Simple Summary
This study describes clinical and genetic characteristics of the largest aggregated cohort of Splicing Factor 3 Subunit B1 (SF3B1)-mutated Uveal Melanoma (UM) in the literature (n = 146). Missense mutations in the spliceosome gene SF3B1 result in an altered splice site recognition and aberrant mRNA transcripts. The SF3B1-mutated UM show early- and late-onset of metastatic disease for which, currently, no distinguishing biomarkers exist. Using a cutoff of 60 months for stratification, we found that a largest basal tumor diameter was more prevalent in the early-onset metastatic disease group. Furthermore, using differential gene expression and the detection of aberrant transcripts, we found that the expression of alpha/beta-Hydrolase domain containing 6 (ABHD6) is associated with early-onset metastatic SF3B1 and aberrant transcripts that are associated with early-onset SF3B1-mutated UM. Our results provide more accurate prognostication and targets for future functional studies in an effort to elucidate pathogenesis of SF3B1-mutated UM.
Abstract
Approximately 25% of all uveal melanoma (UM) contain driver mutations in the gene encoding the spliceosome factor SF3B1, and whilst patients with such SF3B1 mutations generally have an intermediate risk on developing metastatic disease, a third of these patients develop early metastasis within 5 years after diagnosis. We therefore investigated whether clinical and/or genetic variables could be indicative of short progression-free survival (PFS < 60 months) or long PFS (PFS ≥ 60 months) for SF3B1-mutated (SF3B1mut) UM patients. We collected 146 SF3B1mut UM from our Rotterdam Ocular Melanoma Studygroup (ROMS) database and external published datasets. After stratification of all SF3B1mut UM using short PFS vs. long PFS, only largest tumor diameter (LTD) was significantly larger (mean: 17.7 mm (±2.8 SD) in the short PFS SF3B1mut group vs. the long PFS group (mean: 14.7 (±3.7 SD, p = 0.001). Combined ROMS and The Cancer Genome Atlas (TCGA) transcriptomic data were evaluated, and we identified SF3B1mut-specific canonical transcripts (e.g., a low expression of ABHD6 indicative for early-onset metastatic disease) or distinct expression of SF3B1mut UM aberrant transcripts, indicative of early- or late-onset or no metastatic SF3B1mut UM.
1. Introduction
Uveal melanoma (UM) is a highly malignant tumor with metastatic capacity. Metastatic disease is detected either early (<60 months) or late during follow-up. Staging of primary UM has been performed using American Joint Committee on Cancer (AJCC) [1] criteria, but studies show that prognostication of UM patients is also possible through analysis of chromosomal rearrangements [2], sequencing of UM driver genes [3,4], and evaluating gene expression profiles (GEP) [5]. Adding chromosome 3 and 8q status to AJCC classification improves accuracy of prognostication of UM patients [6]. Gain-of-function mutations in guanine nucleotide-binding protein subunit alpha (Gαq) (GNAQ), guanine nucleotide-binding protein alpha 11 (GNA11) (or, more rarely, in cysteinyl leukotriene receptor 1 (CYSLTR2) or phospholipase C beta 4 (PLCBC4)) are considered primary driver events which are found in almost all UM but are not associated with patient prognosis. Mutations in secondary UM driver genes are strongly associated with prognosis of UM patients and affect BRCA1-associated protein 1 (BAP1; associated with the worst prognosis), splicing factor 3b subunit 1 (SF3B1; associated with intermediate prognosis), and eukaryotic translation initiation factor 1A X-Linked (EIF1AX; associated with the most favorable prognosis). BAP1 is an enzyme involved in deubiquitination and interacts with different proteins such as DNA damage repair protein breast cancer type 1 (BRCA1). Splicing Factor 3b Subunit 1 (SF3B1) mutations occur in 15–29% of UM [7,8,9,10] and are cytogenetically characterized by multiple distal chromosomal copy number variations (CNV) such as (partial) loss of chromosome 1p and chromosome 6q and gain of chromosome 6p or chromosome 8q [11,12]. In The Cancer Genome Atlas (TCGA) milestone paper of Robertson et al., most SF3B1-mutated UM were allocated in cluster two comprising disomy chromosome 3 and chromosome 8q gain [12]. Moreover, this cluster analysis was superior in prognostication than the AJJC classification [13]. Finally, EIF1AX is a protein involved in stabilizing the ribosomes during translation, which is also an essential cellular process [14]. These UM tumors occur in approximately 20% of all UM and rarely metastasize.
Mutations in SF3B1 in UM occur mostly at the gene position that encode the amino acid (AA) residues 625, and more rarely affected AA-residues are 666, 700, 783, 781, 742, and 1123 [15]. SF3B1 is involved in splicing of the precursor mRNA, which is an essential cellular process in all eukaryotic species. SF3B1 mutations occur in a heterozygous state and are change-of-function mutations that result in a broad range of aberrantly spliced transcripts due to the mutant SF3B1 protein, as encoded by the mutant allele. The wild-type allele remains active to produce the canonical spliced transcripts. The aberrant transcripts are the result of the use of alternative recognition sites by the mutated spliceosome complex and thereby utilizing (or preferring) a non-canonical splice site due to genomic mutations encoded within the Heat Domains of SF3B1 [16]. Somatic missense mutations predominantly affect the 625 arginine residue within one of the heat domains of SF3B1 [16]. This peculiar preference is in contrast to other malignancies such as breast cancer and leukemia, in which related SF3B1 amino acid substitutions more frequently affect residue K700 and K666, respectively. In the TCGA-Uveal Melanoma cohort (TCGA-UVM) [12], 14 out of the 18 somatic SF3B1 mutations detected reside within the residue R625. In the remaining four cases, SF3B1 mutations reside twice within the residue K666 and once within T663 and H662 and therefore account for 22% of non-R625 SF3B1 mutations.
To predict early- (PFS < 60 months) or late- (PFS ≥ 60 months) onset metastatic disease in the SF3B1 mutated (SF3B1mut) UM, we first set out to describe clinical characteristics of SF3B1mut UM from the updated ROMS cohort (n = 48) [11,17] and compare these to an aggregated cohort distilled from literature. Secondly, we utilize clinical data, whole-transcriptome datasets comprising 106 UM (26 ROMS and 80 TCGA) with mutated secondary driver genes, evaluate differentially expressed genes, and explore differentially expressed aberrantly spliced transcripts that characterize SF3B1mut UM. Finally, we hypothesize that canonical gene expression or aberrantly spliced transcript expression can be used to discriminate between early-onset metastatic disease (defined as progression free survival (PFS) < 60 months) and late-onset metastatic disease defined as PFS ≥ 60 months SF3B1mut UM patients.
2. Materials and Methods
2.1. Generation of a Uniform Clinical Dataset of UM Patients
We combined clinical and genetic variables from 10 publicly available datasets: Alsafadi et al. [8], Royer-Bertrand et al. [18], Johnson et al. [19], Furney et al. [7], Harbour et al. [9], Shain et al. [20], Martin et al. [10], Zehir et al. [21], Rodrigues et al. [22], and Robertson et al. [12] (TCGA-UVM) cohorts. We updated the ROMS dataset used by Yavuzyigitoglu et al. [11] until 2019 and generated a dataset comprising these 11 study groups (Supplementary Figure S1). An overview of the study and study aims is depicted in Figure 1. Clinical and histopathological parameters such as age at diagnosis, gender, ciliary body involvement, presence of epithelioid cells, extraocular extension, closed extracellular matrix patterns, largest tumor diameter (LTD), tumor thickness, T class in TNM [23], inflammation, necrosis, metastatic disease, progression-free survival, patient status, GNAQ/GNA11 gene mutation status, SF3B1 mutation status, and corresponding amino acid changes and/or nucleotide changes were included. The rationale behind PFS cutoff of 60 months was based on a previously observed bimodal metastatic potential [24] and due to an approximately even distribution of samples at risk in the PFS < 60 months and PFS ≥ 60 months groups in survival analyses. Student’s t-test was applied for continuous variables, whereas Fisher’s Exact test was applied for categorical variables. p-value < 0.05 was considered statistically significant.
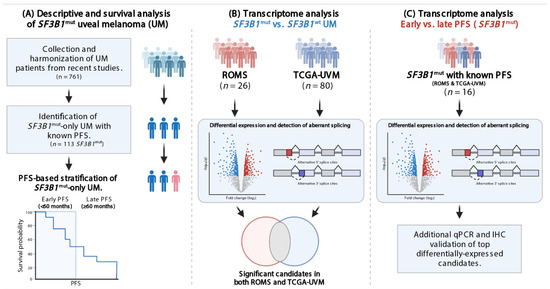
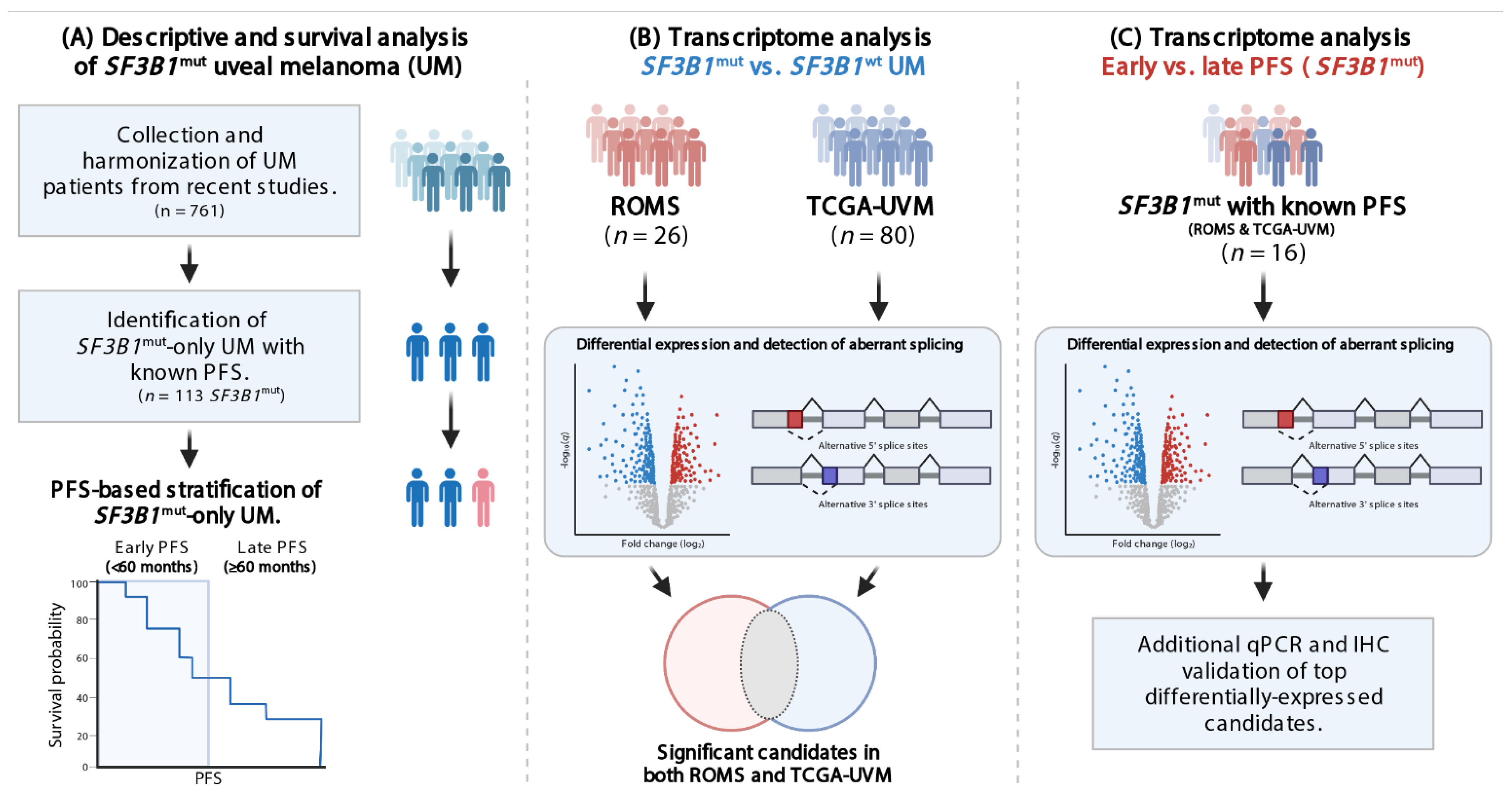
Figure 1.
Flowchart visualizing study design and aims. (A) Data from SF3B1mut UM from 11 cohorts were collected and described with stratification criteria. (B) Differential gene expression analysis (DGE) was performed and the ROMS, and TCGA-UVM data were intersected to investigate SF3B1mut specific transcripts. (C) DGE was performed on SF3B1mut-only samples using the PFS stratification criteria to investigate transcript expression characteristic for early onset (PFS < 60) and late onset (PFS ≥ 60 months). Finally, in silico results were validated in vitro.
2.2. Mutation Analysis
Mutation status of BAP1, SF3B1, GNAQ, GNA11, and EIF1AX was determined for all UM with either BAP1 immunohistochemistry (IHC), Sanger sequencing, and/or next-generation sequencing, as previously described [4,25,26]. Additionally, we used RNA sequencing data from 26 primary UM patients with BAP1, SF3B1, or EIF1AX mutations, which were acquired as described previously by Smit et al. [27]. Further analyses include samples with solely a SF3B1 mutation and for which we exclude UM with concomitant mutations in either BAP1 or EIF1AX (n = 10).
2.3. Survival Analysis
Progression-free survival (PFS) was determined as the interval from treatment until metastasis or metastasis and subsequent death due to UM or until last follow-up. If the interval from treatment to metastatic disease and interval from treatment to death due to UM was reported, we used the interval from treatment to death due to UM or development of metastasis as study endpoints. Patients were censored when they were lost to follow-up or when death from other cause than UM occurred, or occurrence of death was reported without any cause.
2.4. Processing and Analysis of Whole-Transcriptome Data
We used whole-transcriptome sequencing data from 26 UM; information on sample acquisition, preparation, and sequencing has been described previously by Smit et al. [27]. In brief, total RNA was isolated from 5 μm sections of snap-frozen uveal melanoma samples, using the Qiagen miRNeasy isolation kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocols. Consequently, transcripts longer than 200 nucleotides were sequenced on the Ion Proton sequencer (Thermofisher Scientific, Waltham, MA, USA) to produce single-end sequencing reads.
The whole-transcriptome BAM files for the TCGA-UVM cohort (n = 80) were downloaded from NCBI dbGaP website in 2019, under phs000178.v10.p8. Sample acquisition, library preparations and sequencing protocols of the TCGA-UVM cohort were described previously by The Cancer Genome Consortium [12].
Whole-transcriptome BAM files from the TCGA-UVM cohort were sorted on read-names (natural sort) and converted back to unmapped paired-end reads with Samtools (v1.7; htslib v1.9) [28] and Sambamba (v0.7.0) [29]. All whole-transcriptome samples (ROMS and TCGA-UVM) were mapped against the human reference genome (GRCh38; GenBank accession: GCA_000001405.15) using STAR (v2.7.1a) [30] with genomic annotations from GENCODE (v30) [31]. After alignment, duplicate reads were marked, and alignment metrics were obtained using Sambamba (v0.7.0) [29]. To identify potential alignment issues regarding transcript read uniformity, transcript integrity numbers were calculated using tin.py (v2.6.6) [32]. Read counting was performed using featureCounts (v1.6.3) [33] on the GENCODE (v30) genomic annotations; only primary-aligned reads overlapping exonic regions were counted and summarized per gene. General sequence characteristics are visualized in Supplementary Figure S2.
2.5. Differential Gene-Expression Analysis
Raw read counts for GENCODE (v30) transcripts were used as input for DESeq2 (v1.24.0) [34], with the exclusion of pseudogenes, mitochondrial RNA, ribosomal RNA, immunoglobulin (Ig)-variable chain, and T-cell receptor (TcR) genes and inactivated immunoglobulin genes (n = 41140).
For both the ROMS and TCGA-UVM cohort separately, a Wald test between SF3B1mut-only samples and SF3B1wildtype (wt) samples (excluding any double-mutants and/or SRSF2mut samples) was performed. An additional Wald test between SF3B1mut-only samples based on PFS < 60 months with metastatic disease and PFS ≥ 60 months was performed on the combined (ROMS and TCGA-UVM) cohort. Hereafter, PFS < 60 months refers to UM patients with early metastatic disease and late PFS (PFS ≥ 60 months) refers to patients with late or without metastatic disease. During differential gene expression analysis, we corrected for gender batch-effect in all analyses and additionally for cohort batch effect (ROMS/TCGA-UVM) in the combined cohort. To correct for multiple hypothesis testing after DESeq2 analysis, we employed independent hypothesis weighting (IHW; v1.12.0) [35]. Fold-changes (log2) were shrunk using their respective coefficient using apeglm (v1.6.0) [36]. A principal component analysis (PCA) was performed on the top 5000 most-variable (row-wise) genes after correcting for batch effects (Supplementary Figure S3). The top differential candidates were selected based on the following criteria: log2 fold change ≥ |0.5|, adjusted p (q) ≤ 0.05, and average read counts of ≥10 over all samples.
2.6. Differential Gene-Set Enrichment Analysis (GSEA)
Using the R package fgsea (v1.10.0) [37] with 100.000 permutations, we performed gene-set enrichment analysis using the Wald-statistics obtained from the prior DESeq2 analysis for all transcripts with at least 1 read on average to reduce rank ties for low-coverage transcripts. We tested the KEGG (n = 186) and HALLMARK (n = 50) gene sets (version 7.0), which contain 7732 distinct genes in all of the chosen gene sets obtained from the Molecular Signatures Database (MSigDB) [38], for statistically significant enrichment or depletion (q ≤ 0.05). Prior to testing, the ENTREZ identifiers of the gene sets were converted into ENSEMBL identifiers, of which 17 ENTREZ identifiers could not be mapped to ENSEMBL identifiers and were discarded as part of their respective gene set(s).
2.7. Detection of Aberrant Splicing Patterns
To detect SF3B1-related alternative splicing, we performed a strategy using a custom in-house workflow designed around STAR and DEXSeq [39]. This workflow was specifically designed for the detection of aberrant 5′ and 3′ exon shortenings and extensions. The workflow detected aberrant splicing events by incorporating the novel splice-events detected by STAR (SJ.out.tab) during the initial alignment procedure, as novel exonic regions within the GENCODE (v30) genomic annotations. These novel exonic regions were assigned to the nearest neighboring up- and downstream canonical exon (respective to orientation) within GENCODE (v30) annotations and assigned the respective genomic annotations of this neighboring exon, e.g., the gene name and ENSEMBL identifier, among others, and saved as a custom GFF3 file. The new custom GFF3 file contained the novel exonic portions per gene, next to the canonical annotations. We used the subread_to_DEXSeq (https://github.com/vivekbhr/Subread_to_DEXSeq accessed on 25 November 2019) script to further process our custom annotation (GFF3) and create a flattened version, in which each exon is split into its constituent non-overlapping exonic portions. Subsequently, this custom-flattened GFF3 file was used to count the reads per overlapping exonic portion using featureCounts (v1.6.3) [33]. These exon expression read counts were imported into DEXSeq (v1.30.0) [39] to detect differential 5′ and 3′ splice-sites and exons between SF3B1mut and SF3B1wt samples and SF3B1early and SF3B1late samples for both ROMS and the TCGA-UVM cohort.
To determine statistically significant differentially expressed exonic regions, we used the following criteria: adjusted p-value ≤ 0.05 and a log2 fold-change of the splicing event ≥|0.5|. In downstream analysis, we denoted the exonic regions (acceptor/donor) not-yet-present within the GENCODE v30 annotation as novel (acceptor/donor) splicing aberrations.
2.8. Validation of In Silico Results
Within our cohort consisting of 48 ROMS samples, there was tumor material available of 31 Formalin Fixed Paraffin Embedded (FFPE) UM to validate in silico results on with IHC. Of these samples, 20 fresh frozen UM patient samples were available for RNA isolation, as described previously by Smit et al. [27], of which 10 samples were of sufficient quality. Next, seven samples served as an independent validation set. In addition, three samples that were utilized in DGE expression analysis functioned as a validation set of RNA-seq results. Finally, several biomarkers (ABHD6, CSRNP1) derived from the aforementioned DGE analyses were validated with quantitative PCR (qPCR). Then, a 500 ng (nanogram) RNA input was used in cDNA conversions, except for one (PFS < 60 months) SF3B1mut sample, for which a maximum input of 450 ng RNA was possible due to concentration restraints. A real-time PCR (RT-PCR) reaction mix consisted of 5- or 7-times diluted cDNA, 10 μL iTaq Universal SYBR Green Supermix (Bio-rad, Hercules, CA, USA), and 10 μM forward and reverse primers, which were placed in a CFX96 real-time system (Bio-Rad, Hercules, CA, USA). Delta cT values of genes of interest were calculated relative to the control transcript CHMP2A. All qPCR reactions were successfully performed in technical triplicates, except for sample 7, for which only two cT values for CHMP2A could be used. Differential gene expression was calculated using the threshold cycle (Ct) method [40]. A SF3B1mut UM sample with 45.5 months PFS with development of metastatic disease was used as a control during qPCR experiments.
2.9. ABHD6 Immunohistochemistry
Immunohistochemistry was performed with an automated immunohistochemistry staining system (Ventana BenchMark ULTRA, Ventana Medical Systems, Tucson, AZ, USA) using the alkaline phosphatase method and a red chromogen. In brief, following deparaffinization and heat-induced antigen retrieval for 64 min, the tissue sections were incubated with a rabbit polyclonal antibody raised against synthetic peptide of human ABHD6 (1:100, MyBioSource, San Diego, CA, USA) for 1 h at 37 °C, followed by red detection and counterstain with hematoxylin II and bluing reagent according to the manufactures instructions (Ventana). Kidney, tonsil, and the retinal pigment epithelium were used as positive controls for ABHD6 expression. An ophthalmic pathologist independently evaluated the histopathological characterization of the tissue sections and the immunohistochemistry staining. For every section, an immunoreactive score (IRS) was determined. We first determined the intensity of the cytoplasmatic staining (absent, mild, moderate, and intense, scored as 0, 1, 2, or 3, respectively). Next, the percentage of stained cells that showed the predominant intensity, was determined; no positive cells were scored as 0% and less than 10%, 10% to 50%, 51% to 80%, and more than 80% were scored as 1, 2, 3, or 4, respectively. Then, IRS was calculated by multiplying the score for percentage of stained cells with the score for the intensity of the staining [41].
2.10. Statistical Analysis and Code Availability
Analysis was performed using the R statistical platform language (v3.6.3). All used custom R code can be freely requested by contacting the authors.
3. Results
3.1. Establishing a Uniform Clinical Dataset of UM from Various Studies
Updating the ROMS database [11] resulted in seven additional SF3B1mut samples. One UM sample classified as SF3B1mut UM in the Yavuzyigitoglu et al. set was excluded due to melanoma originating from Nevus of Ota. We hypothesized clinical or genetic variables could be indicative for either early-onset metastatic disease SF3B1mut UM (PFS < 60 months) or SF3B1mut UM patients with late-onset metastatic disease (PFS ≥ 60 months). Therefore, we acquired 761 UM from a total of 11 large-scale UM studies (Supplementary Figure S1) and generated a uniform dataset that comprised 146 SF3B1mut UM (Supplementary Table S1).
3.2. Overview of the Clinical Parameters of the ROMS Cohort
The ROMS cohort (n = 48) consisted of 21 males and 27 females with mean age at diagnosis of 56.7 years (±16.8 standard deviation (SD)). The mean largest tumor diameter (LTD) was 13.6 mm (±3 SD), and mean tumor thickness was 6.8 mm (±2.6 SD). Age at diagnosis did not differ between the ROMS and non-ROMS cohorts (p = 0.836). However, LTD, tumor thickness, inflammation, and necrosis were significantly different (all p-values smaller than 0.05) between the ROMS and non-ROMS UM patients (Supplementary Table S2). Finally, mean PFS was 93.5 months (±57.2 SD) in the ROMS cohort, which was significantly longer (p < 0.001) compared to the mean PFS in the non-ROMS cohort of 45.4 months (±33.6 SD). Demographic and genetic variables per dataset are shown in Supplementary Table S1, and these variables stratified for ROMS vs. non-ROMS data are shown in Supplementary Table S2A.
3.3. Stratification of All SF3B1mut UM
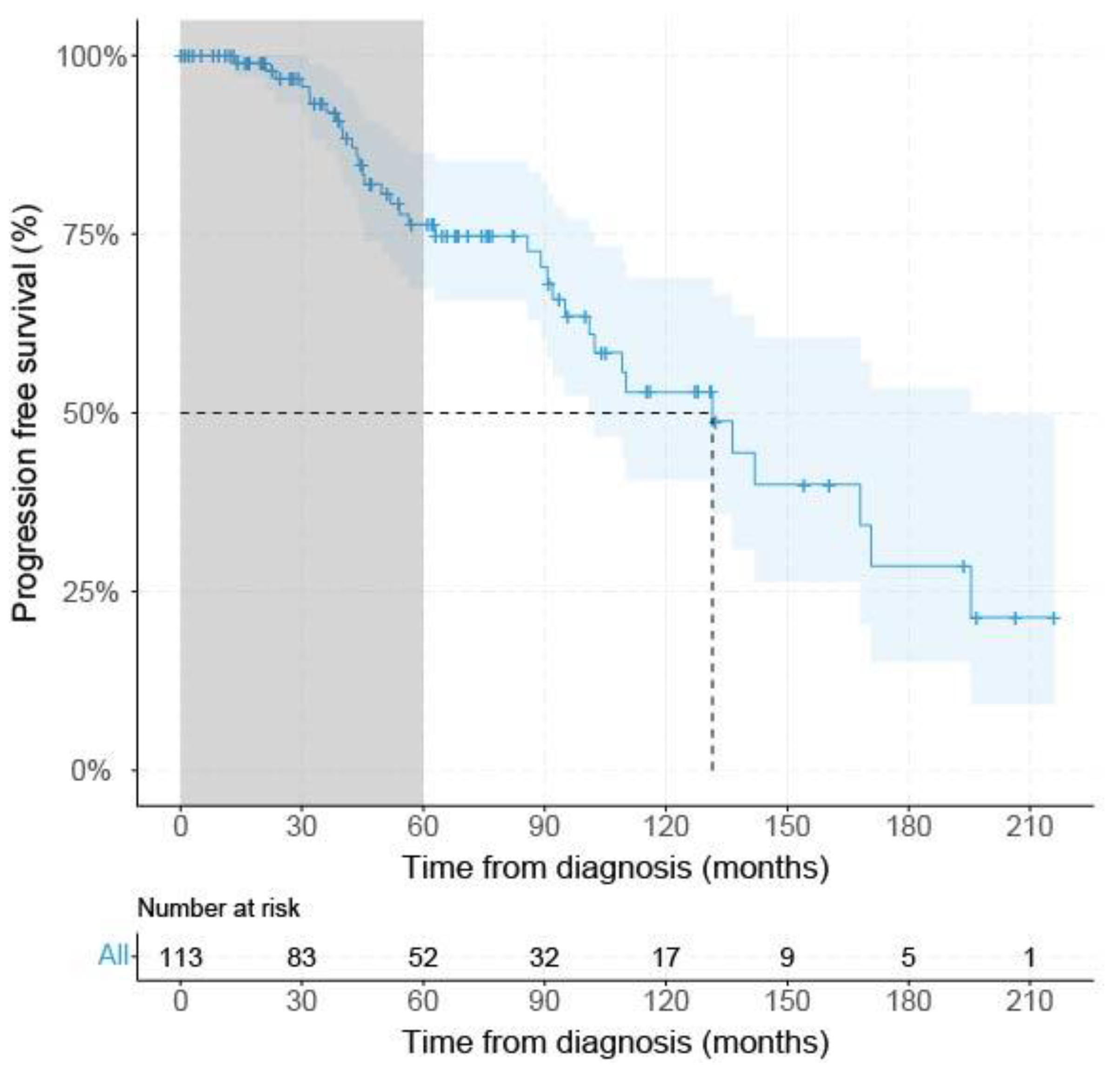
From the 146 acquired UM, survival data were available for 113 patients, 19 patients had PFS < 60 months (with metastatic disease), and 52 UM patients had PFS ≥ 60 months. Only the mean largest tumor diameter was significantly (p < 0.001) larger (17.7 mm (±2.8 SD) in the PFS < 60 group compared to the PFS ≥ 60 months group (mean: 14.7 mm (±3.7 SD) (Table 1). The median PFS of all SF3B1mut UM at risk (n = 113) was 131.5 months (95% CI: 101.0–195.4) using Kaplan–Meier survival analysis (Figure 2).

Table 1.
All SF3B1-mutated UM stratified for PFS < 60 months and PFS ≥ 60 months with description of clinical variables. Age at diagnosis, gender, ciliary body involvement, epithelioid cells present, extraocular extensions, closed extracellular matrix patterns, tumor thickness, T class in TNM category, inflammation, necrosis, GNAQ and GNA11 status, and SF3B1 amino acid mutation did not significantly differ between PFS < 60 months and PFS ≥ 60 months groups (all p > 0.05). Student’s t-test was used for continuous variables and Fisher’s exact test (indicated with *) was used for categorical variables. For overview of all variables, we refer to Supplementary Table S2B.
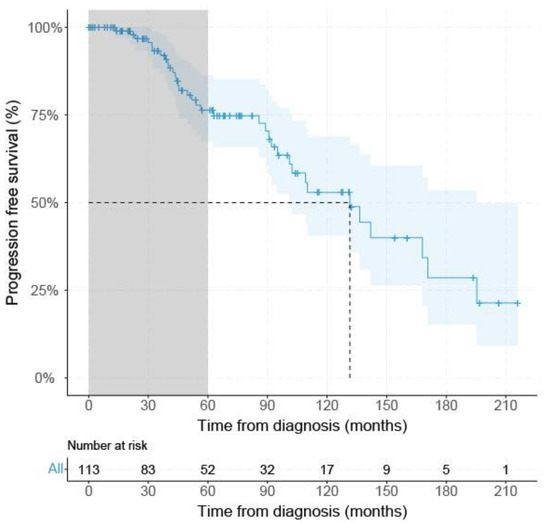
Figure 2.
Kaplan–Meier survival curve of all SF3B1mut UM. Grey area indicates PFS < 60 months. Dashed gray line indicates median progression free survival percentage with corresponding time from either diagnosis or treatment, dependent on the description in the original papers. Blue line shows progression-free survival with a confidence interval of 95% indicated by the blue area and censored data indicated by vertical bar.
3.4. Metastatic Location of SF3B1mut UM
Within the ROMS cohort, 15 UM patients developed metastatic disease mostly located in the liver (n = 8). However, two patients developed both liver and lung metastasis. Two patients showed liver and ossal metastases, and one other patient was diagnosed with only ossal metastasis. One patient had liver and pancreatic metastases. Finally, there was one patient that developed lung, liver, kidney, and subcutaneous metastases (Supplementary Table S2A). In all other datasets, the reported metastatic location in SF3B1mut UM was the liver (Supplementary Table S1), without specifying other metastatic locations.
3.5. Differential Analysis of Canonical Transcripts Reveals SF3B1mut-Exclusive Transcripts
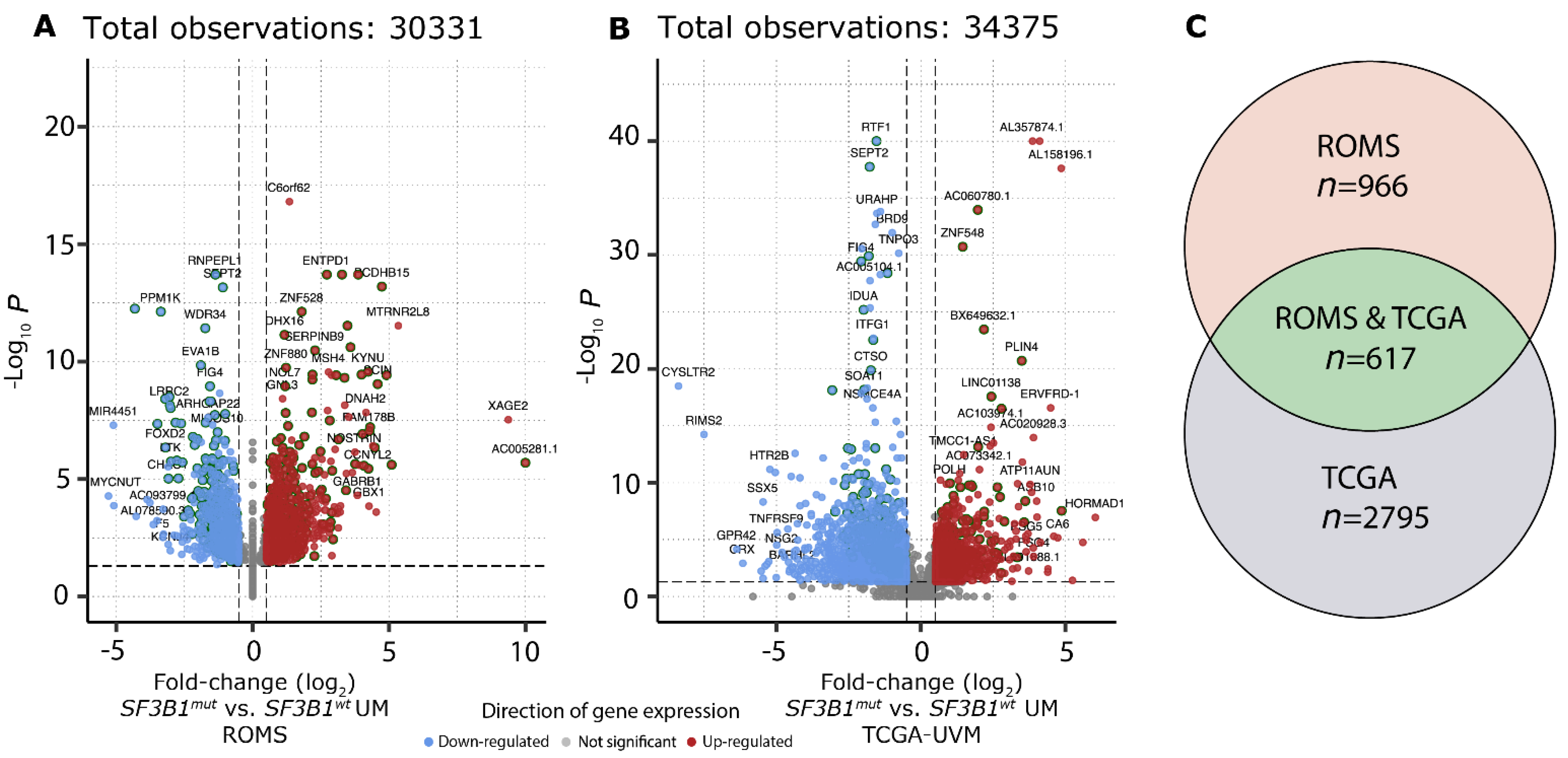
To investigate which canonical transcripts are differentially expressed between SF3B1wt and SF3B1mut in UM, we performed independent differential gene expression analysis on the ROMS (Figure 3A) and TCGA-UVM (Figure 3B) cohorts, respectively, which resulted in 617 gene candidates with a p-adjusted value < 0.05 in both the ROMS and TCGA-UVM cohort independently (Figure 3C). Of these 617 differentially expressed genes, 37 are known cancer genes, of which 21 were downregulated and 16 were upregulated (Supplementary Table S3). Gene-set enrichment analysis (GSEA) revealed upregulation of the spliceosome machinery and downregulation of inflammatory response in both cohorts. Moreover, the downregulation of chemokine signaling, T-cell receptor signaling, and natural killer cell-mediated cytotoxicity pathways within SF3B1mut UM suggests less inflammatory activity in SF3B1mut UM compared to SF3B1wt UM (Supplementary Figure S4, Supplementary Table S4).
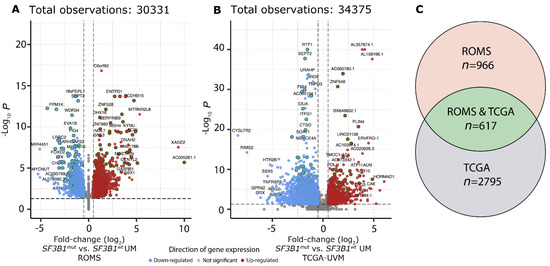
Figure 3.
Differential gene expression in SF3B1mut UM. (A) Volcano plot of the differential expression analysis between SF3B1mut (n = 12) and SF3B1wt (n = 14) UM within the ROMS cohort. Genes significantly down-regulated (blue) and up-regulated (red) in SF3B1mut UM are shown. Gene names for the top 50 genes (based on descending -log10 q-value) and top 20 (based on |log2 fold change|) for both directions are shown. Genes that were found to be differentially expressed in both cohorts (a and b; n = 617) are highlighted by a dark green outer circle. The x-axis displays the log2 fold-change and y-axis displays the adjusted p-value (q) on a -log10 scale. The total amount of tested genes is shown on top. (B) Same as a), except for the differential expression analysis between SF3B1mut (n = 15) and SF3B1wt (n = 61) UM within the TCGA-UVM cohort. (C) Venn diagram displays differential gene expression results for ROMS, ROMS and TCGA, and TCGA cohorts.
3.6. SF3B1mut UM Can Be Stratified Using Differentially Expressed Canonical Transcripts in PFS < 60 and PFS ≥ 60 Months
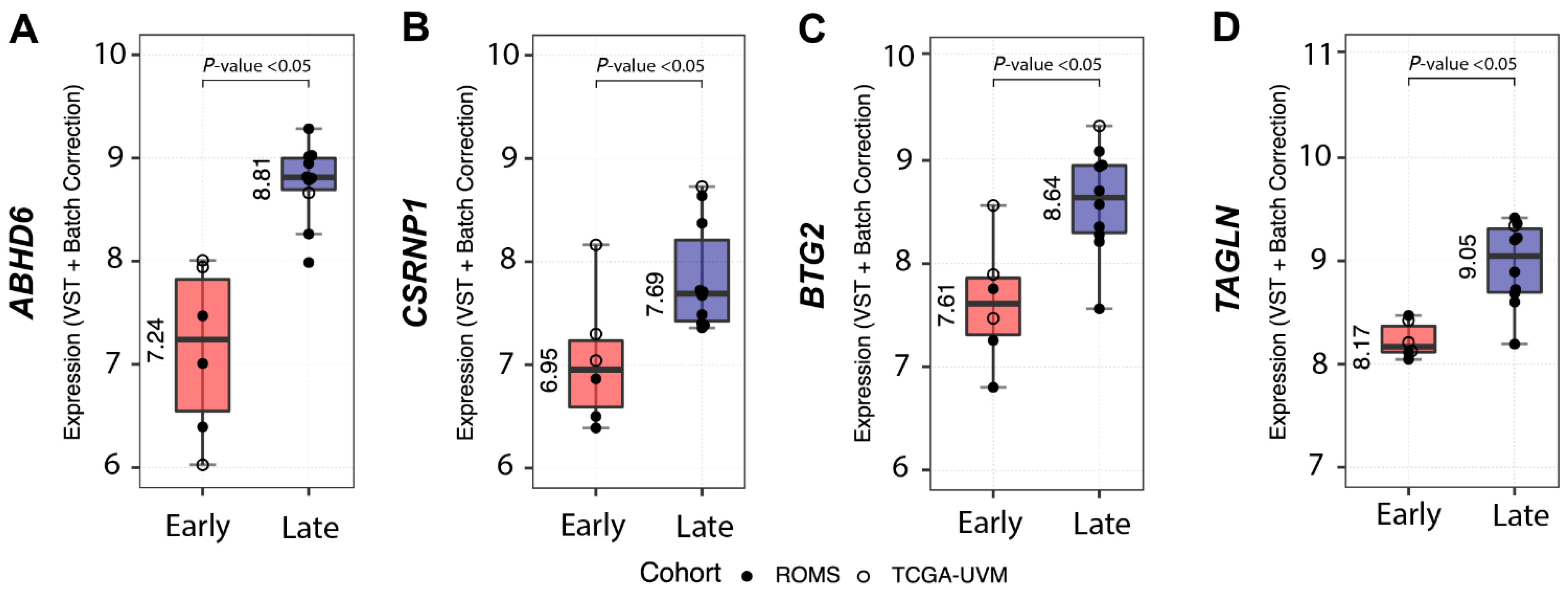
Analysis of differentially expressed full-length transcripts within the SF3B1mut UM group in ROMS and TCGA datasets resulted in 14 gene candidates of which four (ABHD6, CSRNP1, BTG2 and TAGLN) showed decreased expression in the PFS <60 UM group and increased expression in the PFS ≥ 60 months SF3B1mut UM group (Figure 4, Supplementary Table S5).
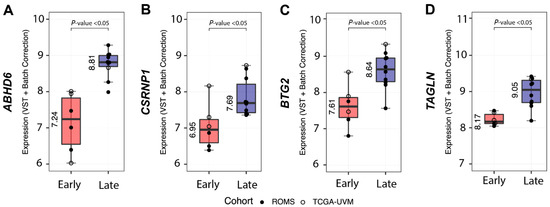
Figure 4.
Differential gene expression between SF3B1mut with short PFS < 60 (Early-onset) vs. SF3B1mut with a long PFS ≥ 60 months (Late-onset). Overview of the most differentially expressed genes between SF3B1mut with an PFS < 60 from the combined (ROMS and TCGA-UVM) cohort. ROMS samples are depicted with closed circles, and TCGA-UVM are depicted with open circles. The boxplots show the variance stabilizing transformation (VST-transformed and batch corrected) expression per PFS category and metastatic status for (A) ABHD6, for (B) CSRNP1, for (C) BT2G, and for (D) TAGLN. All results show p-adjusted value < 0.05.
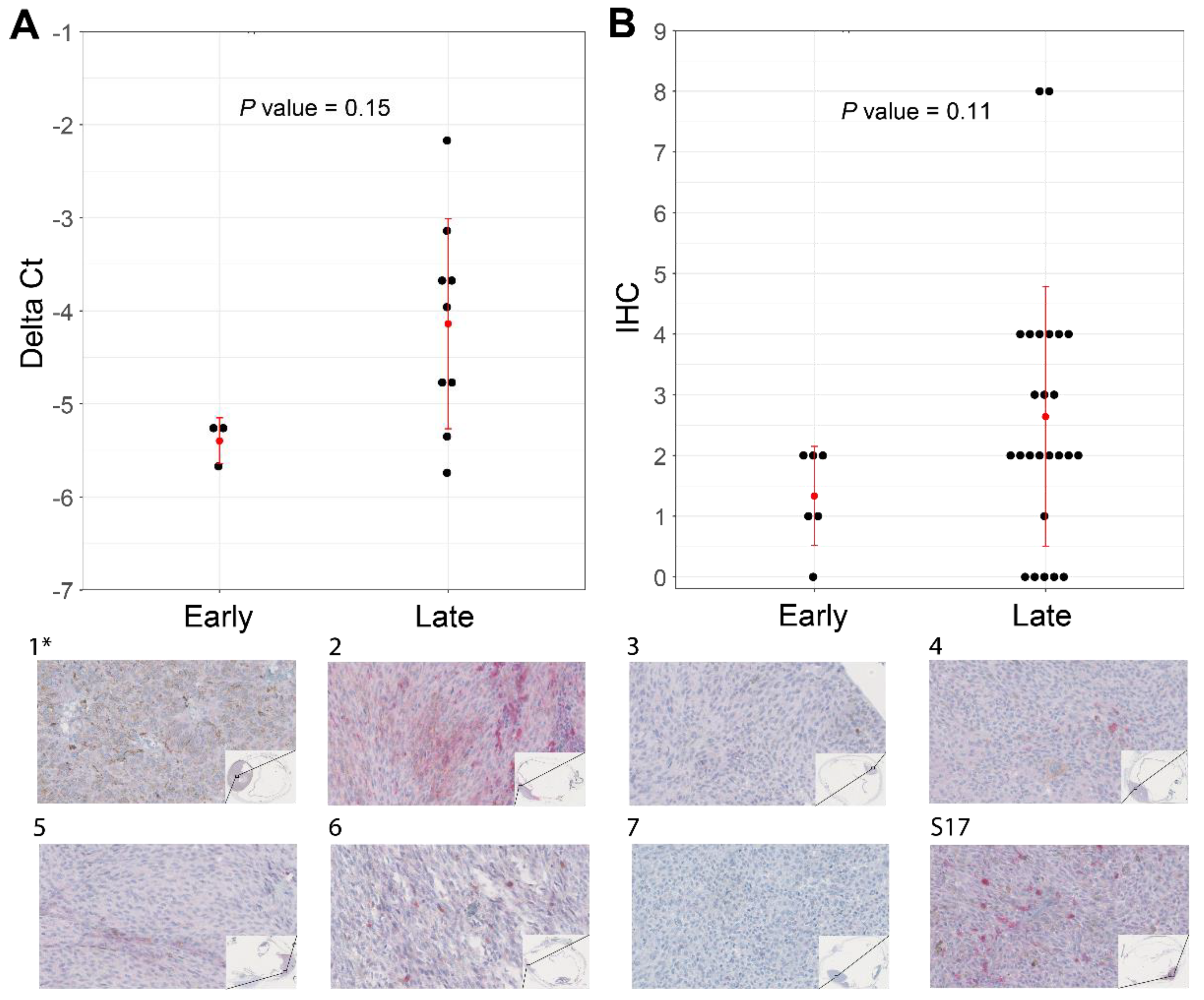
Our attempts to validate the differential expression of ABHD6 and CSRNP1 transcripts with IHC and RT-qPCR was hampered due to the scarcity of N2-stored tumor tissue. Nevertheless, we could collect material for IHC and RT-qPCR from ten SF3B1mut UM samples: one sample had a PFS < 60 months, and nine belonged to the PFS ≥ 60 months group. The expression of ABHD6 was, in general, higher in all validation samples compared to a short PFS reference sample, except for one RT-qPCR sample and for two ABHD6 IHC scores (Figure 5A). Mean delta Ct value was −5.38 (±0.25 SD) in the PFS < 60 months patient sample compared to the mean delta Ct value of −4.14 (±1.13 SD) in the PFS ≥ 60 months group, which was not significantly different between the two groups (p = 0.15) (Figure 5A), and the mean IHC value for the PFS < 60 months group was 1.34 (±0.82 SD) compared to the mean IHC of 2.64 (±2.14 SD), which was also not statistically different between the groups (p = 0.11) (Figure 5B). The RT-qPCR expression of CSRNP1 could not validate the results from our in silico differential gene expression analysis.
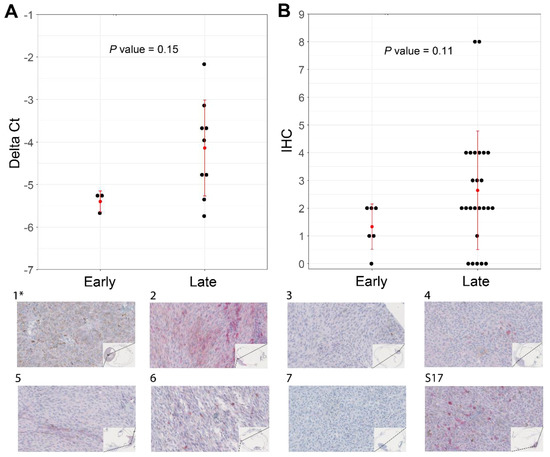
Figure 5.
Increased levels of ABHD6 can be associated with SF3B1mut UM with late-onset metastatic disease (PFS ≥ 60 months). (A) RT-qPCR performed in triplicates using CHMP2A expression as a normalizer of 10 primary UM (see Supplementary Figure S5). (B) ABHD6 IHC on all SF3B1mut UM samples that were available and stratified for early- and late-onset metastatic disease. Red error bars represent standard deviation and red dot represents mean. Wilcoxon rank sum test was used to evaluate statistical difference of delta Ct values and IHC scores shown in scatterplots in panel A and B. p-value < 0.05 was considered statistically significant. (1*–7 and S17) ABHD6 IHC staining of eight primary UM samples, which are a selection of samples in panel A and B. The corresponding delta Ct values and IHC values with regard to histology are represented in Supplementary Figure S5. (40× magnification). (*) indicates the control sample with a PFS < 60 months.
3.7. Differential Analysis of Aberrant-Splicing Reveals SF3B1mut-Exclusive Transcripts
Using our custom DEXSeq pipeline, we next investigated the value of differential (novel) exon usage to discriminate between SF3B1mut and SF3B1wt UM within the ROMS cohort, whilst again using the UVM-TCGA with the same design as validation cohort. Comparing SF3B1mut vs. SF3B1wt samples within the ROMS revealed 2107 differentially expressed exonic regions from 1353 distinct genes, of which 397 exonic regions from 257 distinct genes were also found differentially expressed within the TCGA-UVM (Supplementary Figure S6A–C, Supplementary Table S6). Of these 397 shared differentially expressed exonic regions, we could detect 78 novel acceptor (63 distinct genes) and 19 novel donor (14 distinct genes) splicing aberrations not present within the canonical transcript annotations (GENCODE v30). Amongst these 77 genes containing novel acceptor and donor splicing aberrations, we found known onco- and tumor-suppressor genes such as CDK2, BRD9, NACA, ZNF638, PPP2R5A, NONO, STIP1, and SMARCD2, as well as 21 onco- and tumor-suppressor genes with other forms of differential exon usage (Supplementary Table S6). Using the exon-overlapping read counts of these 77 genes and performing unsupervised clustering (Euclidean distances and Ward.D2 method) on all samples within the ROMS cohort revealed clear separations of SF3B1mut UM vs. SF3B1wildtype (Supplementary Figure S7).
3.8. SF3B1mut UM Can Be Stratified Using Differentially Expressed Aberrant Transcripts in PFS < 60 and PFS ≥ 60 Months
In line with the discriminatory value of using differential exon usage to distinguish SF3B1mut and SF3B1wt UM, we hypothesized whether similar patterns could be indicative of early- or late-onset of metastatic disease in SF3B1mut UM (PFS < 60 months vs. PFS ≥ 60 months) within the ROMS cohort.
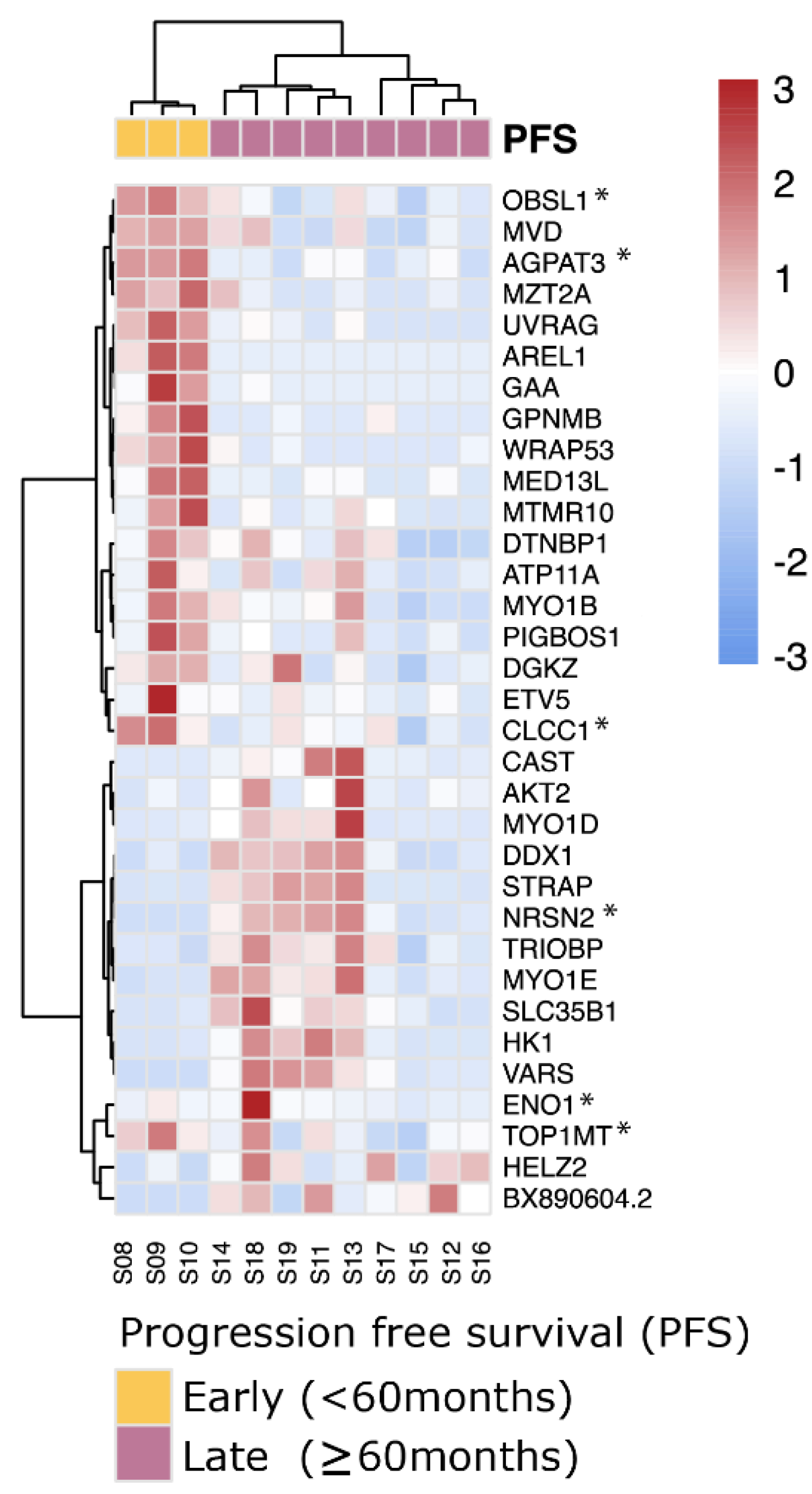
Using the same strategy as before, albeit without the ability of using the TCGA-UVM cohort as validation because of the lack of follow up data, we could detect 34 differential genes with aberrant exon usages in 33 distinct genes between PFS < 60 months and PFS ≥ 60 months SF3B1mut samples (Figure 6). Of these 34 events, 6 were involved with novel acceptor and donor aberrations not present within GENCODE v30 (ENO1, OBSL1, NRSN2, AGPAT3, TOP1MT and CLCC1), and in total, four genes were known onco- and tumor suppressor genes (ETV5, CLCC1, AKT2 and WRAP53). These splicing events supplement the full-length transcript markers shown previously to differentiate between early- and late-onset metastatic disease of SF3B1mut UM (Supplementary Table S7).
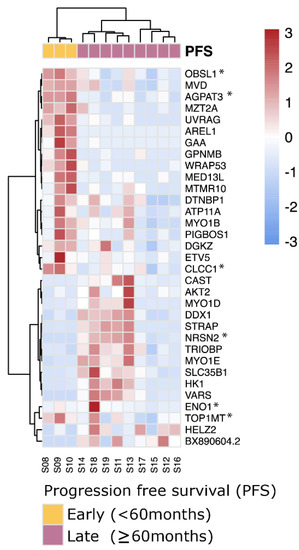
Figure 6.
SF3B1mut UM is characterized by aberrant splicing events. Unsupervised clustering (Euclidean distances and Ward.D2 method) on the differential exon usages between SF3B1mut samples in the ROMS cohort with PFS < 60 months and PFS ≥ 60 months. Values are mean-centered read counts represented with a z score of differentially expressed splicing events in genes (blue, low expression; red, high expression). Top bars represent PFS-status (yellow; PFS < 60 months and purple; PFS ≥ 60 months). Asterisk (*) indicates novel splicing aberrations that are either donors or acceptors.
4. Discussion
In the current study, we describe the largest number of SF3B1mut UM (n = 146) in the literature and report on the prognostic value of clinical and genetic variables, whilst distinguishing between early (PFS < 60 months) and late (PFS ≥ 60 months) metastatic onset SF3B1mut UM. This could provide us with tools to suggest a more stringent treatment protocol or inclusion in specific clinical trials. The question remains if these potentially more stringent screening programs will result in the prolonged overall survival of SF3B1mut UM patients. However, after validation of our results in external cohorts, low expression of ABHD6 could potentially be used as a biomarker to detect and select high-risk SF3B1mut UM.
A high expression of ABHD6 is indicative of a long progression-free survival (PFS ≥ 60 months), whereas a low expression is indicative of early-onset metastatic disease (PFS < 60 months). As described in previous reports, UM with somatic alterations within the spliceosome have a distinct CNV profile and are characterized by aberrant transcripts due to a mutated allele of SF3B1. Considering survival, we have shown that, in particular, SF3B1mut UM gives rise to late-onset metastases [4]. Nevertheless, the SF3B1mut UM patients may also show a less favorable prognosis with early-onset metastatic disease. Although, the majority of the samples with a PFS > 60 months originated from our cohort (n = 9), these UM patients were also present within the Alsafadi et al. (n = 1), Rodrigues et al. (n = 3), Shain et al. (n = 1), and Harbour et al. (n = 2) UM cohorts. Even though this constitutes only a limited number of UM patients within each external cohort, it does illustrate that late-onset metastatic disease occurs in SF3B1mut UM, but only when study follow-up is sufficient. From the clinical parameters, only LTD was significantly larger in the early-onset metastatic UM group compared to the late-onset metastatic UM, which corroborates with earlier study findings, where tumor size was shown to be an independent predictor of UM aggressiveness [17,42]. All included samples in our analyses are mainly derived from larger tumors (Table 1), yet smaller tumors are also represented in our cohort. Consequently, tumor size difference between studies may vary due to selection bias, whereas the discrepancy in necrosis and inflammation classification could be based on non-uniform definition of these variables. When comparing the ROMS SF3B1mut to the other studies for metastases, we did not observe significant differences between clinical and pathological parameters. Interestingly, the ROMS patients have a longer follow-up in contrast to the other studies, possibly explaining difference in median PFS. Regarding metastatic location, there were no associations between extrahepatic metastatic locations and high risk or low risk SF3B1mut UM, most likely due to a restricted number of metastatic samples.
4.1. Unbiased Detection of Pathogenic SF3B1mut UM
In contrast to BAP1 mutations, SF3B1 mutations are change-of-function mutations rather than loss-of-function mutations, with the R625 alteration being the predominant hotspot in UM. However, mutations at other locations encoding K666 or K700 also occur within smaller proportions of UM. At present, it is not clear whether mutations outside the common SF3B1 hotspots are merely passenger mutations, or if these contribute to UM pathogenesis warranting the need for an unbiased diagnostic approach capable of detecting compromised SF3B1 function. One such method could be based on a straightforward PCR primer assay, designed to detect SF3B1mut-specific aberrant transcripts indicative of the transcriptomic hallmark of SF3B1mut UM. This could broaden our understanding and inventory of SF3B1mut UM.
Downstream effects of the SF3B1mut allele are probably vast due to the amounts of aberrant transcripts that are formed. For example, the tumor suppressor BRD9 that is linked to melanomagenesis was recently shown to be downregulated in SF3B1mut UM due to the incorporation of a poison exon, which leads to the non-sense mediated decay of BRD9 [43]. Most likely, there are multiple transcripts that behave as the aforementioned tumor suppressor and are interesting to study. Therefore, differential gene expression in combination with non-sense mediated decay inhibition and queries for stop sequences outside canonical exon borders with sufficient coverage could aid in the detection of novel tumor suppressors. Interestingly, in our differential gene expression analysis, we observed that only ABHD6 expression can be used to discriminate between early-onset and late-onset metastatic SF3B1mut UM. ABHD6 is a lipase involved in endocannabinoid signaling and possibly has other effects in inflammation, metabolic syndromes, and insulin secretion. In contrast to earlier publications [44,45], our results show that the decreased expression of ABHD6 is associated with high-risk SF3B1mut, which we validated using qPCR and IHC in nearly all UM cases tested. However, in order to implement this biomarker in clinical settings, more samples should be tested in another study with sufficient follow-up. For now, the results are promising, and it is possible to identify early onset metastatic SF3B1mut UM.
A limitation of our differential expression analyses to draw convincing conclusions are the low number of samples. However, 617 differentially expressed genes were observed between SF3B1mut and SF3B1wt UM (concordant in both the ROMS and TCGA-UVM cohort), of which 37 are known cancer genes. In addition, GSEA revealed perturbations within spliceosome machinery and inflammatory responses. Moreover, downregulation of chemokine signaling, T-cell receptor signaling, and natural killer cell-mediated cytotoxicity pathways within SF3B1mut UM could indicate a decreased inflammatory function, reflecting potential immunological differences [12]. Furthermore, utilizing the transcriptomic hallmark of dysregulated splicing due to SF3B1 mutations, we could identify 2107 SF3B1mut-specific or significantly perturbed splicing aberrations with donor and/or acceptor extensions or shortenings.
4.2. Potential Biomarkers Capable of Distinguishing between Early- and Late-Onset SF3B1mut UM
Using differential expression analysis on canonical transcripts and detection of SF3B1mut-specific alternatively spliced transcripts between early- and late-onset SF3B1mut, we identified putative biomarkers between these groups of patients. We detected four canonical transcripts (ABHD6, CSRNP1, BTG2, and TAGLN) with significant up-regulated expression within the late-onset UM compared to early-onset UM. We set out to validate the expression of ABHD6 using IHC and rt-qPCR on secondary UM samples. The expression of ABHD6 varies between cancer types and is increased in Ewing sarcoma, prostate cancer, Burkitt lymphoma, and leukemia [46,47]. Moreover, ABHD6 expression in early metastatic UM was significantly downregulated compared to primary non-metastatic uveal melanoma, which corroborates our study findings within SF3B1mut UM [48]. Preferably, more PFS < 60 months SF3B1mut UM cases should be included to draw a convincing conclusion that ABHD6 expression is a prognostic biomarker of PFS in SF3B1mut UM. Due to a lack of IHC staining in samples 3 and 7, we cannot fully conclude ABHD6 IHC is a reliable test to differentiate between early- and late-onset metastasis in SF3B1mut UM, and unfortunately, these results do not corroborate the more reliable qPCR results. An explanation for discrepancy between qPCR and IHC staining in sample 3 and 7 is the fact that the RNA was isolated from a different (N2 stored) part of the tumor, and we cannot exclude some heterogeneity in the tumor specimen. However, pooled data of qPCR results and IHC signaling do support our conclusion that high ABHD6 expression is characteristic for late-onset metastatic disease in SF3B1mut UM. The ABHD6 gene maps to chromosome 3 and the possible involvement of (partial) chromosome 3 deletions could play a role in the ABHD6 deficiency and shorter progression-free survival. However, the log2fold changes are much more than expected from simply losing a copy of the chromosome, nor did we observe a significant change of expression in other chromosome 3 genes such as BAP1. We also detected 34 significantly perturbed splicing aberrations with donor and/or acceptor extensions or shortenings between early- and late-onset metastatic SF3B1mut UM. These findings warrant further research in utilizing such biomarkers to develop classification schemes to distinguish SF3B1mut UM patients with potential early- and late-metastatic onset.
5. Conclusions
This study describes clinical and genetic characteristics of the largest aggregated cohort of SF3B1 mutated UM in literature (n = 146). Patients with SF3B1-mutated UM show early- and late-onset metastatic disease (defined as before or after a follow-up time of 60 months), for which, currently, no biomarkers exist, and for which we identified several promising candidates with potential distinguishing characteristics when implemented within an expression-based classifier. In addition, the largest tumor diameter (LTD) was found to be increased in the early-onset UM. Our results provide more accurate prognostication and targets for future functional studies in an effort to elucidate pathogenesis and clinical stratification of SF3B1-mutated UM.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers14030846/s1, Figure S1: Overview of included cohorts, Figure S2: Overview of whole-transcriptome samples and general sequencing characteristics. (A) Number of included whole-transcriptome samples per cohort (ROMS and TCGA-UVM) and mutational status for BAP1, SF3B1, EIF1AX and SRSF2. (B) Boxplots of general alignment metrics, per cohort, after (re-)alignment against GRCh38 with GENCODE (v30) annotations. Y-axis depicts number of reads in log10-scale per category (x-axis). Per boxplot, the median value is shown (in millions; rounded). (C) Boxplots of the median transcript integrity number (medTIN), this metric provides an overview of the uniformity of the read-coverage over each GENCODE (v30) transcript. In general, medTIN scales according to the quality and sequencing technique of the sample, in which higher numbers represent samples which have a more uniform and higher read-coverage of the transcripts, Figure S3: Principal component analysis of the top 5000 most-variable genes within the analyzed whole-transcriptome cohorts. (A) The upper figure represents an overview of the row-wise variance for each gene (with a row-wide median of at least 2 reads) within the ROMS cohort, the total number of transcripts is indicated within the figure whilst the red dotted horizontal line depicts the border of the 5000 most-variable genes. The middle figure depicts the percentage of variance explained of the first 15 principal components after PCA (using the VST-transformed counts of the top 5000 most-variable genes after batch corrections) whilst lower figure displays the first two principal components. Samples are colored based on their mutational status in BAP1, SF3B1 and EIF1AX whilst males and females are represented by closed and open dots, respectively. SF3B1mut samples with a PFS < 60 months are highlighted by a red asterisk. (B) same as A), except for the TCGA-UVM cohort. (C) Same as (A), except for the combined ROMS and TCGA-UVM cohort for the PFS ≥ 60 and PFS < 60 months SF3B1mut samples. Figure S4: Overview of geneset-enrichment analysis (q ≤ 0.05) between SF3B1mut (n = 12) and SF3B1wt (n = 14) uveal melanoma tissue within the ROMS cohort. Hallmark gene-sets are highlighted in blue. Y-axis depicts normalized enrichments scores (NES) in which NES < 0 means depleted in SF3B1mut samples and vice versa. Genesets that overlap in both ROMS as TCGA cohorts are indicated with an asterisk. Figure S5: Increased levels of ABHD6 can be associated with late metastasizing SF3B1mut UM with late-onset metastatic disease (PFS ≥ 60 months). (A) qPCR performed in triplicates using CHMP2A expression as a normalizer of 10 primary UM of which samples 1–7 are from an independent set whereas samples S15, S13 and S17 were used to validate our RNA-seq in silico results. Asterisk (*) indicates the control sample with a PFS <60 months and pound sign (#) indicates no tumor was present in histological sections. Missing IHC bar charts in samples 1, 3 and 7 indicate zero IHC scores, i.e., no presence of ABHD6. (B) Average results by pooling panel A with corresponding error bars that represent standard deviation. (C) ABHD6 IHC on all SF3B1mut UM samples that were available and stratified for early- and late-onset metastatic disease. Wilcoxon rank sum test was used to evaluate statistical difference of IHC scores shown in boxplots in panel C. p-value < 0.05 was considered statistically significant. (1*-7 and S17): ABHD6 IHC staining of eight primary UM samples which correspond to results in panel A (40x magnification). Figure S6: (A) Volcano-plot of the differential exon usage analysis between SF3B1mut (n = 12) and SF3B1wt (n = 14) uveal melanoma tissue within the ROMS cohort, exonic regions significantly (q ≤ 0.05 and log2 fold change ≥ |0.5|) down-regulated (blue) and up-regulated (red) in SF3B1mut uveal melanoma tissue are shown. Gene names for the top 50 genes (based on descending -log10 q-value) and top 20 (based on |log2 fold change|) for both directions are shown. Genes which were found to be differentially expressed in both cohorts are highlighted by a green circle. The x-axis displays the log2 fold-change and y-axis displays the adjusted p-value (q) shown on a -log10 scale. The total amount of differentially expressed exon usages is shown on top. (B) Same as (A), except for the differential expression analysis between SF3B1mut (n = 15) and SF3B1wt (n = 61) uveal melanoma tissue within the TCGA-UVM cohort. (C) Overlap of differential exon usages between ROMS and TCGA-UVM cohorts within the SF3B1mut and SF3B1wt analysis (performed per cohort). Pink color denotes the number of events (based on genomic location) found to be statistically differentially expressed within both the ROMS and TCGA-UVM cohorts. Figure S7: Unsupervised clustering of the ROMS cohort based upon novel acceptor and donor splicing-aberrations. Unsupervised clustering (Euclidean distances and Ward.D2 method) on the differential exon usages (not found within the GENCODE v30 annotation) between SF3B1mut and SF3B1wt samples in the ROMS cohort. Values are mean-centered read counts represented with a z score of differentially expressed splicing events (blue, low expression; red, high expression). Top bars represent PFS-status (yellow; PFS <60 months and purple; PFS ≥ 60 months) and mutation status. Table S1: overview of included samples and characteristics from all cohorts. Table S2: (A) overview of clinical characteristics of SF3B1mut uveal melanoma from the ten publicly available datasets (non-ROMS) and the ROMS cohort. (B) overview of clinical characteristics of PFS <60 months SF3B1mut uveal melanoma and PFS ≥ 60 months from the ten publicly available datasets. Table S3: Overview of Differential gene expression data SF3B1mut vs. Other. Table S4: Overview of Gene Set Enrichment Analysis data SF3B1mut vs. Other. Table S5: Overview of Differential Expression data SF3B1mut PFS <60 vs. PFS ≥ 60 months. Table S6: Overview of DEXSeq differential expression of ROMS data SF3B1mut vs. Other. Table S7: Overview of DEXSeq differential expression of ROMS data SF3B1mut early vs. SF3B1mut late.
Author Contributions
Conceptualization, W.D., J.v.R., H.J.G.v.d.W. and E.K.; Data curation, W.D. and J.v.R.; Formal analysis, J.v.R., T.B. and H.J.G.v.d.W.; Investigation, W.D., J.Q.N.N., K.N.S. and N.M.v.P.; Methodology, W.D., J.v.R., E.B., H.J.G.v.d.W. and E.K.; Resources, B.E., R.M.V., N.C.N. and D.P.; Software, J.v.R., R.J. and E.M.-S.; Validation, J.V., F.J.M., T.P.P.v.d.B. and R.M.V.; Writing—original draft, W.D. and J.v.R.; Writing—review and editing, A.d.K., H.J.G.v.d.W. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Henkes Foundation [SF3B1-2018], Collaborative Ophthalmic Research Rotterdam [5.2.0 and 5.2.1], Landelijke Stichting voor Blinden en Slechtzienden [2018-4] and Stichting Beheer Het Schild [2018-4].
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Medical ethics committee of the Erasmus Medical Centre (OZR nr 2009-17, MEC-2009-375, 12 November 2009).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data from The Cancer Genome Atlas (TCGA) and repository NCBI GEO Datasets are publicly accessible. The ROMS data are not publicly accessible. Our ethics committee does not allow sharing of individual patient or control genotype information in the public domain.
Acknowledgments
The results shown here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga (accessed on 8 July 2016). Figure 1 was created with BioRender.com accessed on 1 December 2021. The Rotterdam Ocular Melanoma Study Group (ROMS) is a collaborative research group with members from the Rotterdam Eye Hospital, Departments of Ophthalmology, Pathology and Clinical Genetics, of the Erasmus MC, Rotterdam, the Netherlands.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American joint committee on cancer classification of posterior uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology 2013, 120, 2066–2071. [Google Scholar] [CrossRef]
- Prescher, G.; Bornfeld, N.; Hirche, H.; Horsthemke, B.; Jockel, K.H.; Becher, R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar] [PubMed]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of bap1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; van Bodegom, A.; Paridaens, D.; Kilic, E.; de Klein, A.; Rotterdam Ocular Melanoma Study, G. Uveal melanomas with sf3b1 mutations: A distinct subclass associated with late-onset metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [Green Version]
- Dogrusoz, M.; Bagger, M.; van Duinen, S.G.; Kroes, W.G.; Ruivenkamp, C.A.; Bohringer, S.; Andersen, K.K.; Luyten, G.P.; Kiilgaard, J.F.; Jager, M.J. The prognostic value of ajcc staging in uveal melanoma is enhanced by adding chromosome 3 and 8q status. Investig. Ophthalmol. Vis. Sci. 2017, 58, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Furney, S.J.; Pedersen, M.; Gentien, D.; Dumont, A.G.; Rapinat, A.; Desjardins, L.; Turajlic, S.; Piperno-Neumann, S.; de la Grange, P.; Roman-Roman, S.; et al. Sf3b1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013, 3, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsafadi, S.; Houy, A.; Battistella, A.; Popova, T.; Wassef, M.; Henry, E.; Tirode, F.; Constantinou, A.; Piperno-Neumann, S.; Roman-Roman, S.; et al. Cancer-associated sf3b1 mutations affect alternative splicing by promoting alternative branchpoint usage. Nat. Commun. 2016, 7, 10615. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Roberson, E.D.; Anbunathan, H.; Onken, M.D.; Worley, L.A.; Bowcock, A.M. Recurrent mutations at codon 625 of the splicing factor sf3b1 in uveal melanoma. Nat. Genet. 2013, 45, 133–135. [Google Scholar] [CrossRef]
- Martin, M.; Masshofer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; van de Nes, J.; Klein-Hitpass, L.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome sequencing identifies recurrent somatic mutations in eif1ax and sf3b1 in uveal melanoma with disomy 3. Nat. Genet. 2013, 45, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Yavuzyigitoglu, S.; Drabarek, W.; Smit, K.N.; van Poppelen, N.; Koopmans, A.E.; Vaarwater, J.; Brands, T.; Eussen, B.; Dubbink, H.J.; van Riet, J.; et al. Correlation of gene mutation status with copy number profile in uveal melanoma. Ophthalmology 2017, 124, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 2017, 32, 204–220 e215. [Google Scholar] [CrossRef] [Green Version]
- Mazloumi, M.; Vichitvejpaisal, P.; Dalvin, L.A.; Yaghy, A.; Ewens, K.G.; Ganguly, A.; Shields, C.L. Accuracy of the cancer genome atlas classification vs american joint committee on cancer classification for prediction of metastasis in patients with uveal melanoma. JAMA Ophthalmol. 2020, 138, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Martin-Marcos, P.; Zhou, F.; Karunasiri, C.; Zhang, F.; Dong, J.; Nanda, J.; Kulkarni, S.D.; Sen, N.D.; Tamame, M.; Zeschnigk, M.; et al. Eif1a residues implicated in cancer stabilize translation preinitiation complexes and favor suboptimal initiation sites in yeast. Elife 2017, 6. [Google Scholar]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. Cosmic: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [Green Version]
- Darman, R.B.; Seiler, M.; Agrawal, A.A.; Lim, K.H.; Peng, S.; Aird, D.; Bailey, S.L.; Bhavsar, E.B.; Chan, B.; Colla, S.; et al. Cancer-associated sf3b1 hotspot mutations induce cryptic 3’ splice site selection through use of a different branch point. Cell Rep. 2015, 13, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Drabarek, W.; Yavuzyigitoglu, S.; Obulkasim, A.; van Riet, J.; Smit, K.N.; van Poppelen, N.M.; Vaarwater, J.; Brands, T.; Eussen, B.; Verdijk, R.M.; et al. Multi-modality analysis improves survival prediction in enucleated uveal melanoma patients. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3595–3605. [Google Scholar] [CrossRef]
- Royer-Bertrand, B.; Torsello, M.; Rimoldi, D.; El Zaoui, I.; Cisarova, K.; Pescini-Gobert, R.; Raynaud, F.; Zografos, L.; Schalenbourg, A.; Speiser, D.; et al. Comprehensive genetic landscape of uveal melanoma by whole-genome sequencing. Am. J. Hum. Genet. 2016, 99, 1190–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.P.; Kim, I.K.; Esmaeli, B.; Amin-Mansour, A.; Treacy, D.J.; Carter, S.L.; Hodis, E.; Wagle, N.; Seepo, S.; Yu, X.; et al. Systematic genomic and translational efficiency studies of uveal melanoma. PLoS ONE 2017, 12, e0178189. [Google Scholar] [CrossRef] [Green Version]
- Shain, A.H.; Bagger, M.M.; Yu, R.; Chang, D.; Liu, S.; Vemula, S.; Weier, J.F.; Wadt, K.; Heegaard, S.; Bastian, B.C.; et al. The genetic evolution of metastatic uveal melanoma. Nat. Genet. 2019, 51, 1123–1130. [Google Scholar] [CrossRef]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Rodrigues, M.; Mobuchon, L.; Houy, A.; Alsafadi, S.; Baulande, S.; Mariani, O.; Marande, B.; Ait Rais, K.; Van der Kooij, M.K.; Kapiteijn, E.; et al. Evolutionary routes in metastatic uveal melanomas depend on mbd4 alterations. Clin. Cancer Res. 2019, 25, 5513–5524. [Google Scholar] [CrossRef]
- Kivela, T.; Simpson, E.R.; Grossniklaus, H.E.; Jager, M.J.; Singh, A.D.; Caminal, J.M.; Pavlick, A.; Kujala, E.; Coupland, S.E. Uveal melanoma. In Ajcc Cancer Staging Manual, 8th ed.; Amin, M.B., Ed.; Springer: New York, NY, USA, 2017; pp. 805–817. [Google Scholar]
- Szalai, E.; Jiang, Y.; van Poppelen, N.M.; Jager, M.J.; de Klein, A.; Kilic, E.; Grossniklaus, H.E. Association of uveal melanoma metastatic rate with stochastic mutation rate and type of mutation. JAMA Ophthalmol. 2018, 136, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, A.E.; Vaarwater, J.; Paridaens, D.; Naus, N.C.; Kilic, E.; de Klein, A.; Rotterdam Ocular Melanoma Study, g. Patient survival in uveal melanoma is not affected by oncogenic mutations in gnaq and gna11. Br. J. Cancer 2013, 109, 493–496. [Google Scholar] [CrossRef] [Green Version]
- Koopmans, A.E.; Verdijk, R.M.; Brouwer, R.W.; van den Bosch, T.P.; van den Berg, M.M.; Vaarwater, J.; Kockx, C.E.; Paridaens, D.; Naus, N.C.; Nellist, M.; et al. Clinical significance of immunohistochemistry for detection of bap1 mutations in uveal melanoma. Mod. Pathol. 2014, 27, 1321–1330. [Google Scholar] [CrossRef] [Green Version]
- Smit, K.N.; Chang, J.; Derks, K.; Vaarwater, J.; Brands, T.; Verdijk, R.M.; Wiemer, E.A.C.; Mensink, H.W.; Pothof, J.; de Klein, A.; et al. Aberrant microrna expression and its implications for uveal melanoma metastasis. Cancers 2019, 11, 815. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The sequence alignment/map format and samtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Tarasov, A.; Vilella, A.J.; Cuppen, E.; Nijman, I.J.; Prins, P. Sambamba: Fast processing of ngs alignment formats. Bioinformatics 2015, 31, 2032–2034. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Gingeras, T.R. Mapping rna-seq reads with star. Curr. Protoc. Bioinform. 2015, 51, 11.14.1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankish, A.; Diekhans, M.; Ferreira, A.M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. Gencode reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, S.; Li, W. Rseqc: Quality control of rna-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. Featurecounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatiadis, N.; Klaus, B.; Zaugg, J.B.; Huber, W. Data-driven hypothesis weighting increases detection power in genome-scale multiple testing. Nat. Methods 2016, 13, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.; Ibrahim, J.G.; Love, M.I. Heavy-tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Sergushichev, A. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. bioRxiv 2016, 60012, 1–9. [Google Scholar]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Reyes, A.; Huber, W. Detecting differential usage of exons from rna-seq data. Genome Res. 2012, 22, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- van Ipenburg, J.A.; de Waard, N.E.; Naus, N.C.; Jager, M.J.; Paridaens, D.; Verdijk, R.M. Chemokine receptor expression pattern correlates to progression of conjunctival melanocytic lesions. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2950–2957. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Inoue, D.; Chew, G.L.; Liu, B.; Michel, B.C.; Pangallo, J.; D’Avino, A.R.; Hitchman, T.; North, K.; Lee, S.C.; Bitner, L.; et al. Spliceosomal disruption of the non-canonical baf complex in cancer. Nature 2019, 574, 432–436. [Google Scholar] [CrossRef]
- Gruner, B.M.; Schulze, C.J.; Yang, D.; Ogasawara, D.; Dix, M.M.; Rogers, Z.N.; Chuang, C.H.; McFarland, C.D.; Chiou, S.H.; Brown, J.M.; et al. An in vivo multiplexed small-molecule screening platform. Nat. Methods 2016, 13, 883–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Xie, H.; Heier, C.; Huang, J.; Zheng, Q.; Eichmann, T.O.; Schoiswohl, G.; Ni, J.; Zechner, R.; Ni, S.; et al. Enhanced monoacylglycerol lipolysis by abhd6 promotes nsclc pathogenesis. EBioMedicine 2020, 53, 102696. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fei, X.; Xu, J.; Ji, C. An unannotated alpha/beta hydrolase superfamily member, abhd6 differentially expressed among cancer cell lines. Mol. Biol Rep. 2009, 36, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Max, D.; Hesse, M.; Volkmer, I.; Staege, M.S. High expression of the evolutionarily conserved alpha/beta hydrolase domain containing 6 (abhd6) in ewing tumors. Cancer Sci 2009, 100, 2383–2389. [Google Scholar] [CrossRef]
- Fagone, P.; Caltabiano, R.; Russo, A.; Lupo, G.; Anfuso, C.D.; Basile, M.S.; Longo, A.; Nicoletti, F.; De Pasquale, R.; Libra, M.; et al. Identification of novel chemotherapeutic strategies for metastatic uveal melanoma. Sci. Rep. 2017, 7, 44564. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).