Dose-Intense Cisplatin-Based Neoadjuvant Chemotherapy Increases Survival in Advanced Cervical Cancer: An Up-to-Date Meta-Analysis
Abstract
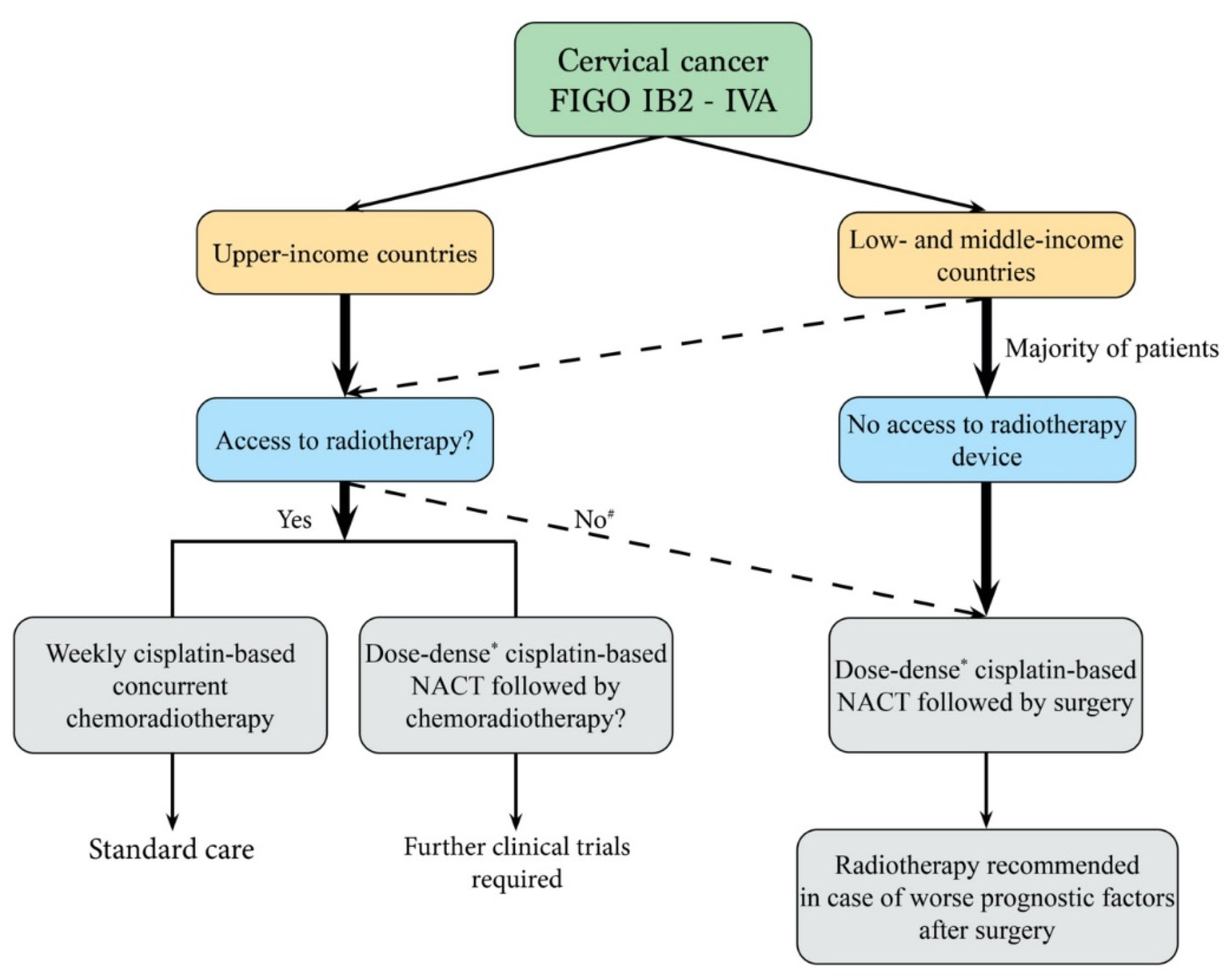
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ERCC1 | Excision Repair Cross-Complementation group 1 |
| PARP1 | Poly (ADP-ribose) Polymerase 1 |
| HSPB1/p | Heat Shock Protein Family B member 1 phosphorylated |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Review |
| MeSH | Medical Subject Headings |
| PICOS | Population, Intervention, Comparator group, Outcomes, and Study design |
| CHSRI | Cochrane Handbook for Systematic Reviews of Interventions |
| NACT | Neoadjuvant Chemotherapy |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Benedet, J.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the Cervix Uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2006, 95 (Suppl. S1), 43–103. [Google Scholar] [CrossRef]
- Gennigens, C.; De Cuypere, M.; Hermesse, J.; Kridelka, F.; Jerusalem, G. Optimal Treatment in Locally Advanced Cervical Cancer. Expert Rev. Anticancer Ther. 2021, 21, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Stutz, E.; Liu, M.; Rogers, S.; Klingbiel, D.; Siebenhüner, A.; Singh, S.; Bodis, S. Concurrent Chemoradiotherapy vs. Radiotherapy Alone in Locally Advanced Cervix Cancer: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2017, 145, 374–385. [Google Scholar] [CrossRef]
- LaVigne, A.W.; Triedman, S.A.; Randall, T.C.; Trimble, E.L.; Viswanathan, A.N. Cervical Cancer in Low and Middle Income Countries: Addressing Barriers to Radiotherapy Delivery. Gynecol. Oncol. Rep. 2017, 22, 16–20. [Google Scholar] [CrossRef]
- Grover, S.; Xu, M.J.; Yeager, A.; Rosman, L.; Groen, R.S.; Chackungal, S.; Rodin, D.; Mangaali, M.; Nurkic, S.; Fernandes, A.; et al. A Systematic Review of Radiotherapy Capacity in Low- and Middle-Income Countries. Front. Oncol. 2015, 4, 380. [Google Scholar] [CrossRef]
- Randall, T.C.; Ghebre, R. Challenges in Prevention and Care Delivery for Women with Cervical Cancer in Sub-Saharan Africa. Front. Oncol. 2016, 6, 160. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.C.; Cheetham, S.; Baxi, S. The Enablers, Barriers and Preferences of Accessing Radiation Therapy Facilities in the Rural Developed World—A Systematic Review. BMC Cancer 2017, 17, 794. [Google Scholar] [CrossRef] [Green Version]
- Mendez, L.C.; Moraes, F.Y.; Castilho, M.S.; Louie, A.V.; Qu, X.M. Lives and Economic Loss in Brazil Due to Lack of Radiotherapy Access in Cervical Cancer: A Cost-Effectiveness Analysis. Clin. Oncol. 2019, 31, e143–e148. [Google Scholar] [CrossRef]
- Chuang, L.T.; Temin, S.; Camacho, R.; Dueñas-Gonzalez, A.; Feldman, S.; Gultekin, M.; Gupta, V.; Horton, S.; Jacob, G.; Kidd, E.A.; et al. Management and Care of Women With Invasive Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline. J. Glob. Oncol. 2016, 2, 311–340. [Google Scholar] [CrossRef]
- Mieog, J.S.D.; van der Hage, J.A.; van de Velde, C.J.H. Neoadjuvant Chemotherapy for Operable Breast Cancer. Br. J. Surg. 2007, 94, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Baert, T.; Vergote, I. Role of Neoadjuvant Chemotherapy in Advanced Epithelial Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- NACCCMA Collaboration Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data from 21 Randomised Trials. Eur. J. Cancer 2003, 39, 2470–2486. [CrossRef] [Green Version]
- Reducing Uncertainties About the Effects of Chemoradiotherapy for Cervical Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data From 18 Randomized Trials. J. Clin. Oncol. 2008, 26, 5802–5812. [CrossRef] [Green Version]
- Scatchard, K.; Forrest, J.; Flubacher, M.; Cornes, P.; Williams, C. Chemotherapy for Metastatic and Recurrent Cervical Cancer. Cochrane Database Syst. Rev. 2012, 10, CD006469. [Google Scholar] [CrossRef]
- Basu, A.; Krishnamurthy, S. Cellular Responses to Cisplatin-Induced DNA Damage. J. Nucleic Acids 2010, 2010, 201367. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-H.; Huang, W.-T.; Kao, W.-C.; Hsiao, S.-Y.; Pan, H.-Y.; Fang, C.-W.; Shiue, Y.-L.; Chou, C.-L.; Li, C.-F. O6-Methylguanine-DNA Methyltransferase Modulates Cisplatin-Induced DNA Double-Strand Breaks by Targeting the Homologous Recombination Pathway in Nasopharyngeal Carcinoma. J. Biomed. Sci. 2021, 28, 2. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef] [Green Version]
- Telli, M.L.; Timms, K.M.; Reid, J.; Hennessy, B.; Mills, G.B.; Jensen, K.C.; Szallasi, Z.; Barry, W.T.; Winer, E.P.; Tung, N.M.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 3764. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Cai, D.; Li, M.; Wu, X. The Homologous Recombination Protein RAD51 Is a Promising Therapeutic Target for Cervical Carcinoma. Oncol. Rep. 2017, 38, 767–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Ji, S.; Hu, Q.; Chen, Q.; Liu, Z.; Wu, J.; Gu, K. The Prognostic Value of Excission Repair Cross-Complementation Group One Enzyme Expression in Locally Advanced Cervical Carcinoma Patients Treated with Cisplatin–Based Treatment: A Meta–Analysis. Int. J. Gynecol. Cancer 2019, 29, 35. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.B.; Prasad, S.B.; Yadav, S.S.; Pandey, L.K.; Singh, S.; Pradhan, S.; Narayan, G. Olaparib Modulates DNA Repair Efficiency, Sensitizes Cervical Cancer Cells to Cisplatin and Exhibits Anti-Metastatic Property. Sci. Rep. 2017, 7, 12876. [Google Scholar] [CrossRef] [PubMed]
- Real, N.E.; Castro, G.N.; Darío Cuello-Carrión, F.; Perinetti, C.; Röhrich, H.; Cayado-Gutiérrez, N.; Guerrero-Gimenez, M.E.; Ciocca, D.R. Molecular Markers of DNA Damage and Repair in Cervical Cancer Patients Treated with Cisplatin Neoadjuvant Chemotherapy: An Exploratory Study. Cell Stress Chaperones 2017, 22, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019; pp. 194–241. [Google Scholar]
- Sardi, J.; Giaroli, A.; Sananes, C.; Rueda, N.G.; Vighi, S.; Ferreira, M.; Bastardas, M.; Paniceres, G.; Di Paola, G. Randomized Trial with Neoadjuvant Chemotherapy in Stage IIIB Squamous Carcinoma Cervix Uteri: An Unexpected Therapeutic Management. Int. J. Gynecol. Cancer 1996, 6, 85–93. [Google Scholar] [CrossRef]
- Sardi, J.E.; Giaroli, A.; Sananes, C.; Ferreira, M.; Soderini, A.; Bermudez, A.; Snaidas, L.; Vighi, S.; Gomez Rueda, N.; di Paola, G. Long-Term Follow-up of the First Randomized Trial Using Neoadjuvant Chemotherapy in Stage Ib Squamous Carcinoma of the Cervix: The Final Results. Gynecol. Oncol. 1997, 67, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sardi; Sananes; Giaroli; Bermúdez; Ferreira; Soderini; Snaidas; Guardado; Anchezar; Ortiz, C.; et al. Neoadjuvant Chemotherapy in Cervical Carcinoma Stage IIB: A Randomized Controlled Trial. Int. J. Gynecol. Cancer 1998, 8, 441–450. [Google Scholar] [CrossRef]
- Tattersall, M.H.; Lorvidhaya, V.; Vootiprux, V.; Cheirsilpa, A.; Wong, F.; Azhar, T.; Lee, H.P.; Kang, S.B.; Manalo, A.; Yen, M.S. Randomized Trial of Epirubicin and Cisplatin Chemotherapy Followed by Pelvic Radiation in Locally Advanced Cervical Cancer. Cervical Cancer Study Group of the Asian Oceanian Clinical Oncology Association. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1995, 13, 444–451. [Google Scholar] [CrossRef]
- Tattersall, M.H.N.; Ramirez, C.; Coppleson, M. A Randomized Trial Comparing Platinum-Based Chemotherapy Followed by Radiotherapy vs. Radiotherapy Alone in Patients with Locally Advanced Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 1992, 2, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, S.C.S.; Bonadio, R.C.; Gabrielli, F.C.G.; Aranha, A.S.; Dias Genta, M.L.N.; Miranda, V.C.; de Freitas, D.; Abdo Filho, E.; Ferreira, P.A.O.; Machado, K.K.; et al. Neoadjuvant Chemotherapy With Cisplatin and Gemcitabine Followed by Chemoradiation Versus Chemoradiation for Locally Advanced Cervical Cancer: A Randomized Phase II Trial. J. Clin. Oncol. 2019, 37, 3124–3131. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, D.; Zhang, J.; Yao, D.; Gao, K.; Wang, H.; Liu, C.; Yu, J.; Li, L. The Efficacy and Safety of Neoadjuvant Chemotherapy in the Treatment of Locally Advanced Cervical Cancer: A Randomized Multicenter Study. Gynecol. Oncol. 2016, 141, 231–239. [Google Scholar] [CrossRef]
- Katsumata, N.; Yoshikawa, H.; Kobayashi, H.; Saito, T.; Kuzuya, K.; Nakanishi, T.; Yasugi, T.; Yaegashi, N.; Yokota, H.; Kodama, S.; et al. Phase III Randomised Controlled Trial of Neoadjuvant Chemotherapy plus Radical Surgery vs Radical Surgery Alone for Stages IB2, IIA2, and IIB Cervical Cancer: A Japan Clinical Oncology Group Trial (JCOG 0102). Br. J. Cancer 2013, 108, 1957–1963. [Google Scholar] [CrossRef] [Green Version]
- Mossa, B.; Mossa, S.; Corosu, L.; Marziani, R. Follow-up in a Long-Term Randomized Trial with Neoadjuvant Chemotherapy for Squamous Cell Cervical Carcinoma. Eur. J. Gynaecol. Oncol. 2010, 31, 497–503. [Google Scholar]
- Chen, H.; Liang, C.; Zhang, L.; Huang, S.; Wu, X. Clinical Efficacy of Modified Preoperative Neoadjuvant Chemotherapy in the Treatment of Locally Advanced (Stage IB2 to IIB) Cervical Cancer: Randomized Study. Gynecol. Oncol. 2008, 110, 308–315. [Google Scholar] [CrossRef]
- Eddy, G.L.; Bundy, B.N.; Creasman, W.T.; Spirtos, N.M.; Mannel, R.S.; Hannigan, E.; O’Connor, D. Treatment of (“bulky”) Stage IB Cervical Cancer with or without Neoadjuvant Vincristine and Cisplatin Prior to Radical Hysterectomy and Pelvic/Para-Aortic Lymphadenectomy: A Phase III Trial of the Gynecologic Oncology Group. Gynecol. Oncol. 2007, 106, 362–369. [Google Scholar] [CrossRef]
- Cai, H.-B.; Chen, H.-Z.; Yin, H.-H. Randomized Study of Preoperative Chemotherapy versus Primary Surgery for Stage IB Cervical Cancer. J. Obstet. Gynaecol. Res. 2006, 32, 315–323. [Google Scholar] [CrossRef]
- Tabata, T.; Takeshima, N.; Nishida, H.; Hirai, Y.; Hasumi, K. A Randomized Study of Primary Bleomycin, Vincristine, Mitomycin and Cisplatin (BOMP) Chemotherapy Followed by Radiotherapy versus Radiotherapy Alone in Stage IIIB and IVA Squamous Cell Carcinoma of the Cervix. Anticancer Res. 2003, 23, 2885–2890. [Google Scholar]
- Benedetti-Panici, P.; Greggi, S.; Colombo, A.; Amoroso, M.; Smaniotto, D.; Giannarelli, D.; Amunni, G.; Raspagliesi, F.; Zola, P.; Mangioni, C.; et al. Neoadjuvant Chemotherapy and Radical Surgery versus Exclusive Radiotherapy in Locally Advanced Squamous Cell Cervical Cancer: Results from the Italian Multicenter Randomized Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 179–188. [Google Scholar] [CrossRef]
- Chang, T.C.; Lai, C.H.; Hong, J.H.; Hsueh, S.; Huang, K.G.; Chou, H.H.; Tseng, C.J.; Tsai, C.S.; Chang, J.T.; Lin, C.T.; et al. Randomized Trial of Neoadjuvant Cisplatin, Vincristine, Bleomycin, and Radical Hysterectomy versus Radiation Therapy for Bulky Stage IB and IIA Cervical Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Herod, J.; Burton, A.; Buxton, J.; Tobias, J.; Luesley, D.; Jordan, S.; Dunn, J.; Poole, C.J. A Randomised, Prospective, Phase III Clinical Trial of Primary Bleomycin, Ifosfamide and Cisplatin (BIP) Chemotherapy Followed by Radiotherapy versus Radiotherapy Alone in Inoperable Cancer of the Cervix. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2000, 11, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Symonds, R.P.; Habeshaw, T.; Reed, N.S.; Paul, J.; Pyper, E.; Yosef, H.; Davis, J.; Hunter, R.; Davidson, S.E.; Stewart, A.; et al. The Scottish and Manchester Randomised Trial of Neo-Adjuvant Chemotherapy for Advanced Cervical Cancer. Eur. J. Cancer Oxf. Engl. 1990 2000, 36, 994–1001. [Google Scholar] [CrossRef]
- Kumar, L.; Grover, R.; Pokharel, Y.H.; Chander, S.; Kumar, S.; Singh, R.; Rath, G.K.; Kochupillai, V. Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer: Two Randomised Studies. Aust. N. Z. J. Med. 1998, 28, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Sundfør, K.; Tropé, C.G.; Högberg, T.; Onsrud, M.; Koern, J.; Simonsen, E.; Bertelsen, K.; Westberg, R. Radiotherapy and Neoadjuvant Chemotherapy for Cervical Carcinoma. A Randomized Multicenter Study of Sequential Cisplatin and 5-Fluorouracil and Radiotherapy in Advanced Cervical Carcinoma Stage 3B and 4A. Cancer 1996, 77, 2371–2378. [Google Scholar] [CrossRef]
- Kigawa, J.; Minagawa, Y.; Ishihara, H.; Itamochi, H.; Kanamori, Y.; Terakawa, N. The Role of Neoadjuvant Intraarterial Infusion Chemotherapy with Cisplatin and Bleomycin for Locally Advanced Cervical Cancer. Am. J. Clin. Oncol. 1996, 19, 225–259. [Google Scholar] [CrossRef]
- Chauvergne, J.; Lhommé, C.; Rohart, J.; Héron, J.F.; Ayme, Y.; Goupil, A.; Fargeot, P.; David, M. [Neoadjuvant chemotherapy of stage IIb or III cancers of the uterine cervix. Long-term results of a multicenter randomized trial of 151 patients]. Bull. Cancer 1993, 80, 1069–1079. [Google Scholar] [PubMed]
- Souhami, L.; Gil, R.A.; Allan, S.E.; Canary, P.C.; Araújo, C.M.; Pinto, L.H.; Silveira, T.R. A Randomized Trial of Chemotherapy Followed by Pelvic Radiation Therapy in Stage IIIB Carcinoma of the Cervix. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 970–977. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO Staging for Carcinoma of the Cervix Uteri. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Ye, Q.; Yang, Y.; Tang, X.; Li, J.; Li, X.; Zhang, Y. Neoadjuvant Chemotherapy Followed by Radical Surgery versus Radiotherapy (with or without Chemotherapy) in Patients with Stage IB2, IIA, or IIB Cervical Cancer: A Systematic Review and Meta-Analysis. Dis. Markers 2020, 2020, 7415056. [Google Scholar] [CrossRef]
- Grover, S.; Longo, J.; Einck, J.; Puri, P.; Brown, D.; Chino, J.; Mahantshetty, U.; Yashar, C.; Erickson, B. The Unique Issues With Brachytherapy in Low- and Middle-Income Countries. Glob. Health Disparities 2017, 27, 136–142. [Google Scholar] [CrossRef]
- Pfister, C.; Gravis, G.; Fléchon, A.; Soulié, M.; Guy, L.; Laguerre, B.; Mottet, N.; Joly, F.; Allory, Y.; Harter, V.; et al. Randomized Phase III Trial of Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients with Muscle-Invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur. Urol. 2021, 79, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Posner, M.; Hitt, R.; Cohen, E.E.W.; Schulten, J.; Lefebvre, J.-L.; Vermorken, J.B. Induction Chemotherapy in Locally Advanced Squamous Cell Carcinoma of the Head and Neck: Role, Controversy, and Future Directions. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Lein, M.-Y.; Yang, S.-N.; Wang, Y.-C.; Lin, Y.-J.; Lin, C.-Y.; Hua, C.-H.; Tsai, M.-H.; Lin, C.-C. Dose-Dense TPF Induction Chemotherapy for Locally Advanced Head and Neck Cancer: A Phase II Study. BMC Cancer 2020, 20, 832. [Google Scholar] [CrossRef]
- Gupta, S.; Maheshwari, A.; Parab, P.; Mahantshetty, U.; Hawaldar, R.; Sastri Chopra, S.; Kerkar, R.; Engineer, R.; Tongaonkar, H.; Ghosh, J.; et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients With Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1548–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenter, G.; Greggi, S.; Vergote, I.; Katsaros, D.; Kobierski, J.; Massuger, L.; van Doorn, H.C.; Landoni, F.; Van Der Velden, J.; Reed, N.S.; et al. Results from Neoadjuvant Chemotherapy Followed by Surgery Compared to Chemoradiation for Stage Ib2-IIb Cervical Cancer, EORTC 55994. J. Clin. Oncol. 2019, 37, 5503. [Google Scholar] [CrossRef]



| Country Author Year Trial | Median Follow-up (Months) | Comparison | Experimental * Arm | Control Arm ** | Figostage | NACT Regimen | Planned Duration of NACT | Cisplatin (mg/m2/3 Weeks) | Radiotherapy | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age # | n | Age # | ||||||||
| Brazil da Costa [32] 2019 Phase II | 31.7 | NACT+CRT vs. CRT | 55 | 48 (22–69) | 52 | 45 (20–67) | IIB-IVA a | CCDP 50 mg/m2 Gem 1000 mg/m2, Day 1, 8 | Every 21 days for 3 cycles | 50 | Weekly CCDP 40 mg/m2 with RT 45–50.4 Gy over 6 weeks followed by BT 28–30 Gy |
| China Yang [33] 2016 Phase III | 32 | NACT+surgery vs. surgery | 109 | 47 (23–66) | 110 | 48 (26–68) | IB2-II a | CCDP 70 mg/m2 IRI 180 mg/m2 OR CCDP 70 Pacl 175 mg/m2 | Every 21 days for 1–2 cycles | 70 | Postoperative RT of 48–50 Gy if risk factors. |
| Japan Katsumata [34] 2013 Phase III | 49 | NACT+surgery vs. surgery | 67 | 47 (28–70) | 67 | 46 (22–67) | IB2-IIB b | CCDP 70 mg/m2 BLM 35 mg/m2 VCR 0.7 mg/m2 MMC 7 mg/m2 | Every 21 days for 2 cycles | 70 | Postoperative RT of 45–50.4 Gy if risk factors. BT if surgical margins positive |
| Italy Mossa [35] 2010 Phase III | 84 | NACT+surgery/RT vs. surgery/RT | 159 | 48.5 (32–65) | 129 | 48.5 (32–65) | IB-IIIB b | CCDP 50 mg/m2 VCR 1 mg/m2 BLM 75 mg/m2 | Every 21 days for 3 cycles | 50 | 50 Gy over 5–6 weeks followed by BT a maximum 30 Gy |
| China Chen [36] 2008 Phase II | NA | NACT+surgery vs. surgery | 72 | 44 (25–74) | 70 | 44 (25–74) | IB2-IIB b | CCDP 100 mg/m2 MMC 20 mg/m2 5FU 120 mg/kg | Every 14 days for 2–3 cycles | 150 | Postoperative pelvic RT at a dose of 45 Gy if risk factors after surgery |
| American Eddy [37] 2007 Phase III | 62 | NACT+surgery vs. surgery | 145 | NA | 143 | NA | IB2 b | CCDP 50 mg/m2 VCR 1 mg/m2 | Every 10 days for 3 cycles | 105 | Postoperative pelvic RT if risk factors after surgery |
| China Cai [38] 2006 Phase II | 62 | NACT+surgery vs. surgery | 52 | 45.6 | 54 | 44.8 | IB b | CCDP 75 mg/m2 5FU 120 mg/kg | Every 21 days for 2 cycles | 75 | Postoperative RT at a dose of 45 Gy if risk factors after surgery |
| Italy Tabata [39] 2003 Phase II | NA | NACT+RT vs. RT | 32 | 57 (35–68) | 29 | 59 (44–70) | IIIB-IVA b | CCDP 70 mg/m2 BLM 5 mg/m2 VCR 0.7 mg/m2 MMC 7 mg/m2 | Every 28 days for 3 cycles | 52.5 | 50 Gy in 25 F followed by BT |
| Italy Benedetti-Panici [40] 2002 Phase III | 40 | NACT+surgery vs. RT | 227 | 49 (25–70) | 214 | 52 (28–69) | IB2-III b | CCDP 160 mg/m2 BLM 15 mg/m2, Day 1, 8 | Every 21 days for 2 cycles | 160 § | Median total dose of 70 Gy delivered to point A over 62 days |
| China Chang [41] 2000 Phase II | 39 | NACT+surgery vs. RT | 68 | 46 (33–69) | 52 | 47 (32–70) | IB2-IIA b | CCDP 50 mg/m2 VCR 1 mg/m2 BLM 75 mg/m2 | Every 10 days for 3 cycles | 105 | 50–54 Gy followed by BT, or 70 Gy without BT |
| England Herod [42] 2000 Phase III | 108 | NACT+RT vs. RT | 86 | 47 (24–74) | 86 | 46 (27–73) | IB-IVA b | CCDP 50 mg/m2 BLM 30 mg IFOS 5 g/m2 Mesna 6 g/m2 | Every 21 days for 2–3 cycles | 50 | According to institutional policy |
| England Symonds [43] 2000 Phase III | 65 | NACT+RT vs. RT | 100 | 49 (25–69) | 104 | 48 (24–70) | IIB-IVA b | CCDP 50 mg/m2 MTX 100 mg/m2 | Every 14 days for 3 cycles | 75 | 40–45 Gy in 20 F over 28 days followed by BT 24–33.75 Gy |
| Argentina Sardi [29] 1998 Phase II | 84 | NACT+RT vs. RT | 73 | 42.9 | 74 | 41.5 | IIB c | CCDP 50 mg/m2 VCR 1 mg/m2 BLM 75 mg/m2 | Every 10 days for 3 cycles | 105 | 50–60 Gy in 28–30 F over 45–50 days, followed by BT 25–35 Gy |
| India Kumar [44] 1998 Phase II | NA | NACT+RT vs. RT | 88 | 45 (30–65) | 85 | 45.5 (21–65) | IIB-IVA c | CCDP 50 mg/m2 IFOS 5 g/m2 BLM 15 mg/m2 Mesna 3 g/m2 | Every 21 days for 2 cycles | 50 | 40 Gy in 22 F + 10 Gy in 5 F over 35 days followed by BT 30 Gy |
| Argentina Sardi [28] 1997 Phase II | 67 | NACT+ surgery ± RT vs. Surgery±RT | 102 | 39 (23–68) | 103 | 41 (24–69) | IB c | CCDP 50 mg/m2 VCR 1 mg/m2 BLM 75 mg/m2 | Every 10 days for 3 cycles | 105 | 50–60 Gy over 45–50 days followed by BT 25–35 Gy |
| Argentina Sardi [27] 1996 Phase II | 28 | NACT+RT vs. RT | 54 | 48.2 | 54 | 49.6 | IIIB c | CCDP 50 mg/m2 VCR 1 mg/m2 BLM 75 mg/m2 | Every 10 days for 3 cycles | 105 | 50–60 Gy over 45–50 days followed by BT 25–35 Gy |
| Norway Sundfor [45] 1996 Phase II | 46 | NACT+RT vs. RT | 47 | 52.7 (25–70) | 47 | 52.2 (26–70) | IIIB-IVA c | CCDP 100 mg/m2 5FU 5000 mg/m2 | Every 21 days for 3 cycles | 100 | 64.8 Gy in 36 F over 50 days |
| Japan Kigawa [46] 1996 Phase II | 42 | NACT &±surgery ±RT vs. RT | 25 | 55.6 (41–67) | 25 | 60.2 (43–69) | IIB-IIIB c | CCDP 50 mg/m2 BLM 30 mg/m2 | Every 21 days for 2–3 cycles | 50 | 50 Gy in 25 F over 35 days followed by BT 24–38 Gy |
| Australia Tattersall [30] 1995 Phase II | 16 | NACT+RT vs. RT | 129 | 47 (26–75) | 131 | 52 (27–78) | IIB-IVA c | CCDP 60 mg/m2 EPI 110 mg/m2 | Every 21 days for 2–3 cycles | 60 | 40–55 Gy over 28–35 days followed by BT 30–35 Gy |
| France Chauvergne [47] 1993 Phase III | 84 | NACT+RT vs. RT | 75 | 54.3 | 76 | 54 | IIB-IIIB c | CCDP 80 mg/m2 CLB 20 mg/m2 VCR 0.7 mg/m2 MTX 30 mg/m2 | Every 21 days for 2–4 cycles | 80 | 45 Gy followed by BT |
| Australia Tattersall 1992 [31] Phase II | 37 | NACT+RT vs. RT | 34 | 54 (33–70) | 37 | 56 (23–70) | IIB-IVA c | CCDP 50 mg/m2 VBL 4 mg/m2 BLM 45 mg/m2 | Every 21 days for 3 cycles | 50 | 40–55 Gy in 20–25 F over 28–35 days |
| Brazil Souhami [48] 1991 Phase II | 44 | NACT+RT vs. RT | 39 | 50 (24–69) | 52 | 49 (26–69) | IIIB c | CCDP 50 mg/m2 VCR 1 mg/m2 BLM 120U MMC 10 mg/m2 | Every 21 days for 3 cycles | 50 | 50 Gy in 25 F over 35 days followed by BT 40 Gy |
| RR for Fixed Effect | [95%CI] | |
|---|---|---|
| Overall analysis | 0.97 | [0.90–1.05] |
| Dose-intense cisplatin ≥ 72.5 mg/m2/3 weeks | 0.87 | [0.76–0.98] |
| Dose-intense cisplatin ≥ 105 mg/m2/3 weeks | 0.79 | [0.67–0.93] |
| Triplet cisplatin-based chemotherapy (yes) | 0.97 | [0.83–1.13] |
| Chemotherapy duration (≤6 weeks) | 0.91 | [0.80; 1.04] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.T.; Winterman, S.; Playe, M.; Benbara, A.; Zelek, L.; Pamoukdjian, F.; Bousquet, G. Dose-Intense Cisplatin-Based Neoadjuvant Chemotherapy Increases Survival in Advanced Cervical Cancer: An Up-to-Date Meta-Analysis. Cancers 2022, 14, 842. https://doi.org/10.3390/cancers14030842
Nguyen VT, Winterman S, Playe M, Benbara A, Zelek L, Pamoukdjian F, Bousquet G. Dose-Intense Cisplatin-Based Neoadjuvant Chemotherapy Increases Survival in Advanced Cervical Cancer: An Up-to-Date Meta-Analysis. Cancers. 2022; 14(3):842. https://doi.org/10.3390/cancers14030842
Chicago/Turabian StyleNguyen, Van Tai, Sabine Winterman, Margot Playe, Amélie Benbara, Laurent Zelek, Frédéric Pamoukdjian, and Guilhem Bousquet. 2022. "Dose-Intense Cisplatin-Based Neoadjuvant Chemotherapy Increases Survival in Advanced Cervical Cancer: An Up-to-Date Meta-Analysis" Cancers 14, no. 3: 842. https://doi.org/10.3390/cancers14030842
APA StyleNguyen, V. T., Winterman, S., Playe, M., Benbara, A., Zelek, L., Pamoukdjian, F., & Bousquet, G. (2022). Dose-Intense Cisplatin-Based Neoadjuvant Chemotherapy Increases Survival in Advanced Cervical Cancer: An Up-to-Date Meta-Analysis. Cancers, 14(3), 842. https://doi.org/10.3390/cancers14030842







