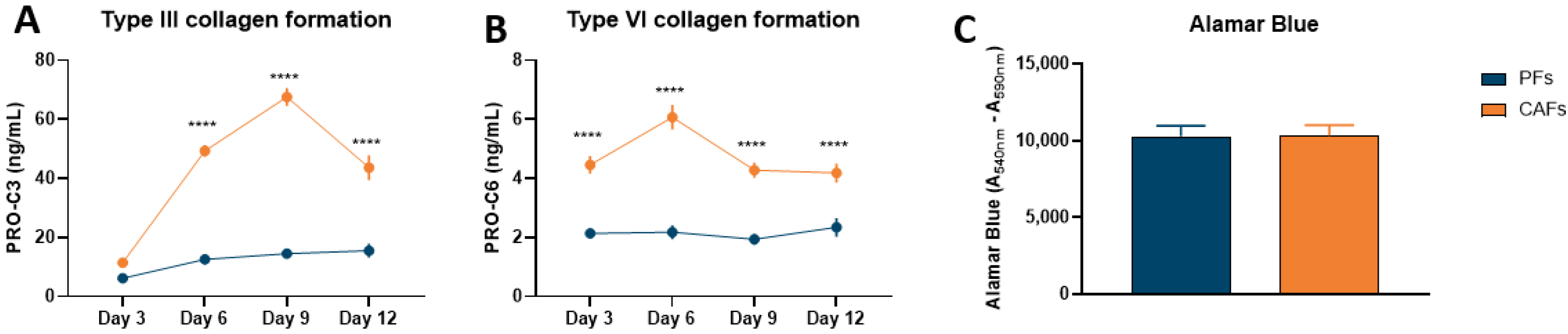
3.1. Pancreatic CAFs Have Greater Fibrotic Potential Than PFs
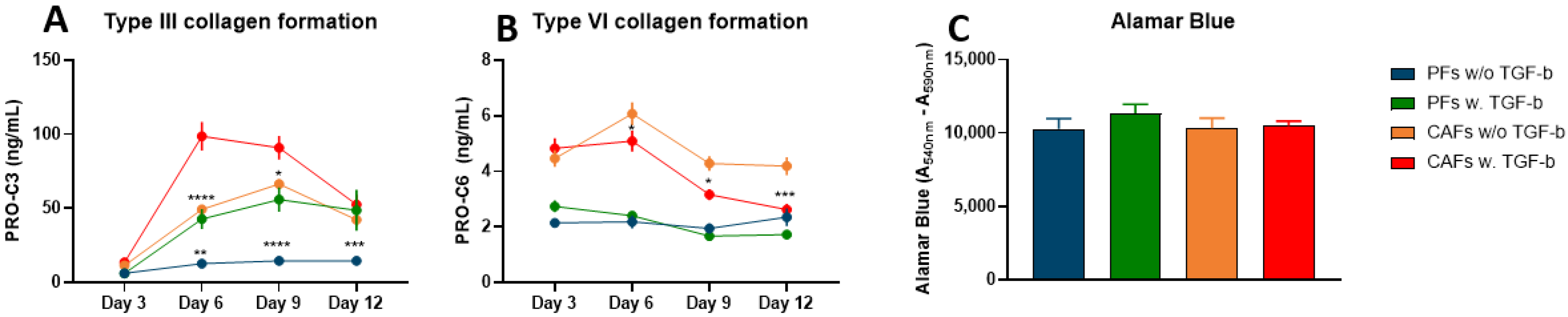
3.2. TGF-ß Induces Type III Collagen Formation, but Not Type VI Collagen Formation
3.3. Type III and VI Collagen Production Is Inhibited by ALK5i in CAFs
3.4. Collagen Fibers from CAFs Are More Aligned Than Collagen Fibers Produced by PFs
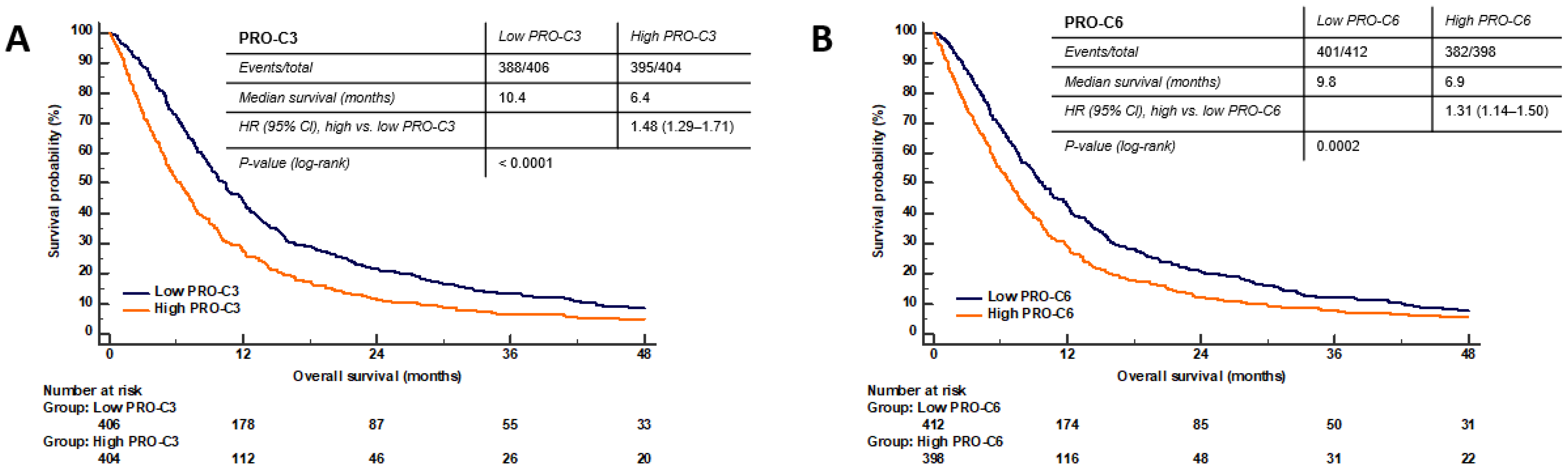
3.5. PRO-C3 and PRO-C6 Are Prognostic for OS in PDAC—Translational Value of the PDAC SiaJ Model
3.6. High Serum PRO-C3 Levels Are Predictive of Short OS in Patients with PDAC
3.7. High Serum PRO-C6 Levels Are Predictive of Short OS in Patients with PDAC
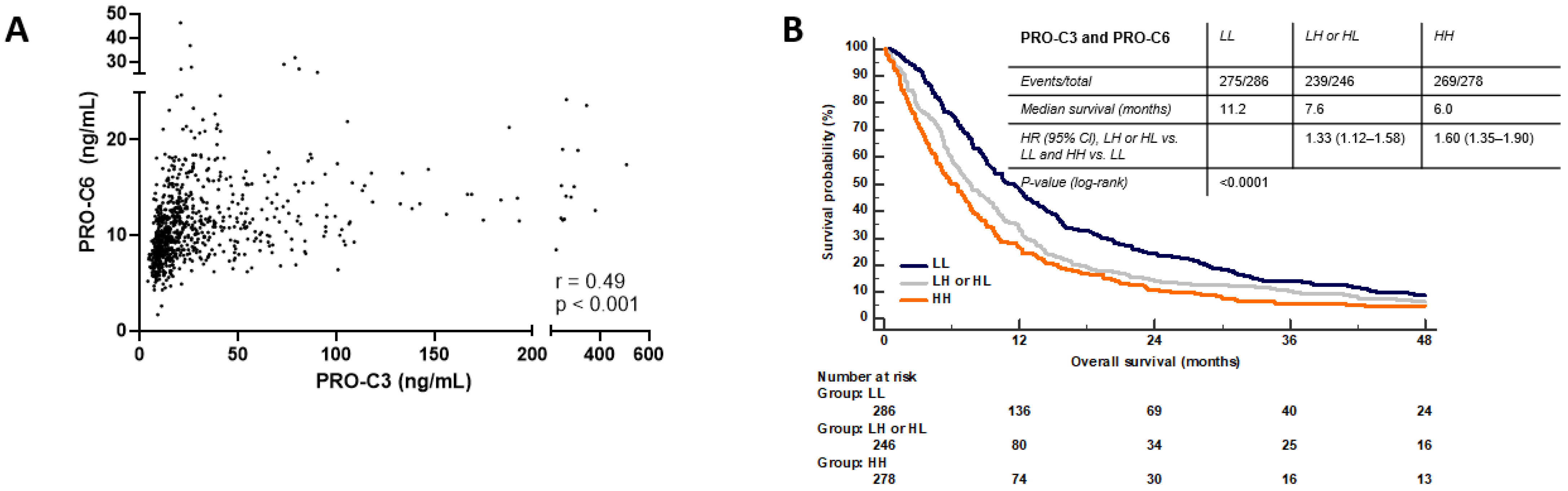
3.8. Combination PRO-C3 and PRO-C6 Are Complementary
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA J. Am. Med. Assoc. 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer — clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Young, C.D.; Zhou, H.; Wang, X. Transforming growth factor-β signaling in fibrotic diseases and cancer-associated fibroblasts. Biomolecules 2020, 10, 1666. [Google Scholar] [CrossRef]
- Pankova, D.; Chen, Y.; Terajima, M.; Schliekelman, M.J.; Baird, B.N.; Fahrenholtz, M.; Sun, L.; Gill, B.J.; Vadakkan, T.J.; Kim, M.P.; et al. Cancer-Associated Fibroblasts Induce a Collagen Cross-link Switch in Tumor Stroma. Mol. Cancer Res. 2016, 14, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drifka, C.R.; Loeffler, A.G.; Mathewson, K.; Keikhosravi, A.; Eickhoff, J.C.; Liu, Y.; Weber, S.M.; John Kao, W.; Eliceiri, K.W. Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection. Oncotarget 2016, 7, 76197–76213. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front. Cell Dev. Biol. 2019, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cannon, A.; Thompson, C.; Hall, B.R.; Jain, M.; Kumar, S.; Batra, S.K. Desmoplasia in pancreatic ductal adenocarcinoma: Insight into pathological function and therapeutic potential. Genes Cancer 2018, 9, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khomiak, A.; Brunner, M.; Kordes, M.; Lindblad, S.; Miksch, R.C.; Öhlund, D.; Regel, I. Recent discoveries of diagnostic, prognostic and predictive biomarkers for pancreatic cancer. Cancers 2020, 12, 3234. [Google Scholar] [CrossRef]
- Bai, J.P.F.; Bell, R.; Buckman, S.; Burckart, G.J.; Eichler, H.-G.; Fang, K.C.; Goodsaid, F.M.; Jusko, W.J.; Lesko, L.L.; Meibohm, B.; et al. Translational biomarkers: From preclinical to clinical a report of 2009 AAPS/ACCP Biomarker Workshop. AAPS J. 2011, 13, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Amur, S.; Frueh, F.W.; Lesko, L.J.; Huang, S.-M. Integration and use of biomarkers in drug development, regulation and clinical practice: A US regulatory perspective. Biomark. Med. 2008, 2, 305–311. [Google Scholar] [CrossRef]
- Petersen, E.V.; Chudakova, D.A.; Skorova, E.Y.; Anikin, V.; Reshetov, I.V.; Mynbaev, O.A. The Extracellular Matrix-Derived Biomarkers for Diagnosis, Prognosis, and Personalized Therapy of Malignant Tumors. Front. Oncol. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Sinkeviciute, D.; Madsen, D.H.; Önnerfjord, P.; Hansen, M.; Schmidt, H.; Karsdal, M.A.; Svane, I.M.; Willumsen, N. Granzyme B degraded type IV collagen products in serum identify melanoma patients responding to immune checkpoint blockade. Cancers 2020, 12, 2786. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Madsen, D.H.; Hansen, M.; Schmidt, H.; Svane, I.M.; Karsdal, M.A.; Willumsen, N. Non-invasive biomarkers derived from the extracellular matrix associate with response to immune checkpoint blockade (anti-CTLA-4) in metastatic melanoma patients. J. Immunother. Cancer 2018, 6, 152. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Bager, C.L.; Karsdal, M.A.; Chondros, D.; Taverna, D.; Willumsen, N. Blood-based extracellular matrix biomarkers as predictors of survival in patients with metastatic pancreatic ductal adenocarcinoma receiving pegvorhyaluronidase alfa. J. Transl. Med. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Hurkmans, D.P.; Jensen, C.; Koolen, S.L.W.; Aerts, J.; Karsdal, M.A.; Mathijssen, R.H.J.; Willumsen, N. Blood-based extracellular matrix biomarkers are correlated with clinical outcome after PD-1 inhibition in patients with metastatic melanoma. J. Immunother. Cancer 2020, 8, e001193. [Google Scholar] [CrossRef]
- Jensen, C.; Holm Nielsen, S.; Eslam, M.; Genovese, F.; Nielsen, M.J.; Vongsuvanh, R.; Uchila, R.; van der Poorten, D.; George, J.; Karsdal, M.A.; et al. Cross-Linked Multimeric Pro-Peptides of Type III Collagen (PC3X) in Hepatocellular Carcinoma—A Biomarker That Provides Additional Prognostic Value in AFP Positive Patients. J. Hepatocell. Carcinoma 2020, 7, 301–313. [Google Scholar] [CrossRef]
- Nissen, N.I.; Kehlet, S.; Johansen, A.Z.; Chen, I.M.; Karsdal, M.; Johansen, J.S.; Diab, H.M.H.; Jørgensen, L.N.; Sun, S.; Manon-Jensen, T.; et al. Noninvasive prognostic biomarker potential of quantifying the propeptides of Type XI collagen alpha-1 chain (PRO-C11) in patients with pancreatic ductal adenocarcinoma. Int. J. Cancer 2021, 149, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.I.; Kehlet, S.; Boisen, M.K.; Liljefors, M.; Jensen, C.; Johansen, A.Z.; Johansen, J.S.; Erler, J.T.; Karsdal, M.; Mortensen, J.H.; et al. Prognostic value of blood-based fibrosis biomarkers in patients with metastatic colorectal cancer receiving chemotherapy and bevacizumab. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Chen, I.M.; Willumsen, N.; Dehlendorff, C.; Johansen, A.Z.; Jensen, B.V.; Hansen, C.P.; Hasselby, J.P.; Bojesen, S.E.; Pfeiffer, P.; Nielsen, S.E.; et al. Clinical value of serum hyaluronan and propeptide of type III collagen in patients with pancreatic cancer. Int. J. Cancer 2020, 146, 2913–2922. [Google Scholar] [CrossRef]
- Bager, C.L.; Willumsen, N.; Leeming, D.J.; Smith, V.; Karsdal, M.A.; Dornan, D.; Bay-Jensen, A.C. Collagen degradation products measured in serum can separate ovarian and breast cancer patients from healthy controls: A preliminary study. Cancer Biomark. 2015, 15, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willumsen, N.; Bager, C.L.; Leeming, D.J.; Smith, V.; Karsdal, M.A.; Dornan, D.; Bay-Jensen, A.-C.C. Extracellular matrix specific protein fingerprints measured in serum can seperate pancreatic cancer patients from healthy controls. BMC Cancer 2013, 13, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppila, S.; Stenback, F.; Risteli, J.; Jukkola, A. Risteli L Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J. Pathol. 1998, 268, 262–268. [Google Scholar] [CrossRef]
- Hilska, M.; Peltonen, J.; Gullichsen, R.; Paajanen, H.; Laato, M. The Distribution of Collagen Types I, III, and IV in Normal and Malignant Colorectal Mucosa. Eur. J. Surg. 1998, 164, 457–464. [Google Scholar] [CrossRef]
- Nurmenniemi, S.; Koivula, M.; Nyberg, P.; Tervahartiala, T.; Sorsa, T. Type I and III collagen degradation products in serum predict patient survival in head and neck squamous cell carcinoma. Oral Oncol. 2012, 48, 136–140. [Google Scholar] [CrossRef]
- Basso, D.; Belluco, C.; Mazza, S.; Greco, E. Colorectal cancer metastatic phenotype stimulates production by fibroblasts of N-terminal peptide of type III collagen: Clinical implications for prognosis. Clin. Chim. Acta 2001, 312, 135–142. [Google Scholar] [CrossRef]
- Chen, P.; Cescon, M.; Bonaldo, P. Collagen VI in cancer and its biological mechanisms. Trends Mol. Med. 2013, 19, 410–417. [Google Scholar] [CrossRef]
- Willumsen, N.; Bager, C.; Karsdal, M.A. Matrix Metalloprotease Generated Fragments of Type VI Collagen Have Serum Biomarker Potential in Cancer—A Proof of Concept Study. Transl. Oncol. 2019, 12, 693–698. [Google Scholar] [CrossRef]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Chen, H.; Zhao, L.; Hu, J.; Yang, W.; Li, G.; Cheng, C.; Zhao, Z.; Zhang, T.; Li, L.; et al. Cancer-Associated Fibroblast (CAF) Heterogeneity and Targeting Therapy of CAFs in Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Froeling, F.E.M.; Kocher, H.M. Homeostatic restoration of desmoplastic stroma rather than its ablation slows pancreatic cancer progression. Gastroenterology 2015, 148, 849–850. [Google Scholar] [CrossRef] [Green Version]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Transl. Res. 2019, 209, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Z.C.; Peng, Y.X.; Wang, Z.B.; Fish, P.V.; Kaar, J.L.; Koepsel, R.R.; Russell, A.J.; Lareu, R.R.; Raghunath, M. The Scar-in-a-Jar: Studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br. J. Pharmacol. 2009, 158, 1196–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rønnow, S.R.; Dabbagh, R.Q.; Genovese, F.; Nanthakumar, C.B.; Barrett, V.J.; Good, R.B.; Brockbank, S.; Cruwys, S.; Jessen, H.; Sorensen, G.L.; et al. Prolonged Scar-in-a-Jar: An in vitro screening tool for anti-fibrotic therapies using biomarkers of extracellular matrix synthesis. Respir. Res. 2020, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Juhl, P.; Bondesen, S.; Hawkins, C.L.; Karsdal, M.A.; Bay-Jensen, A.C.; Davies, M.J.; Siebuhr, A.S. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-β, PDGF and IL-6 in a model for skin fibrosis. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Franco-Barraza, J.; Beacham, D.A.; Amatangelo, M.D.; Cukierman, E. Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. Curr. Protoc. Cell Biol. 2016, 71, 10.9.1–10.9.34. [Google Scholar] [CrossRef]
- Boudaoud, A.; Burian, A.; Borowska-Wykręt, D.; Uyttewaal, M.; Wrzalik, R.; Kwiatkowska, D.; Hamant, O. FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat. Protoc. 2014, 9, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Jensen, B.V.; Bojesen, S.E.; Johansen, A.Z.; Schultz, N.A.; Hansen, C.P.; Hasselby, J.P.; Holländer, N.H.; Nissen, M.H.B.; Bjerregaard, J.K.; et al. Identification of new biomarkers in patients with pancreatic cancer (BIOPAC): A study protocol of an open cohort study. J. Cancer Sci. Ther. 2019, 11, 232. [Google Scholar]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M.; Costa, J.; Di Leo, A.; Mayer, R.J. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 2005, 23, 9067–9072. [Google Scholar] [CrossRef] [Green Version]
- Asano, T.; Yamada, S.; Fujii, T.; Yabusaki, N.; Nakayama, G.; Sugimoto, H.; Koike, M.; Fujiwara, M.; Kodera, Y. The Charlson age comorbidity index predicts prognosis in patients with resected pancreatic cancer. Int. J. Surg. 2017, 39, 169–175. [Google Scholar] [CrossRef]
- Elahi-Gedwillo, K.Y.; Carlson, M.; Zettervall, J.; Provenzano, P.P. Antifibrotic therapy disrupts stromal barriers and modulates the immune landscape in pancreatic ductal adenocarcinoma. Cancer Res. 2019, 79, 372–386. [Google Scholar] [CrossRef] [Green Version]
- Hauge, A.; Rofstad, E.K. Antifibrotic therapy to normalize the tumor microenvironment. J. Transl. Med. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Huang, H.; Brekken, R.A. The next wave of stroma-targeting therapy in pancreatic cancer. Cancer Res. 2019, 79, 328–330. [Google Scholar] [CrossRef] [Green Version]
- Hanley, C.J.; Noble, F.; Ward, M.; Bullock, M.; Drifka, C.; Mellone, M.; Manousopoulou, A.; Johnston, H.E.; Hayden, A.; Thirdborough, S.; et al. A subset of myofibroblastic cancer-associated fibroblasts regulate collagen fiber elongation, which is prognostic in multiple cancers. Oncotarget 2016, 7, 6159–6174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lareu, R.R.; Arsianti, I.; Subramhanya, H.K.; Yanxian, P.; Raghunath, M. In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: A preliminary study. Tissue Eng. 2007, 13, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Philippi, C.; Vogelmann, R.; Seidel, B.; Lutz, M.P.; Adler, G.; Wedlich, D. Down-Regulation of E-Cadherin Gene Expression by Collagen Type I and Type III in Pancreatic Cancer Cell Lines 1. Biochemistry 2001, 61, 3508–3517. [Google Scholar]
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman-Baust, C.A.; Weeraratna, A.T.; Rangel, L.B.A.; Pizer, E.S.; Cho, K.R.; Schwartz, D.R.; Shock, T.; Morin, P.J. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell 2003, 3, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Varma, R.R.; Hector, S.M.; Clark, K.; Greco, W.R.; Hawthorn, L.; Pendyala, L. Gene expression profiling of a clonal isolate of oxaliplatin-resistant ovarian carcinoma cell line A2780/C10. Oncol. Rep. 2005, 14, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Morley, T.S.; Scherer, P.E. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol. Med. 2013, 5, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Espina, V.; Williams, T.W.; Lin, Y.; Berry, D.; Jelicks, L.A.; Lee, H.; Temple, K.; Graves, R.; Pollard, J.; et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Investig. 2005, 115, 1163–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, C.Y.; Wang, J.; Axell-House, D.; Soni, P.; Chu, M.-L.; Chipitsyna, G.; Sarosiek, K.; Sendecki, J.; Hyslop, T.; Al-Zoubi, M.; et al. Clinical Significance of Serum COL6A3 in Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Surg. 2014, 18, 7–15. [Google Scholar] [CrossRef]
- Arafat, H.; Lazar, M.; Salem, K.; Chipitsyna, G.; Gong, Q.; Pan, T.-C.; Zhang, R.-Z.; Yeo, C.J.; Chu, M.-L. Tumor-specific expression and alternative splicing of the Col6a3 Gene in Pancreatic Cancer. Surgery 2011, 150, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Z.; Raghunath, M. Focus on collagen: In vitro systems to study fibrogenesis and antifibrosis state of the art. Fibrogenesis Tissue Repair 2009, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β receptors: In and beyond TGF-β signaling. Cell. Signal. 2018, 52, 112–120. [Google Scholar] [CrossRef]
- Caja, L.; Tan, E.-J. Epithelium to Mesenchyme Transition. In Encyclopedia of Cancer; Boffetta, P., Hainaut, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 14–23. ISBN 978-0-12-812485-7. [Google Scholar]
- Valkenburg, K.C.; De Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Piperigkou, Z.; Passi, A.; Götte, M.; Rousselle, P.; Vlodavsky, I. Extracellular matrix-based cancer targeting. Trends Mol. Med. 2021, 27, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronopoulos, A.; Robinson, B.; Sarper, M.; Cortes, E.; Auernheimer, V.; Lachowski, D.; Attwood, S.; García, R.; Ghassemi, S.; Fabry, B.; et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat. Commun. 2016, 7, 12630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zion, O.; Genin, O.; Kawada, N.; Yoshizato, K.; Roffe, S.; Nagler, A.; Iovanna, J.L.; Halevy, O.; Pines, M. Inhibition of Transforming Growth Factor β Signaling by Halofuginone as a Modality for Pancreas Fibrosis Prevention. Pancreas 2009, 38, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Juárez, P.; Mohammad, K.S.; Yin, J.J.; Fournier, P.G.J.; McKenna, R.C.; Davis, H.W.; Peng, X.H.; Niewolna, M.; Javelaud, D.; Chirgwin, J.M.; et al. Halofuginone inhibits the establishment and progression of melanoma bone metastases. Cancer Res. 2012, 72, 6247–6256. [Google Scholar] [CrossRef] [Green Version]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Piao, X.-M.; Hwang, B.; Jeong, P.; Byun, Y.J.; Kang, H.W.; Seo, S.P.; Kim, W.T.; Lee, J.-Y.; Ha, Y.-S.; Lee, Y.-S.; et al. Collagen type VI-α1 and 2 repress the proliferation, migration and invasion of bladder cancer cells. Int. J. Oncol. 2021, 59, 1–13. [Google Scholar] [CrossRef]
- Di Martino, J.S.; Nobre, A.R.; Mondal, C.; Taha, I.; Farias, E.F.; Fertig, E.J.; Naba, A.; Aguirre-Ghiso, J.A.; Bravo-Cordero, J.J. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat. Cancer 2021, 3, 90–107. [Google Scholar] [CrossRef]
- Drifka, C.R.; Loeffler, A.G.; Esquibel, C.R.; Weber, S.M.; Eliceiri, K.W.; Kao, W.J. Human pancreatic stellate cells modulate 3D collagen alignment to promote the migration of pancreatic ductal adenocarcinoma cells. Biomed. Microdevices 2016, 18, 105. [Google Scholar] [CrossRef]
- Drifka, C.R.; Tod, J.; Loeffler, A.G.; Liu, Y.; Thomas, G.J.; Eliceiri, K.W.; Kao, W.J. Periductal stromal collagen topology of pancreatic ductal adenocarcinoma differs from that of normal and chronic pancreatitis. Mod. Pathol. 2015, 28, 1470–1480. [Google Scholar] [CrossRef] [Green Version]
- Bolm, L.; Zghurskyi, P.; Lapshyn, H.; Petrova, E.; Zemskov, S.; Vashist, Y.K.; Deichmann, S.; Honselmann, K.C.; Bronsert, P.; Keck, T.; et al. Alignment of stroma fibers, microvessel density and immune cell populations determine overall survival in pancreatic cancer-An analysis of stromal morphology. PLoS ONE 2020, 15, e0234568. [Google Scholar] [CrossRef]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariathasan, S.; Turley, S.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 22, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Nielsen, S.H.; Leeming, D.J.; Langholm, L.L.; Nielsen, M.J.; Manon-Jensen, T.; Siebuhr, A.; Gudmann, N.S.; Rønnow, S.; Sand, J.M.; et al. The good and the bad collagens of fibrosis – Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017, 121, 43–56. [Google Scholar] [CrossRef]
- Han, C.; Liu, T.; Yin, R. Biomarkers for cancer-associated fibroblasts. Biomark. Res. 2020, 8, 64. [Google Scholar] [CrossRef]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Sebastian, A.; Hum, N.R.; Martin, K.A.; Gilmore, S.F.; Peran, I.; Byers, S.W.; Wheeler, E.K.; Coleman, M.A.; Loots, G.G. Single-Cell Transcriptomic Analysis of Tumor-Derived Fibroblasts and Normal Tissue-Resident Fibroblasts Reveals Fibroblast Heterogeneity in Breast Cancer. Cancers 2020, 12, 1307. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Voisin, V.; Poon, S.; Karamboulas, C.; Bui, N.H.B.; Meens, J.; Dmytryshyn, J.; Ho, V.W.; Tang, K.H.; Paterson, J.; et al. Distinct fibroblast functional states drive clinical outcomes in ovarian cancer and are regulated by TCF21. J. Exp. Med. 2020, 217, e20191094. [Google Scholar] [CrossRef] [PubMed]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osswald, A.; Hedrich, V.; Sommergruber, W. 3D-3 Tumor Models in Drug Discovery for Analysis of Immune Cell Infiltration BT—Target Identification and Validation in Drug Discovery: Methods and Protocols; Moll, J., Carotta, S., Eds.; Springer: New York, NY, USA, 2019; pp. 151–162. ISBN 978-1-4939-9145-7. [Google Scholar]
- Fontana, F.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. In Vitro 3D Cultures to Model the Tumor Microenvironment. Cancers 2021, 13, 2970. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Goossens, N.; Nakagawa, S.; Sun, X.; Hoshida, Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015, 4, 256–269. [Google Scholar] [CrossRef]


| Clinical Variables (PDAC) | Study Population (n = 810) |
|---|---|
| Age, (years) | |
| Median (min, max) | 66 (37–89) |
| Gender, n (%) | |
| Male | 433 (53%) |
| Female | 377 (47%) |
| Number of metastatic sites, n (%) | |
| 0 site | 376 (46%) |
| ≥1 site | 434 (54%) |
| Liver metastasis (of all patients with metastasis, n = 434), n (%) | |
| Yes | 331 (76%) |
| No | 103 (24%) |
| BMI | |
| Median (min, max) | 23 (14–39) |
| Stage | |
| 1 | 15 (2%) |
| 2 | 123 (15%) |
| 3 | 237 (29%) |
| 4 | 431 (53%) |
| Unknown | 4 (<1 %) |
| Diabetes | |
| Yes | 198 (24%) |
| No | 603 (74%) |
| Unknown | 9 (1%) |
| Tobacco | |
| Ever | 484 (60%) |
| Never | 251 (31%) |
| Unknown | 75 (9%) |
| Alcohol | |
| <DHAR | 554 (68%) |
| >DHAR | 179 (22%) |
| Unknown | 77 (10%) |
| CA19-9 (U/mL) | |
| ≤median (≤506 U/mL) | 395 (49%) |
| >median (>506 U/mL) | 387 (48%) |
| Unknown | 28 (3%) |
| Performance status, n (%) | |
| 0 | 294 (36%) |
| 1 | 335 (41%) |
| 2 | 89 (11%) |
| 3 | 5 (<1%) |
| Unknown | 87 (11%) |
| The Charlson age comorbidity index | |
| <4 | 538 (66%) |
| ≥4 | 258 (32%) |
| Unknown | 14 (2%) |
| Table 2: Uni- and Multivariate Analysis (Overall Survival), n = 810 | Univariate | Multivariate | Multivariate * Kombi | ||||
|---|---|---|---|---|---|---|---|
| Variables | HR (95% Cl) | p-Value | HR (95% Cl) | p-Value | HR (95% Cl) | p-Value | |
| PRO-C3 | Continuous | 1.00 (1.00–1.00) | 0.0036 | - | - | - | - |
| >median vs. ≤median | 1.48 (1.29–1.71) | <0.0001 | 1.24 (1.04–1.47) | 0.0149 | - | - | |
| PRO-C6 | Continuous | 1.05 (1.03–1.07) | <0.0001 | - | - | - | - |
| >median vs. ≤median | 1.31 (1.14–1.50) | 0.0002 | 1.15 (0.97–1.36) | 0.1139 | - | - | |
| PRO-C3 and PRO-C6 | High + high vs. low + lowLow + high or high + low vs. low + low | 1.60 (1.35–1.90) 1.33 (1.12–1.58) | <0.0001 0.0014 | - | - | 1.42 (1.18–1.71) 1.19 (0.99–1.43) | 0.0002 0.0703 |
| Age | Per year increase | 1.01 (1.00–1.02) | 0.0199 | 1.01 (1.00–1.36) | 0.1694 | 1.01 (1.00–1.02) | 0.1916 |
| Gender | Female vs. male | 0.97 (0.85–1.12) | 0.7063 | - | - | - | - |
| Number of metastatic sites | ≥1 vs. 0 | 2.56 (2.21–2.97) | <0.0001 | 1.52 (1.18–2.00) | 0.0011 | 1.51 (1.18–1.95) | 0.0013 |
| Liver metastasis | Yes vs. no | 2.37 (2.05–2.75) | <0.0001 | 1.28 (1.01–1.62) | 0.0396 | 1.28 (1.02–1.63) | 0.0363 |
| BMI | Continuous | 0.99 (0.97–1.01) | 0.2344 | - | - | - | - |
| Stage | 3 + 4 vs. 1 + 2 | 2.85 (2.33–3.50) | <0.0001 | 1.97 (1.52–2.55) | <0.0001 | 1.97 (1.52–2.56) | <0.0001 |
| Diabetes | Yes vs. no | 1.08 (0.92–1.27) | 0.3431 | - | - | - | - |
| Tobacco | Ever vs. never | 1.06 (0.91–1.24) | 0.4448 | - | - | - | - |
| Alcohol | >DHAR vs. <DHAR | 1.05 (0.88–1.24) | 0.6112 | - | - | - | - |
| CA19-9 | >median vs. ≤ median | 2.01 (1.69–2.39) | <0.0001 | 1.53 (1.30–1.80) | <0.0001 | 1.54 (1.23–1.70) | <0.0001 |
| PS | 1 + 2+3 vs. 0 | 1.58 (1.36–1.85) | <0.0001 | 1.45 (1.24–1.71) | <0.0001 | 1.44 (1.23–1.69) | <0.0001 |
| CACI | High (≥4 vs. <4) | 1.14 (0.98–1.33) | 0.0860 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nissen, N.I.; Johansen, A.Z.; Chen, I.; Johansen, J.S.; Pedersen, R.S.; Hansen, C.P.; Karsdal, M.A.; Willumsen, N. Collagen Biomarkers Quantify Fibroblast Activity In Vitro and Predict Survival in Patients with Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 819. https://doi.org/10.3390/cancers14030819
Nissen NI, Johansen AZ, Chen I, Johansen JS, Pedersen RS, Hansen CP, Karsdal MA, Willumsen N. Collagen Biomarkers Quantify Fibroblast Activity In Vitro and Predict Survival in Patients with Pancreatic Ductal Adenocarcinoma. Cancers. 2022; 14(3):819. https://doi.org/10.3390/cancers14030819
Chicago/Turabian StyleNissen, Neel I., Astrid Z. Johansen, Inna Chen, Julia S. Johansen, Rasmus S. Pedersen, Carsten P. Hansen, Morten A. Karsdal, and Nicholas Willumsen. 2022. "Collagen Biomarkers Quantify Fibroblast Activity In Vitro and Predict Survival in Patients with Pancreatic Ductal Adenocarcinoma" Cancers 14, no. 3: 819. https://doi.org/10.3390/cancers14030819
APA StyleNissen, N. I., Johansen, A. Z., Chen, I., Johansen, J. S., Pedersen, R. S., Hansen, C. P., Karsdal, M. A., & Willumsen, N. (2022). Collagen Biomarkers Quantify Fibroblast Activity In Vitro and Predict Survival in Patients with Pancreatic Ductal Adenocarcinoma. Cancers, 14(3), 819. https://doi.org/10.3390/cancers14030819






