Intraductal Carcinoma of the Prostate as a Cause of Prostate Cancer Metastasis: A Molecular Portrait
Abstract
:Simple Summary
Abstract
1. Introduction
2. IDC-P Is an Independent Parameter for Metastasis and Survival
2.1. IDC-P Predicts the Presence of Lymph Node Metastasis at Diagnosis
2.2. IDC-P Predicts the Occurrence of Distant Metastasis
2.3. IDC-P Predicts Poor Prognosis in Men with Metastatic PCa
2.4. IDC-P Predicts Death from PCa
3. Treatment Response
3.1. Response to Neoadjuvant Chemotherapy, Androgen-Deprivation Therapy and Androgen Receptor Axis-Targeted Therapy
3.2. Response to Chemotherapy or Androgen Receptor Axis-Targeted Therapy
3.3. Response to Adjuvant Radiation Therapy
4. Molecular Markers of IDC-P
4.1. IDC-P without Associated Invasive Carcinoma
4.2. IDC-P Compared to High-Grade Intraepithelial Neoplasia (HGPIN) and to Adjacent Invasive Carcinoma
4.2.1. PTEN and ERG (TMPRSS2:ERG)
4.2.2. Multimarker Studies
4.2.3. Novel Diagnostic Biomarker: Raman Microspectroscopy
4.3. Cases with IDC-P Compared to Cases without IDC-P
4.4. IDC-P and Deleterious Germline and Somatic Alterations of DNA-Damage Repair Genes
4.5. IDC-P and Its Impact on PCa Outcome
4.6. Commercial Classification Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Park, S.H.; Eber, M.R.; Peters, C.M.; Shiozawa, Y. Skeletal complications in cancer patients with bone metastases. Int. J. Urol. 2016, 23, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.K.; Dayyani, F.; Gallick, G.E. Steps in prostate cancer progression that lead to bone metastasis. Int. J. Cancer 2011, 128, 2545–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2015, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- McNeal, J.E.; Yemoto, C.E. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am. J. Surg. Pathol. 1996, 20, 802–814. [Google Scholar] [CrossRef]
- Rhamy, R.K.; Buchanan, R.D.; Spalding, M.J. Intraductal carcinoma of the prostate gland. J. Urol. 1973, 109, 457–460. [Google Scholar] [CrossRef]
- Kovi, J.; Jackson, M.A.; Heshmat, M.Y. Ductal spread in prostatic carcinoma. Cancer 1985, 56, 1566–1573. [Google Scholar] [CrossRef]
- Porter, L.H.; Lawrence, M.G.; Ilic, D.; Clouston, D.; Bolton, D.M.; Frydenberg, M.; Murphy, D.G.; Pezaro, C.; Risbridger, G.P.; Taylor, R.A. Systematic Review Links the Prevalence of Intraductal Carcinoma of the Prostate to Prostate Cancer Risk Categories. Eur. Urol. 2017, 72, 492–495. [Google Scholar] [CrossRef]
- Seipel, A.H.; Wiklund, F.; Wiklund, N.P.; Egevad, L. Histopathological features of ductal adenocarcinoma of the prostate in 1,051 radical prostatectomy specimens. Virchows Arch. 2013, 462, 429–436. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Fine, S.W.; Algaba, F.; Aron, M.; Baydar, D.E.; Beltran, A.L.; Brimo, F.; Cheville, J.C.; Colecchia, M.; et al. The 2019 Genitourinary Pathology Society (GUPS) White Paper on Contemporary Grading of Prostate Cancer. Arch. Pathol. Lab. Med. 2021, 145, 461–493. [Google Scholar] [CrossRef]
- van Leenders, G.; van der Kwast, T.H.; Iczkowski, K.A. The 2019 International Society of Urological Pathology Consensus Conference on Prostate Cancer Grading. Eur. Urol. 2021, 79, 707–709. [Google Scholar] [CrossRef]
- Varma, M. Intraductal Carcinoma of the Prostate: A Guide for the Practicing Pathologist. Adv. Anat. Pathol. 2021, 28, 276–287. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Sun, G.L.; Ma, D.L.; Wei, C.; Shang, H.J.; Liu, Z.; Li, R.; Wang, T.; Wang, S.G.; Liu, J.H.; et al. The presence of intraductal carcinoma of the prostate is closely associated with poor prognosis: A systematic review and meta-analysis. Asian J. Androl. 2021, 23, 103–108. [Google Scholar] [CrossRef]
- Karakoc, S.; Celik, S.; Kaya, N.; Bozkurt, O.; Ellidokuz, H.; Tuna, B.; Yorukoglu, K.; Mungan, M.U. Prognostic value of intraductal carcinoma for adjuvant radiotherapy candidates after radical prostatectomy. Int. J. Clin. Pract. 2021, 75, e14099. [Google Scholar] [CrossRef]
- Trinh, V.Q.; Benzerdjeb, N.; Chagnon-Monarque, S.; Dionne, N.; Delouya, G.; Kougioumoutzakis, A.; Sirois, J.; Albadine, R.; Latour, M.; Mes-Masson, A.-M.; et al. Retrospective study on the benefit of adjuvant radiotherapy in men with intraductal carcinoma of prostate. Radiat. Oncol. 2019, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.H.; Hashimoto, K.; Lawrence, M.G.; Pezaro, C.; Clouston, D.; Wang, H.; Papargiris, M.; Thorne, H.; Li, J.; kConFab; et al. Intraductal carcinoma of the prostate can evade androgen deprivation, with emergence of castrate-tolerant cells. BJU Int. 2018, 121, 971–978. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, N.; Shen, P.; Gong, J.; Li, X.; Zhao, T.; Liao, B.; Liu, L.; Liu, Z.; Zhang, X.; et al. The presence and clinical implication of intraductal carcinoma of prostate in metastatic castration resistant prostate cancer. Prostate 2015, 75, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Abascal-Junquera, J.M.; Fumado-Ciutat, L.; Gasa-Galmes, B.; Costa-Planells, M.; Munarriz-Polo, M.; Sanroma-Salva, A.; Polaina-Barroso, L.; Sola-Marques, C.; Juanpere-Rodero, N.; Lloreta-Trull, J.; et al. Concomitant intraductal carcinoma of the prostate and response to hormonal therapy in metastatic prostate carcinoma. Actas. Urol. Esp. 2021, 45, 455–460. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; True, L.D.; Higano, C.S.; Rademacher, B.L.; Garzotto, M.; Beer, T.M. Histologic changes associated with neoadjuvant chemotherapy are predictive of nodal metastases in patients with high-risk prostate cancer. Am. J. Clin. Pathol. 2010, 133, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Ke, Z.B.; Chen, Y.H.; Wu, Y.P.; Chen, S.H.; Wei, Y.; Zheng, Q.S.; Huang, J.B.; Li, X.D.; Xue, X.Y. Risk Factors for Pathologically Confirmed Lymph Nodes Metastasis in Patients With Clinical T2N0M0 Stage Prostate Cancer. Front. Oncol. 2020, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Kryvenko, O.N.; Gupta, N.S.; Virani, N.; Schultz, D.; Gomez, J.; Amin, A.; Lane, Z.; Epstein, J.I. Gleason score 7 adenocarcinoma of the prostate with lymph node metastases: Analysis of 184 radical prostatectomy specimens. Arch. Pathol. Lab. Med. 2013, 137, 610–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downes, M.R.; Xu, B.; van der Kwast, T.H. Gleason grade patterns in nodal metastasis and corresponding prostatectomy specimens: Impact on patient outcome. Histopathology 2019, 75, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.R.; Xu, B.; van der Kwast, T.H. Cribriform architecture prostatic adenocarcinoma in needle biopsies is a strong independent predictor for lymph node metastases in radical prostatectomy. Eur. J. Cancer 2021, 148, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, J.; Kristiansen, A.; Wiklund, P.; Gronberg, H.; Egevad, L. Tracking the origin of metastatic prostate cancer. Eur. Urol. 2015, 67, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Dinerman, B.F.; Khani, F.; Golan, R.; Bernstein, A.N.; Cosiano, M.F.; Margolis, D.J.; Hu, J.C. Population-based study of the incidence and survival for intraductal carcinoma of the prostate. Urol. Oncol. 2017, 35, 673.e9–673.e14. [Google Scholar] [CrossRef] [PubMed]
- Trinh, V.Q.; Sirois, J.; Benzerdjeb, N.; Mansoori, B.K.; Grosset, A.A.; Albadine, R.; Latour, M.; Mes-Masson, A.M.; Hovington, H.; Bergeron, A.; et al. The impact of intraductal carcinoma of the prostate on the site and timing of recurrence and cancer-specific survival. Prostate 2018, 78, 697–706. [Google Scholar] [CrossRef]
- Tom, M.C.; Nguyen, J.K.; Luciano, R.; Mian, O.Y.; Stephans, K.L.; Ciezki, J.P.; Smile, T.D.; Wei, W.; McKenney, J.K.; Magi-Galluzzi, C.; et al. Impact of Cribriform Pattern and Intraductal Carcinoma on Gleason 7 Prostate Cancer Treated with External Beam Radiotherapy. J. Urol. 2019, 202, 710–716. [Google Scholar] [CrossRef]
- Van der Kwast, T.; Al Daoud, N.; Collette, L.; Sykes, J.; Thoms, J.; Milosevic, M.; Bristow, R.G.; Van Tienhoven, G.; Warde, P.; Mirimanoff, R.O.; et al. Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur. J. Cancer 2012, 48, 1318–1325. [Google Scholar] [CrossRef]
- Kweldam, C.F.; Wildhagen, M.F.; Steyerberg, E.W.; Bangma, C.H.; van der Kwast, T.H.; van Leenders, G.J. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod. Pathol. 2015, 28, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Chua, M.L.K.; Lo, W.; Pintilie, M.; Murgic, J.; Lalonde, E.; Bhandari, V.; Mahamud, O.; Gopalan, A.; Kweldam, C.F.; van Leenders, G.; et al. A Prostate Cancer "Nimbosus": Genomic Instability and SChLAP1 Dysregulation Underpin Aggression of Intraductal and Cribriform Subpathologies. Eur. Urol. 2017, 72, 665–674. [Google Scholar] [CrossRef]
- Kimura, K.; Tsuzuki, T.; Kato, M.; Saito, A.M.; Sassa, N.; Ishida, R.; Hirabayashi, H.; Yoshino, Y.; Hattori, R.; Gotoh, M. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate 2014, 74, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Hansum, T.; Hollemans, E.; Verhoef, E.I.; Bangma, C.H.; Rietbergen, J.; Osanto, S.; Pelger, R.C.M.; van Wezel, T.; van der Poel, H.; Bekers, E.; et al. Comedonecrosis Gleason pattern 5 is associated with worse clinical outcome in operated prostate cancer patients. Mod. Pathol. 2021, 34, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Hollemans, E.; Verhoef, E.I.; Bangma, C.H.; Rietbergen, J.; Osanto, S.; Pelger, R.C.M.; van Wezel, T.; van der Poel, H.; Bekers, E.; Helleman, J.; et al. Cribriform architecture in radical prostatectomies predicts oncological outcome in Gleason score 8 prostate cancer patients. Mod. Pathol. 2021, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Hollemans, E.; Verhoef, E.I.; Bangma, C.H.; Rietbergen, J.; Roobol, M.J.; Helleman, J.; van Leenders, G. Clinical outcome comparison of Grade Group 1 and Grade Group 2 prostate cancer with and without cribriform architecture at the time of radical prostatectomy. Histopathology 2020, 76, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Velho, P.I.; Lim, D.; Wang, H.; Park, J.C.; Kaur, H.B.; Almutairi, F.; Carducci, M.A.; Denmeade, S.R.; Markowski, M.C.; Isaacs, W.B.; et al. Molecular Characterization and Clinical Outcomes of Primary Gleason Pattern 5 Prostate Cancer After Radical Prostatectomy. JCO Precis. Oncol. 2019, 3, PO.19.00081. [Google Scholar] [CrossRef]
- Zhao, T.; Liao, B.; Yao, J.; Liu, J.; Huang, R.; Shen, P.; Peng, Z.; Gui, H.; Chen, X.; Zhang, P.; et al. Is there any prognostic impact of intraductal carcinoma of prostate in initial diagnosed aggressively metastatic prostate cancer? Prostate 2015, 75, 225–232. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, P.; Sun, G.; Chen, N.; Liu, J.; Tang, X.; Huang, R.; Cai, D.; Gong, J.; Zhang, X.; et al. The prognostic implication of intraductal carcinoma of the prostate in metastatic castration-resistant prostate cancer and its potential predictive value in those treated with docetaxel or abiraterone as first-line therapy. Oncotarget 2017, 8, 55374–55383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Liu, J.; Sun, G.; Zhang, M.; Chen, J.; Shen, P.; Liu, Z.; Liao, B.; Zhang, X.; Gong, J.; et al. The Prognostic Value of the Proportion and Architectural Patterns of Intraductal Carcinoma of the Prostate in Patients with De Novo Metastatic Prostate Cancer. J. Urol. 2019, 201, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, G.; Liao, B.; Zhang, X.; Armstrong, C.M.; Yin, X.; Liu, J.; Chen, J.; Yang, Y.; Zhao, P.; et al. Novel nomograms for castration-resistant prostate cancer and survival outcome in patients with de novo bone metastatic prostate cancer. BJU Int. 2018, 122, 994–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, M.; Tsuzuki, T.; Kimura, K.; Hirakawa, A.; Kinoshita, F.; Sassa, N.; Ishida, R.; Fukatsu, A.; Kimura, T.; Funahashi, Y.; et al. The presence of intraductal carcinoma of the prostate in needle biopsy is a significant prognostic factor for prostate cancer patients with distant metastasis at initial presentation. Mod. Pathol. 2016, 29, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Hirakawa, A.; Sato, H.; Hanazawa, R.; Naito, Y.; Tochigi, K.; Sano, T.; Ishida, S.; Funahashi, Y.; Fujita, T.; et al. Grade group 2 (10% >/= GP4) patients have very similar malignant potential with grade group 1 patients, given the risk of intraductal carcinoma of the prostate. Int. J. Clin. Oncol. 2021, 26, 764–769. [Google Scholar] [CrossRef]
- Kato, M.; Kimura, K.; Hirakawa, A.; Kobayashi, Y.; Ishida, R.; Kamihira, O.; Majima, T.; Funahashi, Y.; Sassa, N.; Matsukawa, Y.; et al. Prognostic parameter for high risk prostate cancer patients at initial presentation. Prostate 2018, 78, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeter, T.; Vlatkovic, L.; Waaler, G.; Servoll, E.; Nesland, J.M.; Axcrona, K.; Axcrona, U. Intraductal Carcinoma of the Prostate on Diagnostic Needle Biopsy Predicts Prostate Cancer Mortality: A Population-Based Study. Prostate 2017, 77, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Kanamaru, H.; Yoshimura, K.; Okubo, K.; Kamoto, T.; Yoshida, O. Incidence of lymph node metastasis and its impact on long-term prognosis in clinically localized prostate cancer. Int. J. Urol. 1998, 5, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T. Intraductal carcinoma of the prostate: A comprehensive and updated review. Int. J. Urol. 2015, 22, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Mori, K.; Mostafaei, H.; Quhal, F.; Motlagh, R.S.; Pradere, B.; Laukhtina, E.; D’Andrea, D.; Saika, T.; Shariat, S.F. The Prognostic Impact of Intraductal Carcinoma of the Prostate: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 909–917. [Google Scholar] [CrossRef]
- Iczkowski, K.A.; van Leenders, G.; Tarima, S.; Wu, R.; Van der Kwast, T.; Berney, D.M.; Evans, A.J.; Wheeler, T.M.; Ro, J.Y.; Samaratunga, H.; et al. Cribriform prostate cancer: Morphologic criteria enabling a diagnosis, based on survey of experts. Ann. Diagn. Pathol. 2021, 52, 151733. [Google Scholar] [CrossRef] [PubMed]
- Iczkowski, K.A.; Paner, G.P.; Van der Kwast, T. The New Realization About Cribriform Prostate Cancer. Adv. Anat. Pathol. 2018, 25, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.A.; de La Taille, A.; Bagiella, E.; Olsson, C.A.; O’Toole, K.M. Cribriform carcinoma of the prostate and cribriform prostatic intraepithelial neoplasia: Incidence and clinical implications. Am. J. Surg. Pathol. 1998, 22, 840–848. [Google Scholar] [CrossRef]
- Efstathiou, E.; Abrahams, N.A.; Tibbs, R.F.; Wang, X.; Pettaway, C.A.; Pisters, L.L.; Mathew, P.F.; Do, K.A.; Logothetis, C.J.; Troncoso, P. Morphologic characterization of preoperatively treated prostate cancer: Toward a post-therapy histologic classification. Eur. Urol. 2010, 57, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Hirakawa, A.; Kobayashi, Y.; Yamamoto, A.; Ishida, R.; Kamihira, O.; Sano, T.; Majima, T.; Ishida, S.; Funahashi, Y.; et al. Response of intraductal carcinoma of the prostate to androgen deprivation therapy predicts prostate cancer prognosis in radical prostatectomy patients. Prostate 2020, 80, 284–290. [Google Scholar] [CrossRef]
- McKay, R.R.; Xie, W.; Ye, H.; Fennessy, F.M.; Zhang, Z.; Lis, R.; Calagua, C.; Rathkopf, D.; Laudone, V.P.; Bubley, G.J.; et al. Results of a Randomized Phase II Trial of Intense Androgen Deprivation Therapy prior to Radical Prostatectomy in Men with High-Risk Localized Prostate Cancer. J. Urol. 2021, 206, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hollemans, E.; Verhoef, E.I.; Bangma, C.H.; Rietbergen, J.; Helleman, J.; Roobol, M.J.; van Leenders, G. Large cribriform growth pattern identifies ISUP grade 2 prostate cancer at high risk for recurrence and metastasis. Mod. Pathol. 2019, 32, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Elfandy, H.; Armenia, J.; Pederzoli, F.; Pullman, E.; Pertega-Gomes, N.; Schultz, N.; Viswanathan, K.; Vosoughi, A.; Blattner, M.; Stopsack, K.H.; et al. Genetic and Epigenetic Determinants of Aggressiveness in Cribriform Carcinoma of the Prostate. Mol. Cancer Res. 2019, 17, 446–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberts, A.R.; Bokhorst, L.P.; Kweldam, C.F.; Schoots, I.G.; van der Kwast, T.H.; van Leenders, G.J.; Roobol, M.J. Biopsy undergrading in men with Gleason score 6 and fatal prostate cancer in the European Randomized study of Screening for Prostate Cancer Rotterdam. Int. J. Urol. 2017, 24, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, R.R.; Berchuck, J.; Kwak, L.; Xie, W.; Silver, R.; Bubley, G.J.; Chang, P.K.; Wagner, A.; Zhang, Z.; Kibel, A.S.; et al. Outcomes of Post-Neoadjuvant Intense Hormone Therapy and Surgery for High Risk Localized Prostate Cancer: Results of a Pooled Analysis of Contemporary Clinical Trials. J. Urol. 2021, 205, 1689–1697. [Google Scholar] [CrossRef]
- Wilkinson, S.; Ye, H.; Karzai, F.; Harmon, S.A.; Terrigino, N.T.; VanderWeele, D.J.; Bright, J.R.; Atway, R.; Trostel, S.Y.; Carrabba, N.V.; et al. Nascent Prostate Cancer Heterogeneity Drives Evolution and Resistance to Intense Hormonal Therapy. Eur. Urol. 2021, 80, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Kato, M.; Matsui, H.; Ishida, R.; Kimura, T.; Funahashi, Y.; Sassa, N.; Matsukawa, Y.; Kamihira, O.; Hattori, R.; et al. Efficacy of docetaxel in castration-resistant prostate cancer patients with intraductal carcinoma of the prostate. Int. J. Clin. Oncol 2018, 23, 584–590. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kato, M.; Hattori, K.; Naito, Y.; Tochigi, K.; Sano, T.; Kawanishi, H.; Ishikawa, T.; Yuba, T.; Hattori, R.; et al. Propensity score-matched comparison of docetaxel and androgen receptor axis-targeted agents in patients with castration-resistant intraductal carcinoma of the prostate. BJU Int. 2020, 125, 702–708. [Google Scholar] [CrossRef]
- Cohen, R.J.; McNeal, J.E.; Baillie, T. Patterns of differentiation and proliferation in intraductal carcinoma of the prostate: Significance for cancer progression. Prostate 2000, 43, 11–19. [Google Scholar] [CrossRef]
- Guo, C.C.; Epstein, J.I. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod. Pathol. 2006, 19, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Grypari, I.M.; Logotheti, S.; Lazaris, A.C.; Kallidonis, P.; Fokaefs, E.; Melachrinou, M.; Zolota, V.; Tzelepi, V. Isolated Intraductal Carcinoma of the Prostate in Prostatectomy Specimens: Report of 2 Cases and Review of the Literature. Int. J. Surg. Pathol. 2020, 28, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Khani, F.; Wobker, S.E.; Hicks, J.L.; Robinson, B.D.; Barbieri, C.E.; De Marzo, A.M.; Epstein, J.I.; Pritchard, C.C.; Lotan, T.L. Intraductal carcinoma of the prostate in the absence of high-grade invasive carcinoma represents a molecularly distinct type of in situ carcinoma enriched with oncogenic driver mutations. J. Pathol. 2019, 249, 79–89. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Antonarakis, E.S.; Bismar, T.A.; Guedes, L.B.; Cheng, H.H.; Tretiakova, M.S.; Vakar-Lopez, F.; Klemfuss, N.; Konnick, E.Q.; Mostaghel, E.A.; et al. Genomic Characterization of Prostatic Ductal Adenocarcinoma Identifies a High Prevalence of DNA Repair Gene Mutations. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miyai, K.; Divatia, M.K.; Shen, S.S.; Miles, B.J.; Ayala, A.G.; Ro, J.Y. Clinicopathological analysis of intraductal proliferative lesions of prostate: Intraductal carcinoma of prostate, high-grade prostatic intraepithelial neoplasia, and atypical cribriform lesion. Hum. Pathol. 2014, 45, 1572–1581. [Google Scholar] [CrossRef]
- Lotan, T.L.; Gumuskaya, B.; Rahimi, H.; Hicks, J.L.; Iwata, T.; Robinson, B.D.; Epstein, J.I.; De Marzo, A.M. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod. Pathol. 2013, 26, 587–603. [Google Scholar] [CrossRef] [Green Version]
- Morais, C.L.; Han, J.S.; Gordetsky, J.; Nagar, M.S.; Anderson, A.E.; Lee, S.; Hicks, J.L.; Zhou, M.; Magi-Galluzzi, C.; Shah, R.B.; et al. Utility of PTEN and ERG immunostaining for distinguishing high-grade PIN from intraductal carcinoma of the prostate on needle biopsy. Am. J. Surg. Pathol. 2015, 39, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.B.; Yoon, J.; Liu, G.; Tian, W. Atypical intraductal proliferation and intraductal carcinoma of the prostate on core needle biopsy: A comparative clinicopathological and molecular study with a proposal to expand the morphological spectrum of intraductal carcinoma. Histopathology 2017, 71, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Hickman, R.A.; Yu, H.; Li, J.; Kong, M.; Shah, R.B.; Zhou, M.; Melamed, J.; Deng, F.M. Atypical Intraductal Cribriform Proliferations of the Prostate Exhibit Similar Molecular and Clinicopathologic Characteristics as Intraductal Carcinoma of the Prostate. Am. J. Surg. Pathol. 2017, 41, 550–556. [Google Scholar] [CrossRef]
- Haffner, M.C.; Weier, C.; Xu, M.M.; Vaghasia, A.; Gurel, B.; Gumuskaya, B.; Esopi, D.M.; Fedor, H.; Tan, H.L.; Kulac, I.; et al. Molecular evidence that invasive adenocarcinoma can mimic prostatic intraepithelial neoplasia (PIN) and intraductal carcinoma through retrograde glandular colonization. J. Pathol. 2016, 238, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Mosquera, J.M.; Perner, S.; Demichelis, F.; Kim, R.; Hofer, M.D.; Mertz, K.D.; Paris, P.L.; Simko, J.; Collins, C.; Bismar, T.A.; et al. Morphological features of TMPRSS2-ERG gene fusion prostate cancer. J. Pathol. 2007, 212, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Suleman, K.; Wang, L.; Siddiqui, J.; Sercia, L.; Magi-Galluzzi, C.; Palanisamy, N.; Chinnaiyan, A.M.; Zhou, M.; Shah, R.B. ETS gene aberrations in atypical cribriform lesions of the prostate: Implications for the distinction between intraductal carcinoma of the prostate and cribriform high-grade prostatic intraepithelial neoplasia. Am. J. Surg. Pathol. 2010, 34, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Bettendorf, O.; Schmidt, H.; Staebler, A.; Grobholz, R.; Heinecke, A.; Boecker, W.; Hertle, L.; Semjonow, A. Chromosomal imbalances, loss of heterozygosity, and immunohistochemical expression of TP53, RB1, and PTEN in intraductal cancer, intraepithelial neoplasia, and invasive adenocarcinoma of the prostate. Genes Chromosomes Cancer 2008, 47, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.R.; Satturwar, S.; Trudel, D.; van der Kwast, T.H. Evaluation of ERG and PTEN protein expression in cribriform architecture prostate carcinomas. Pathol. Res. Pract. 2017, 213, 34–38. [Google Scholar] [CrossRef]
- Schneider, T.M.; Osunkoya, A.O. ERG expression in intraductal carcinoma of the prostate: Comparison with adjacent invasive prostatic adenocarcinoma. Mod. Pathol. 2014, 27, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Pan, X.; Zhang, M.; Yin, X.; Gong, J.; Chen, X.; Xu, M.; Zhou, Q.; Chen, N. The expression profile and heterogeneity analysis of ERG in 633 consecutive prostate cancers from a single center. Prostate 2019, 79, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Torabi-Nezhad, S.; Malekmakan, L.; Mashayekhi, M.; Daneshian, A. Histopathological features of intra-ductal carcinoma of prostatic and high grade prostatic intraepithelialneoplasia and correlation with PTEN and P63. Prostate 2016, 76, 394–401. [Google Scholar] [CrossRef]
- Shah, R.B.; Shore, K.T.; Yoon, J.; Mendrinos, S.; McKenney, J.K.; Tian, W. PTEN loss in prostatic adenocarcinoma correlates with specific adverse histologic features (intraductal carcinoma, cribriform Gleason pattern 4 and stromogenic carcinoma). Prostate 2019, 79, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, H.J.; Sellner, L.N.; Turbett, G.R.; Thompson, C.A.; Redmond, S.L.; McNeal, J.E.; Cohen, R.J. Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adenocarcinoma, using molecular markers of cancer progression. Prostate 2000, 44, 265–270. [Google Scholar] [CrossRef]
- Grosset, A.A.; Dallaire, F.; Nguyen, T.; Birlea, M.; Wong, J.; Daoust, F.; Roy, N.; Kougioumoutzakis, A.; Azzi, F.; Aubertin, K.; et al. Identification of intraductal carcinoma of the prostate on tissue specimens using Raman micro-spectroscopy: A diagnostic accuracy case-control study with multicohort validation. PLoS Med. 2020, 17, e1003281. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Nakamura, N.; Vazquez, F.; Batt, D.B.; Perera, S.; Roberts, T.M.; Sellers, W.R. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 2110–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairns, P.; Okami, K.; Halachmi, S.; Halachmi, N.; Esteller, M.; Herman, J.G.; Jen, J.; Isaacs, W.B.; Bova, G.S.; Sidransky, D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997, 57, 4997–5000. [Google Scholar] [PubMed]
- Suzuki, H.; Freije, D.; Nusskern, D.R.; Okami, K.; Cairns, P.; Sidransky, D.; Isaacs, W.B.; Bova, G.S. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998, 58, 204–209. [Google Scholar] [PubMed]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Lotan, T.L.; Gurel, B.; Sutcliffe, S.; Esopi, D.; Liu, W.; Xu, J.; Hicks, J.L.; Park, B.H.; Humphreys, E.; Partin, A.W.; et al. PTEN protein loss by immunostaining: Analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin. Cancer Res. 2011, 17, 6563–6573. [Google Scholar] [CrossRef] [Green Version]
- Ahearn, T.U.; Pettersson, A.; Ebot, E.M.; Gerke, T.; Graff, R.E.; Morais, C.L.; Hicks, J.L.; Wilson, K.M.; Rider, J.R.; Sesso, H.D.; et al. A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [Green Version]
- Hamid, A.A.; Gray, K.P.; Huang, Y.; Bowden, M.; Pomerantz, M.; Loda, M.; Sweeney, C.J. Loss of PTEN Expression Detected by Fluorescence Immunohistochemistry Predicts Lethal Prostate Cancer in Men Treated with Prostatectomy. Eur. Urol. Oncol. 2019, 2, 475–482. [Google Scholar] [CrossRef]
- Choucair, K.; Ejdelman, J.; Brimo, F.; Aprikian, A.; Chevalier, S.; Lapointe, J. PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity. BMC Cancer 2012, 12, 543. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Jiang, X. Post-translational regulation of PTEN. Oncogene 2008, 27, 5454–5463. [Google Scholar] [CrossRef] [Green Version]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Gasparrini, S.; Cimadamore, A.; Mazzucchelli, R.; Scarpelli, M.; Massari, F.; Raspollini, M.R.; Galosi, A.B.; Lopez-Beltran, A.; Cheng, L.; Montironi, R. Pathology and molecular updates in tumors of the prostate: Towards a personalized approach. Expert Rev. Mol. Diagn 2017, 17, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Lokman, U.; Erickson, A.M.; Vasarainen, H.; Rannikko, A.S.; Mirtti, T. PTEN Loss but Not ERG Expression in Diagnostic Biopsies Is Associated with Increased Risk of Progression and Adverse Surgical Findings in Men with Prostate Cancer on Active Surveillance. Eur. Urol. Focus 2017, 4, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deramaudt, T.B.; Remy, P.; Stiegler, P. Identification of interaction partners for two closely-related members of the ETS protein family, FLI and ERG. Gene 2001, 274, 169–177. [Google Scholar] [CrossRef]
- Hägglöf, C.; Hammarsten, P.; Strömvall, K.; Egevad, L.; Josefsson, A.; Stattin, P.; Granfors, T.; Bergh, A. TMPRSS2-ERG expression predicts prostate cancer survival and associates with stromal biomarkers. PLoS ONE 2014, 9, e86824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Li, L.Y.; Zhou, F.J.; Xie, K.J.; Shao, C.K.; Su, Z.L.; Sun, Q.P.; Chen, M.K.; Pang, J.; Zhou, X.F.; et al. ERG rearrangement for predicting subsequent cancer diagnosis in high-grade prostatic intraepithelial neoplasia and lymph node metastasis. Clin. Cancer Res. 2012, 18, 4163–4172. [Google Scholar] [CrossRef] [Green Version]
- Hoogland, A.M.; Jenster, G.; van Weerden, W.M.; Trapman, J.; van der Kwast, T.; Roobol, M.J.; Schroder, F.H.; Wildhagen, M.F.; van Leenders, G.J. ERG immunohistochemistry is not predictive for PSA recurrence, local recurrence or overall survival after radical prostatectomy for prostate cancer. Mod. Pathol. 2012, 25, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Kron, K.; Liu, L.; Trudel, D.; Pethe, V.; Trachtenberg, J.; Fleshner, N.; Bapat, B.; van der Kwast, T. Correlation of ERG expression and DNA methylation biomarkers with adverse clinicopathologic features of prostate cancer. Clin. Cancer Res. 2012, 18, 2896–2904. [Google Scholar] [CrossRef] [Green Version]
- Attard, G.; Clark, J.; Ambroisine, L.; Fisher, G.; Kovacs, G.; Flohr, P.; Berney, D.; Foster, C.S.; Fletcher, A.; Gerald, W.L.; et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene 2008, 27, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Latour, M.; Amin, M.B.; Billis, A.; Egevad, L.; Grignon, D.J.; Humphrey, P.A.; Reuter, V.E.; Sakr, W.A.; Srigley, J.R.; Wheeler, T.M.; et al. Grading of invasive cribriform carcinoma on prostate needle biopsy: An interobserver study among experts in genitourinary pathology. Am. J. Surg. Pathol. 2008, 32, 1532–1539. [Google Scholar] [CrossRef]
- Jermyn, M.; Desroches, J.; Aubertin, K.; St-Arnaud, K.; Madore, W.J.; De Montigny, E.; Guiot, M.C.; Trudel, D.; Wilson, B.C.; Petrecca, K.; et al. A review of Raman spectroscopy advances with an emphasis on clinical translation challenges in oncology. Phys. Med. Biol. 2016, 61, R370-r400. [Google Scholar] [CrossRef]
- Jermyn, M.; Desroches, J.; Mercier, J.; St-Arnaud, K.; Guiot, M.C.; Leblond, F.; Petrecca, K. Raman spectroscopy detects distant invasive brain cancer cells centimeters beyond MRI capability in humans. Biomed. Opt. Express 2016, 7, 5129–5137. [Google Scholar] [CrossRef] [Green Version]
- Jermyn, M.; Desroches, J.; Mercier, J.; Tremblay, M.A.; St-Arnaud, K.; Guiot, M.C.; Petrecca, K.; Leblond, F. Neural networks improve brain cancer detection with Raman spectroscopy in the presence of operating room light artifacts. J. Biomed. Opt. 2016, 21, 94002. [Google Scholar] [CrossRef] [Green Version]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.C.; Petrecca, K.; Leblond, F. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015, 7, 274ra219. [Google Scholar] [CrossRef]
- Aubertin, K.; Trinh, V.Q.; Jermyn, M.; Baksic, P.; Grosset, A.A.; Desroches, J.; St-Arnaud, K.; Birlea, M.; Vladoiu, M.C.; Latour, M.; et al. Mesoscopic characterization of prostate cancer using Raman spectroscopy: Potential for diagnostics and therapeutics. BJU Int. 2018, 122, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.; Zorn, K.; Tremblay, J.P.; Desroches, J.; Aubertin, K.; Marple, E.; Kent, K.; Leblond, F.; Trudel, D.; Lesage, F. Integration of a Raman spectroscopy system to a robotic-assisted surgical system for real time tissue characterization during radical prostatectomy procedures. J. Biomed. Opt. 2019, 24, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plante, A.; Dallaire, F.; Grosset, A.A.; Nguyen, T.; Birlea, M.; Wong, J.; Daoust, F.; Roy, N.; Kougioumoutzakis, A.; Azzi, F.; et al. Dimensional reduction based on peak fitting of Raman micro spectroscopy data improves detection of prostate cancer in tissue specimens. J. Biomed. Opt. 2021, 26, 116501. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, R.; Kweldam, C.F.; Livingstone, J.; Lalonde, E.; Yamaguchi, T.N.; Huang, V.; Yousif, F.; Fraser, M.; Bristow, R.G.; van der Kwast, T.; et al. Cribriform and intraductal prostate cancer are associated with increased genomic instability and distinct genomic alterations. BMC Cancer 2018, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Salles, D.C.; Vidotto, T.; Faisal, F.A.; Tosoian, J.J.; Guedes, L.B.; Muranyi, A.; Bai, I.; Singh, S.; Yan, D.; Shanmugam, K.; et al. Assessment of MYC/PTEN Status by Gene-Protein Assay in Grade Group 2 Prostate Biopsies. J. Mol. Diagn. 2021, 23, 1030–1041. [Google Scholar] [CrossRef]
- Olkhov-Mitsel, E.; Siadat, F.; Kron, K.; Liu, L.; Savio, A.J.; Trachtenberg, J.; Fleshner, N.; van der Kwast, T.; Bapat, B. Distinct DNA methylation alterations are associated with cribriform architecture and intraductal carcinoma in Gleason pattern 4 prostate tumors. Oncol. Lett. 2017, 14, 390–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Sun, G.; Zhu, S.; Dai, J.; Chen, J.; Zhang, M.; Ni, Y.; Zhang, H.; Shen, P.; Zhao, X.; et al. Circulating tumour DNA reveals genetic traits of patients with intraductal carcinoma of the prostate. BJU Int. 2021. [Google Scholar] [CrossRef]
- Lee, S.; Han, J.S.; Chang, A.; Ross, H.M.; Montironi, R.; Yorukoglu, K.; Lane, Z.; Epstein, J.I. Small cell-like change in prostatic intraepithelial neoplasia, intraductal carcinoma, and invasive prostatic carcinoma: A study of 7 cases. Hum. Pathol. 2013, 44, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Sarsik, B.; Karadeniz, N.; Simsir, A.; Akyol, R.; Yorukoglu, K.; Sen, S. Acinar Adenocarcinoma of Prostate with Predominant Ttf-1 Positive Intraductal Component. Turk. Patoloji Derg. 2017, 33, 70–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, E.; Goh, C.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Dadaev, T.; Govindasami, K.; Guy, M.; Ellis, S.; Frost, D.; et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur. Urol. 2015, 68, 186–193. [Google Scholar] [CrossRef]
- Willems, A.J.; Dawson, S.J.; Samaratunga, H.; De Luca, A.; Antill, Y.C.; Hopper, J.L.; Thorne, H.J. Loss of heterozygosity at the BRCA2 locus detected by multiplex ligation-dependent probe amplification is common in prostate cancers from men with a germline BRCA2 mutation. Clin. Cancer Res. 2008, 14, 2953–2961. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.R.; Wallis, C.J.; Toi, A.; Trachtenberg, J.; Sun, P.; Narod, S.A.; Nam, R.K. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br. J. Cancer 2014, 111, 1238–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.G.; Risbridger, G.P.; Bristow, R.G.; Sandhu, S. The Evolving Narrative of DNA Repair Gene Defects: Distinguishing Indolent from Lethal Prostate Cancer. Eur. Urol. 2017, 71, 748–749. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- Risbridger, G.P.; Taylor, R.A.; Clouston, D.; Sliwinski, A.; Thorne, H.; Hunter, S.; Li, J.; Mitchell, G.; Murphy, D.; Frydenberg, M.; et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur. Urol. 2015, 67, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Fraser, M.; Livingstone, J.; Espiritu, S.M.; Thorne, H.; Huang, V.; Lo, W.; Shiah, Y.J.; Yamaguchi, T.N.; Sliwinski, A.; et al. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat. Commun. 2017, 8, 13671. [Google Scholar] [CrossRef]
- Isaacsson Velho, P.; Silberstein, J.L.; Markowski, M.C.; Luo, J.; Lotan, T.L.; Isaacs, W.B.; Antonarakis, E.S. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer. Prostate 2018, 78, 401–407. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Shaukat, F.; Isaacsson Velho, P.; Kaur, H.; Shenderov, E.; Pardoll, D.M.; Lotan, T.L. Clinical Features and Therapeutic Outcomes in Men with Advanced Prostate Cancer and DNA Mismatch Repair Gene Mutations. Eur. Urol. 2019, 75, 378–382. [Google Scholar] [CrossRef]
- Lozano, R.; Salles, D.C.; Sandhu, S.; Aragon, I.M.; Thorne, H.; Lopez-Campos, F.; Rubio-Briones, J.; Gutierrez-Pecharroman, A.M.; Maldonado, L.; di Domenico, T.; et al. Association between BRCA2 alterations and intraductal and cribriform histologies in prostate cancer. Eur. J. Cancer 2021, 147, 74–83. [Google Scholar] [CrossRef]
- Uysal-Onganer, P.; Kawano, Y.; Caro, M.; Walker, M.M.; Diez, S.; Darrington, R.S.; Waxman, J.; Kypta, R.M. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol. Cancer 2010, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaikhibrahim, Z.; Offermann, A.; Braun, M.; Menon, R.; Syring, I.; Nowak, M.; Halbach, R.; Vogel, W.; Ruiz, C.; Zellweger, T.; et al. MED12 overexpression is a frequent event in castration-resistant prostate cancer. Endocr. Relat. Cancer 2014, 21, 663–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.J.; Vujcic, T.; Huang, X.; et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef]
- Chen, S.; Huang, V.; Xu, X.; Livingstone, J.; Soares, F.; Jeon, J.; Zeng, Y.; Hua, J.T.; Petricca, J.; Guo, H.; et al. Widespread and Functional RNA Circularization in Localized Prostate Cancer. Cell 2019, 176, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Spieker, A.J.; Gordetsky, J.B.; Maris, A.S.; Dehan, L.M.; Denney, J.E.; Arnold Egloff, S.A.; Scarpato, K.; Barocas, D.A.; Giannico, G.A. PTEN expression and morphological patterns in prostatic adenocarcinoma. Histopathology 2021, 79, 1061–1071. [Google Scholar] [CrossRef]
- Prensner, J.R.; Iyer, M.K.; Sahu, A.; Asangani, I.A.; Cao, Q.; Patel, L.; Vergara, I.A.; Davicioni, E.; Erho, N.; Ghadessi, M.; et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genet. 2013, 45, 1392–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, S.G.; Carm, K.T.; Bogaard, M.; Olsen, L.G.; Bakken, A.C.; Løvf, M.; Lothe, R.A.; Axcrona, K.; Axcrona, U.; Skotheim, R.I. High expression of SCHLAP1 in primary prostate cancer is an independent predictor of biochemical recurrence, despite substantial heterogeneity. Neoplasia 2021, 23, 634–641. [Google Scholar] [CrossRef]
- Greenland, N.Y.; Zhang, L.; Cowan, J.E.; Carroll, P.R.; Stohr, B.A.; Simko, J.P. Correlation of a Commercial Genomic Risk Classifier with Histological Patterns in Prostate Cancer. J. Urol. 2019, 202, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.S.; Morgan, T.M.; Wallington, D.G.; Chinnaiyan, A.M.; Spratt, D.E.; Mehra, R. Correlation between cribriform/intraductal prostatic adenocarcinoma and percent Gleason pattern 4 to a 22-gene genomic classifier. Prostate 2020, 80, 146–152. [Google Scholar] [CrossRef]
- Klein, E.A.; Cooperberg, M.R.; Magi-Galluzzi, C.; Simko, J.P.; Falzarano, S.M.; Maddala, T.; Chan, J.M.; Li, J.; Cowan, J.E.; Tsiatis, A.C.; et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur. Urol. 2014, 66, 550–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, J.; Rosner, I.L.; Brand, T.C.; Zhang, N.; Tsiatis, A.C.; Moncur, J.; Ali, A.; Chen, Y.; Knezevic, D.; Maddala, T.; et al. A Biopsy-based 17-gene Genomic Prostate Score Predicts Recurrence After Radical Prostatectomy and Adverse Surgical Pathology in a Racially Diverse Population of Men with Clinically Low- and Intermediate-risk Prostate Cancer. Eur. Urol. 2015, 68, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Eeden, S.K.; Lu, R.; Zhang, N.; Quesenberry, C.P., Jr.; Shan, J.; Han, J.S.; Tsiatis, A.C.; Leimpeter, A.D.; Lawrence, H.J.; Febbo, P.G.; et al. A Biopsy-based 17-gene Genomic Prostate Score as a Predictor of Metastases and Prostate Cancer Death in Surgically Treated Men with Clinically Localized Disease. Eur. Urol. 2018, 73, 129–138. [Google Scholar] [CrossRef]
- Covas Moschovas, M.; Chew, C.; Bhat, S.; Sandri, M.; Rogers, T.; Dell’Oglio, P.; Roof, S.; Reddy, S.; Sighinolfi, M.C.; Rocco, B.; et al. Association Between Oncotype DX Genomic Prostate Score and Adverse Tumor Pathology After Radical Prostatectomy. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef]
- Knudsen, B.S.; Kim, H.L.; Erho, N.; Shin, H.; Alshalalfa, M.; Lam, L.L.C.; Tenggara, I.; Chadwich, K.; Van Der Kwast, T.; Fleshner, N.; et al. Application of a Clinical Whole-Transcriptome Assay for Staging and Prognosis of Prostate Cancer Diagnosed in Needle Core Biopsy Specimens. J. Mol. Diagn. 2016, 18, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Yousefi, K.; Haddad, Z.; Abdollah, F.; Lam, L.L.; Shin, H.; Alshalalfa, M.; Godebu, E.; Wang, S.; Shabaik, A.; et al. Evaluation of a genomic classifier in radical prostatectomy patients with lymph node metastasis. Res. Rep. Urol. 2016, 8, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Erho, N.; Crisan, A.; Vergara, I.A.; Mitra, A.P.; Ghadessi, M.; Buerki, C.; Bergstralh, E.J.; Kollmeyer, T.; Fink, S.; Haddad, Z.; et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE 2013, 8, e66855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnes, R.J.; Bergstralh, E.J.; Davicioni, E.; Ghadessi, M.; Buerki, C.; Mitra, A.P.; Crisan, A.; Erho, N.; Vergara, I.A.; Lam, L.L.; et al. Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. J. Urol. 2013, 190, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Den, R.B.; Feng, F.Y.; Showalter, T.N.; Mishra, M.V.; Trabulsi, E.J.; Lallas, C.D.; Gomella, L.G.; Kelly, W.K.; Birbe, R.C.; McCue, P.A.; et al. Genomic prostate cancer classifier predicts biochemical failure and metastases in patients after postoperative radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1038–1046. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.A.; Yousefi, K.; Haddad, Z.; Choeurng, V.; Buerki, C.; Stephenson, A.J.; Li, J.; Kattan, M.W.; Magi-Galluzzi, C.; Davicioni, E. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur. Urol. 2015, 67, 778–786. [Google Scholar] [CrossRef]
- Den, R.B.; Yousefi, K.; Trabulsi, E.J.; Abdollah, F.; Choeurng, V.; Feng, F.Y.; Dicker, A.P.; Lallas, C.D.; Gomella, L.G.; Davicioni, E.; et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J. Clin. Oncol. 2015, 33, 944–951. [Google Scholar] [CrossRef]
- Ross, A.E.; Johnson, M.H.; Yousefi, K.; Davicioni, E.; Netto, G.J.; Marchionni, L.; Fedor, H.L.; Glavaris, S.; Choeurng, V.; Buerki, C.; et al. Tissue-based Genomics Augments Post-prostatectomy Risk Stratification in a Natural History Cohort of Intermediate- and High-Risk Men. Eur. Urol. 2016, 69, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.G.; Leo, M.C.; Haddad, Z.; Yousefi, K.; du Plessis, M.; Chen, C.; Choeurng, V.; Abdollah, F.; Robbins, B.; Ra, S.; et al. Validation of a Genomic Classifier for Predicting Post-Prostatectomy Recurrence in a Community Based Health Care Setting. J. Urol. 2016, 195, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Choeurng, V.; Howard, L.; De Hoedt, A.; du Plessis, M.; Yousefi, K.; Lam, L.L.; Buerki, C.; Ra, S.; Robbins, B.; et al. Utilization of a Genomic Classifier for Prediction of Metastasis Following Salvage Radiation Therapy after Radical Prostatectomy. Eur. Urol. 2016, 70, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.A.; Haddad, Z.; Yousefi, K.; Lam, L.L.; Wang, Q.; Choeurng, V.; Palmer-Aronsten, B.; Buerki, C.; Davicioni, E.; Li, J.; et al. Decipher Genomic Classifier Measured on Prostate Biopsy Predicts Metastasis Risk. Urology 2016, 90, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.L.; Martin, N.E.; Choeurng, V.; Palmer-Aronsten, B.; Kolisnik, T.; Beard, C.J.; Orio, P.F.; Nezolosky, M.D.; Chen, Y.W.; Shin, H.; et al. Utilization of biopsy-based genomic classifier to predict distant metastasis after definitive radiation and short-course ADT for intermediate and high-risk prostate cancer. Prostate Cancer Prostatic Dis. 2017, 20, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Spratt, D.E.; Yousefi, K.; Deheshi, S.; Ross, A.E.; Den, R.B.; Schaeffer, E.M.; Trock, B.J.; Zhang, J.; Glass, A.G.; Dicker, A.P.; et al. Individual Patient-Level Meta-Analysis of the Performance of the Decipher Genomic Classifier in High-Risk Men After Prostatectomy to Predict Development of Metastatic Disease. J. Clin. Oncol. 2017, 35, 1991–1998. [Google Scholar] [CrossRef]
- Van den Broeck, T.; Moris, L.; Gevaert, T.; Tosco, L.; Smeets, E.; Fishbane, N.; Liu, Y.; Helsen, C.; Margrave, J.; Buerki, C.; et al. Validation of the Decipher Test for Predicting Distant Metastatic Recurrence in Men with High-risk Nonmetastatic Prostate Cancer 10 Years After Surgery. Eur. Urol. Oncol. 2019, 2, 589–596. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Davicioni, E.; Crisan, A.; Jenkins, R.B.; Ghadessi, M.; Karnes, R.J. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur. Urol. 2015, 67, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Badani, K.; Thompson, D.J.; Buerki, C.; Davicioni, E.; Garrison, J.; Ghadessi, M.; Mitra, A.P.; Wood, P.J.; Hornberger, J. Impact of a genomic classifier of metastatic risk on postoperative treatment recommendations for prostate cancer patients: A report from the DECIDE study group. Oncotarget 2013, 4, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Badani, K.K.; Kemeter, M.J.; Febbo, P.G.; Lawrence, H.J.; Denes, B.S.; Rothney, M.P.; Rothberg, M.B.; Brown, G.A. The Impact of a Biopsy Based 17-Gene Genomic Prostate Score on Treatment Recommendations in Men with Newly Diagnosed Clinically Prostate Cancer Who are Candidates for Active Surveillance. Urol. Pract. 2015, 2, 181–189. [Google Scholar] [CrossRef]
- Michalopoulos, S.N.; Kella, N.; Payne, R.; Yohannes, P.; Singh, A.; Hettinger, C.; Yousefi, K.; Hornberger, J. Influence of a genomic classifier on post-operative treatment decisions in high-risk prostate cancer patients: Results from the PRO-ACT study. Curr. Med. Res. Opin. 2014, 30, 1547–1556. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Shin, H.; Yousefi, K.; Thompson, D.J.; Hornberger, J.; Hyatt, A.S.; Badani, K.K.; Morgan, T.M.; Feng, F.Y. Impact of a Genomic Classifier of Metastatic Risk on Postprostatectomy Treatment Recommendations by Radiation Oncologists and Urologists. Urology 2015, 86, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.L.; du Plessis, M.; Santiago-Jiménez, M.; Yousefi, K.; Thompson, D.J.S.; Karsh, L.; Lane, B.R.; Franks, M.; Chen, D.Y.T.; Bandyk, M.; et al. Decipher test impacts decision making among patients considering adjuvant and salvage treatment after radical prostatectomy: Interim results from the Multicenter Prospective PRO-IMPACT study. Cancer 2017, 123, 2850–2859. [Google Scholar] [CrossRef]
- Vince, R.A., Jr.; Jiang, R.; Qi, J.; Tosoian, J.J.; Takele, R.; Feng, F.Y.; Linsell, S.; Johnson, A.; Shetty, S.; Hurley, P.; et al. Impact of Decipher Biopsy testing on clinical outcomes in localized prostate cancer in a prospective statewide collaborative. Prostate Cancer Prostatic Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Gandhi, J.S.; Moch, H.; Aron, M.; Comperat, E.; Paner, G.P.; McKenney, J.K.; Amin, M.B. Similarities and Differences in the 2019 ISUP and GUPS Recommendations on Prostate Cancer Grading: A Guide for Practicing Pathologists. Adv. Anat. Pathol. 2021, 28, 1–7. [Google Scholar] [CrossRef]
- van Leenders, G.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N.; et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87–e99. [Google Scholar] [CrossRef] [PubMed]
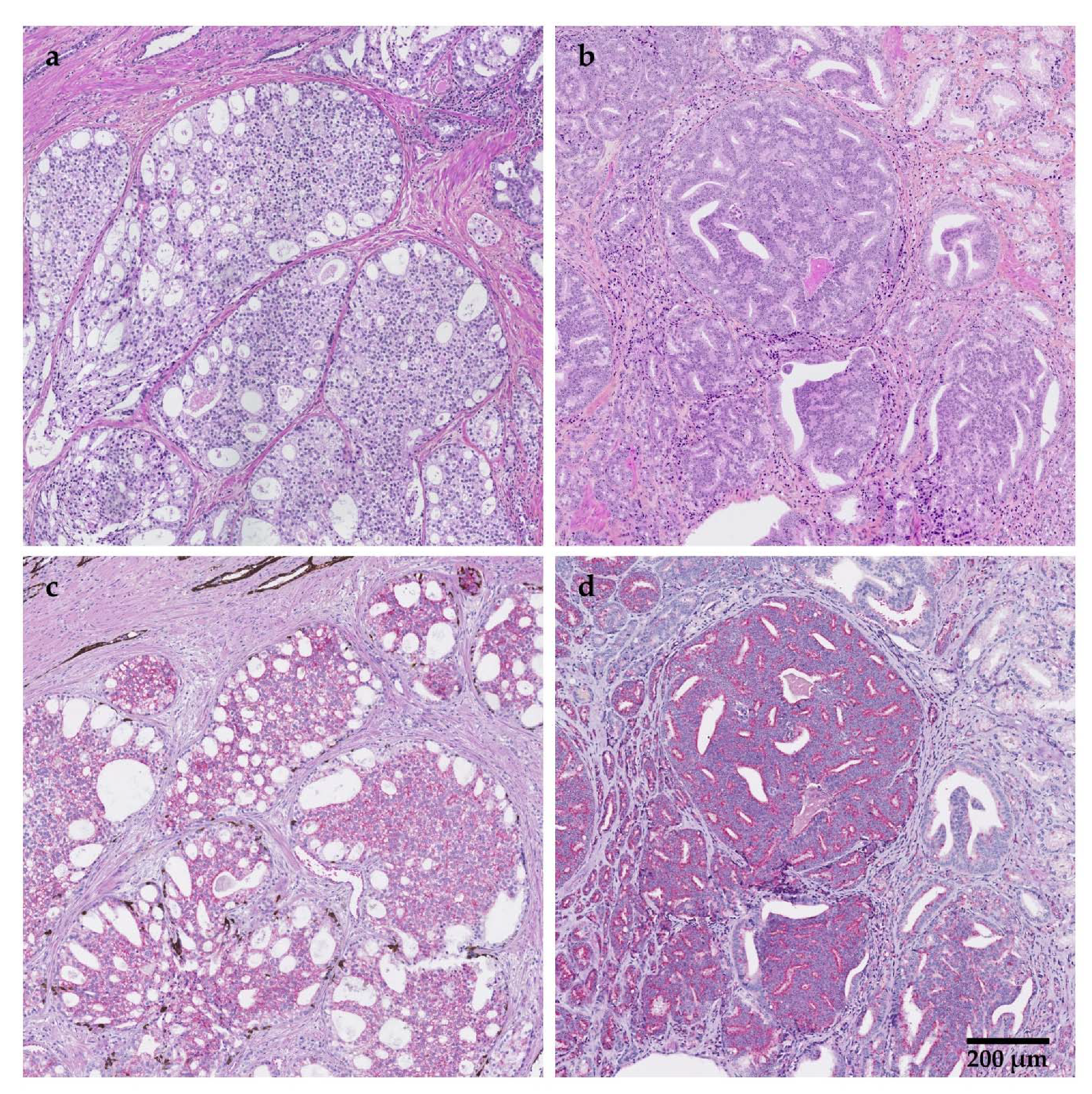

| Method | Type of Alteration | Gene or Chromosome | Mutation/ Alteration | % Positivity (n/n) |
|---|---|---|---|---|
| NGS | Activating SNV (PI3K/MAPK pathways) | PIK3CA AKT1 MAP2K1 KRAS BRAF | p.H1047R p.E17K p.I99_K104del p.G13P p.K601E | 57% (4/7) |
| Other SNV | PTEN | Splicing | 14% (1/7) | |
| GW CNA analysis | CNA | PTEN | Loss | 29% (2/7) |
| IHC | Protein loss | PTEN | – | 47% (7/15) |
| NGS | Other SNV (DNA repair genes) | BRCA2 CDK12 CHEK2 | p.L1740 * p.K756Q, p.K504 * p.I157T | 29% (2/7) |
| Other SNV | FOXA1 | p.F266V pF396 * | 29% (2/7) | |
| Other SNV | SPOP | p.F133V | 14% (1/7) | |
| GW CNA analysis | CNA | CDKN2A RB1 CCND1 | Loss Loss Gain | 71% (5/7) |
| CNA | MYC | Gain | 57% (4/7) | |
| CNA | TP53 | Loss | 14% (1/7) | |
| CNA | CHD1 | Loss | 14% (1/7) | |
| CNA | TSC2 | Gain | 14% (1/7) | |
| CNA | Chr. 8 (8p) | LOH | 43% (3/7) | |
| IHC | Protein overexpression | ERG | – | 7% (1/15) |
| Specimen Type | Method | % Positivity (n/n) | Ref. |
|---|---|---|---|
| TMPRSS2:ERG fusion | |||
| RP | Sanger sequencing | 100% (4/4) | [70] |
| RP | FISH | 94% (82/87) | [71] |
| RP | FISH (break-apart probe) | 75% (36/48) | [72] |
| PTEN loss | |||
| RP | Sanger sequencing | 75% (3/4) | [70] |
| RP | Microsatellite analysis: PTEN (10q23) LOH | 48% (13/27) | [73] |
| ERG protein overexpression | |||
| RP | IHC | 63% (20/32) | [74] |
| RP | IHC | 61% (28/46) | [69] |
| RP | IHC | 58% (26/45) | [66] |
| Bx | IHC | 58% (29/50) | [67] |
| Bx | IHC | 55% (33/60) | [68] |
| 15 Bx; 8 RP; 6 TURP; 2 RCP | IHC | 35% (11/31) | [75] |
| Bx | IHC | 10% (12/128) | [76] |
| PTEN protein loss | |||
| RP | IHC | 89% (32/36) | [77] |
| RP | IHC | 84% (38/45) | [66] |
| Bx | IHC | 76% (38/50) | [67] |
| RP | IHC | 75% (18/24) | [69] |
| Bx | IHC | 75% (61/81) | [78] |
| Bx | IHC | 72% (43/60) | [68] |
| RP | IHC | 72% (23/32) | [74] |
| Other | |||
| RP | CGH: −1q23 → q32, −5p, −6cen → q22, +7p, +7q, −8p, +8q21.1 → qter, −10p, −10q, −10q21 → qter, −13q, −13q14 → qter, −16q, −16q13 → qter, −17p, −18q, +19p, +19q | 73% (8/11) | [73] |
| RP | Microsatellite analysis: TP53 (17p13.1) LOH | 60% (16/27) | [73] |
| RP | Microsatellite analysis: RB1 (13q14.2) LOH | 81% (22/27) | [73] |
| RP | Microsatellite analysis: 3pter–3p24.2, 5q21–22, 6q21–22, 7q31, 8p22, 10q23–24, 11p15.5, 16q23.1–16qter, 18q21, 18q21.33, 21q22.1–22.3 LOH | 60% (12/20) | [79] |
| RP | Raman microspectroscopy | Accuracy, sensitivity, and specificity >85% | [80] |
| N (Cohort) | Specimen | IDC-P alone or with CC | Gene or Alteration | Result | Ref. |
|---|---|---|---|---|---|
| 266 (TCGA, SU2C/PCF Dream Team) | RP | IDC-P/CC vs. NC4 | PTENloss | 39% vs. 25.5% p = 0.024 | [54] |
| SPOPmut | 17.1% vs. 2.9% p < 0.001 | ||||
| ATMmut | 7.3% vs. 0.98% p = 0.019 | ||||
| EZH2 methylation | logFC= 0.48, q < 0.001 | ||||
| TIMP2 methylation | logFC= −0.34, q = 0.01 | ||||
| TIMP3 methylation | logFC= −0.52, q < 0.001 | ||||
| SLIT2 methylation | logFC= −0.46, q = 0.01 | ||||
| 260 (TCGA) | RP | IDC-P/CC | Higher PGA: 779 gene deletions | q-value < 0.1 | [107] |
| Higher PGA: 317 gene amplifications | q-value < 0.1 | ||||
| 88 (TCGA) | RP | IDC-P/CC | FOXA1mut | 15% vs. 5% p = 0.007 | |
| TP53mut | 19% vs. 10% p = 0.035 | ||||
| SPOPmut | 19% vs. 10% p = 0.035 | ||||
| 277 | Bx | IDC-P (n = 31) | MYC amplification | uOR: 2.54 95% CI: 1.10–5.88 p = 0.02 | [108] |
| PTEN loss | uOR: 5.01 95% CI: 2.26–11.47 p < 0.0001 | ||||
| MYC amplification and PTEN loss | uOR: 13.33 95% CI: 3.85–49.67 p < 0.0001 | ||||
| 91 | RP | IDC-P/CC (n = 61) | APC methylation | Median PMR: 47.3% vs. 31.7% p = 0.045 | [109] |
| RASSF15 methylation | Median PMR: 99.2% vs. 69.5% p = 0.003 | ||||
| TBX15 methylation | Median PMR: 21.6% vs. 10.0% p = 0.013 | ||||
| 245 | Liquid Bx | IDC-P | DDR pathway alterations | 11.8% (19/161) vs. 2.4% (2/84) p = 0.024 | [110] |
| HR pathway alterations | 11.2% (18/161) vs. 2.4% (2/84) p = 0.032 | ||||
| NCOR2 alterations | 21.1% (34/161) vs. 6.0% (5/84) p = 0.004 | ||||
| 7 | 3 RP, 3 Bx, 1 RCP | IDC-P | TTF-1 overexpression | 100% (3/3) | [110] |
| 1 | Bx | IDC-P | TTF-1 overexpression | 100% (1/1) | [110] |
| Specimen | Method | Alteration | Result | Ref. |
|---|---|---|---|---|
| PDX | Histology review | IDC-P in BRCA2mut and BRCAX grafts vs. sporadic PCas | 61% (27/44) vs. 8% (5/62) p = 0.04 | [121] |
| RP | Microdissection and WGS | In BRCAmut cases MED12L/MED12 amplification in IDC-P+ vs. − | 75% (6/8) vs. 17% (1/6) | [122] |
| Saliva | NGS | % IDC-P in cases with and without germline mutations of DDR genes | 24% (5/21) vs. 9% (12/129) p = 0.06 | [123] |
| Saliva | NGS | % IDC-P in cases with germline mutations of MMR genes | 23% (3/13 IDC-P+) | [124] |
| 6 Bx, 6 RP, 1 LN (IDC-P = 3) | NGS | MSH6 germline mutation in IDC-P cases | 1/3 | [124] |
| MSH6 somatic mutations in IDC-P cases | 1/3 | |||
| TP53 somatic mutations in IDC-P cases | 1/3 | |||
| MSH2 somatic mutation + LOH in IDC-P cases | 1/3 | |||
| MSH6 somatic mutation + LOH in IDC-P cases | 1/3 | |||
| 135 RP, 39 Bx (IDC-P = 79) | FISH | Bi-allelic BRCA2 loss (LOH + gBRCA2 or bi-allelicDel) | mOR: 4.3 95% CI: 1.1–16.2 p = 0.031 | [125] |
| PTEN homozygous loss | mOR: 5.2 95% CI: 2.1–13.1 p < 0.001 |
| N | IDC-P alone or with CC | Method | Measure | Result | Ref. |
|---|---|---|---|---|---|
| 476 | IDC-P/CC | CNA analysis | PGA | 34% vs. 16% p = 0.033 | [30] |
| 156 | IDC-P/CC | Microarray analysis | SCHLAP1 expression | FC 3.23 p < 0.001 | |
| 393 | IDC-P/CC | RNA-ISH | Detection of IDC-P/CC using SCHLAP1 expression | Accuracy: 82.4% p < 0.001 | |
| 318 | IDC-P/CC | SNP microarray | PGA | p < 0.0001 | [128] |
| 333 (TCGA) and 215 (CPC-GENE) | IDC-P/CC | Ragnum signature | Hypoxia | p < 0.0001 | |
| 144 | IDC-P/CC | Total RNA-seq | SCHLAP1:UBE2E3 fusion | FDR:0.0015 | [129] |
| 163 | IDC-P | IHC | PTEN protein loss | 86% (30/35) | [130] |
| IDC-P/CC | IHC | BCR cumulative incidence | mHR: 5.06, 95%CI: 2.21–11.6 p < 0.001 |
| N | Specimen | IDC-P alone or with CC | Measure | Result | Ref. |
|---|---|---|---|---|---|
| 319 | Bx | IDC-P/CC | Increase in GPS | No Gleason pattern 4: mean GPS = 22.3; IDC-P/CC: mean GPS = 41.8 p < 0.001 | [133] |
| 48 | RP | IDC-P/CC | % cases with high-risk Decipher score | 56% vs. 22% p = 0.007 | [134] |
| IDC-P | Increase in Decipher score | OR: 1.92, 95% CI: 0.65–5.67, p = 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantazopoulos, H.; Diop, M.-K.; Grosset, A.-A.; Rouleau-Gagné, F.; Al-Saleh, A.; Boblea, T.; Trudel, D. Intraductal Carcinoma of the Prostate as a Cause of Prostate Cancer Metastasis: A Molecular Portrait. Cancers 2022, 14, 820. https://doi.org/10.3390/cancers14030820
Pantazopoulos H, Diop M-K, Grosset A-A, Rouleau-Gagné F, Al-Saleh A, Boblea T, Trudel D. Intraductal Carcinoma of the Prostate as a Cause of Prostate Cancer Metastasis: A Molecular Portrait. Cancers. 2022; 14(3):820. https://doi.org/10.3390/cancers14030820
Chicago/Turabian StylePantazopoulos, Helen, Mame-Kany Diop, Andrée-Anne Grosset, Frédérique Rouleau-Gagné, Afnan Al-Saleh, Teodora Boblea, and Dominique Trudel. 2022. "Intraductal Carcinoma of the Prostate as a Cause of Prostate Cancer Metastasis: A Molecular Portrait" Cancers 14, no. 3: 820. https://doi.org/10.3390/cancers14030820
APA StylePantazopoulos, H., Diop, M.-K., Grosset, A.-A., Rouleau-Gagné, F., Al-Saleh, A., Boblea, T., & Trudel, D. (2022). Intraductal Carcinoma of the Prostate as a Cause of Prostate Cancer Metastasis: A Molecular Portrait. Cancers, 14(3), 820. https://doi.org/10.3390/cancers14030820







