Prognostic and Predictive Molecular Markers in Cholangiocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Biomarkers in CCA
3. Prognostic Serum Biomarkers
3.1. Serum Proteins
3.2. Inflammatory Biomarkers
3.3. Circulating Nucleic Acids
3.4. Single-Nucleotide Polymorphisms
3.5. Other Biomarkers with Potentially Prognostic Value
4. Prognostic Tumor Tissue Biomarkers
4.1. Cell Surface Molecules
4.2. Signaling Molecules
4.3. Mucins
4.4. Tumor Stroma and Microenvironment
4.5. Non-Coding RNA
5. Predictive Biomarkers of Treatment Response and Novel Therapeutic Strategies
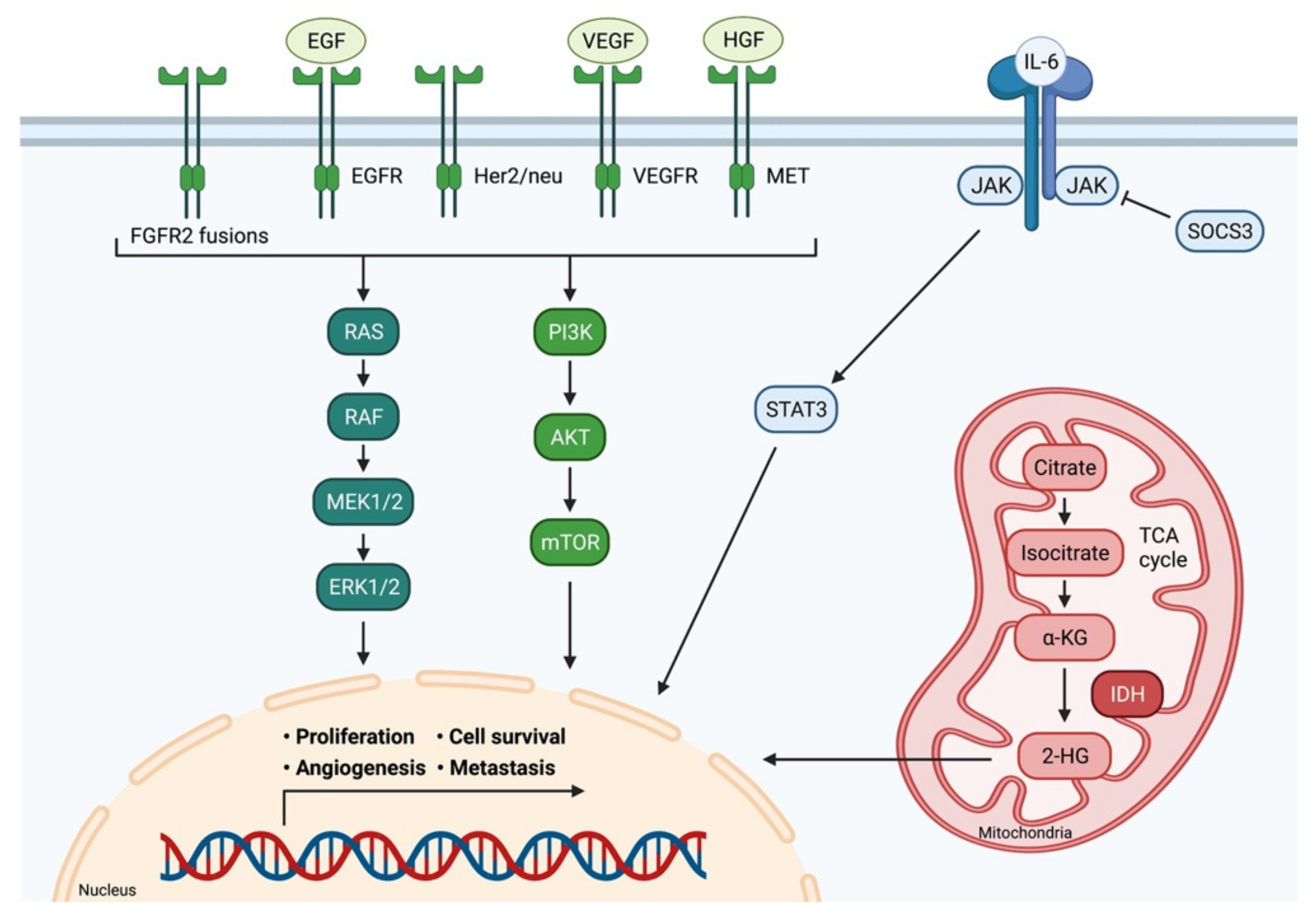
5.1. Fibroblast Growth Factor Receptor
5.2. C-met-Encoded Receptor for Hepatocyte Growth Factor
5.3. Tyrosine Kinases
5.4. Angiogenesis
5.5. Isocitrate Dehydrogenase-1 and -2
5.6. KRAS
5.7. Immunotherapy
6. Conclusions
| Name | Occurrence | Expression | Associated Prognostic Value | Reference |
|---|---|---|---|---|
| Proteins/Cytokines | ||||
| CA19-9 | CCA (all subtypes) | Increased | OS | [31,32,33,34] |
| CEA | mostly iCCA, but also all CCA subtypes | Increased | OS | [36,38,39,40,41,42] |
| CYFRA | iCCA, gallbladder cancer | Increased | OS | [43,158] |
| Osteopontin | CCA (all subtypes) | Increased | OS | [45] |
| iCCA | Low level of circulating osteopontin/volume; Decreased expression in tumor tissue | OS | [46] | |
| Urokinase plasminogen activator receptor (uPAR) | CCA (all subtypes) | Elevated serum levels; Increased expression in tumor tissue | OS | [48] |
| 2-hydroxyglutarate (2-HG) | iCCA | Elevated serum levels | IDH1/2 mutation status, tumor burden | [151] |
| Nardilysin (NRDC) | iCCA | Elevated serum levels and mRNA expression in tumor tissue | OS, DFS | [87] |
| IL-6 | iCCA | Elevated serum levels | DFS | [47] |
| Circulating Nucleic Acids | ||||
| miR-21 | CCA (all subtypes) | Elevated serum levels | OS, clinical staging, metastasis | [159] |
| miR-192 | Liver fluke-associated CCA | Elevated serum levels | OS, lymph node metastasis | [160] |
| miR-106a | CCA | Decreased serum levels | OS, lymph node metastasis | [161] |
| miR-26a | CCA | Elevated serum levels | OS, clinical stage, metastasis, differentiation status | [162] |
| Panel (miR-29, miR-122, miR-155, miR-192 | CCA | Elevated serum levels | OS | [56] |
| Single-Nucleotide Polymorphisms (SNPs) | ||||
| CXCR1 +860 C>G | pCCA | Heterozygous polymorphism | OS, DFS | [65] |
| G protein subunit-β 3 (GNB3) 825 C>T | eCCA | Heterozygous polymorphism | OS | [66] |
| EZH2 rs887569 TT genotype | CCA | Homozygous polymorphism | OS | [163] |
| NRF2 rs6726395 GG genotype | CCA | Homozygous polymorphism | OS | [164] |
| Name | Occurrence | Expression | Associated Prognostic Value | Reference |
|---|---|---|---|---|
| Cell Surface Molecules | ||||
| CD 155 | CCA | Increased | OS, DFS, histological grading, lymph node metastasis | [77] |
| CD44 | Liver fluke-associated CCA | Increased | OS | [78] |
| CD55, CD97 | iCCA | Increased | OS, histological grading, lymph node metastasis, venous invasion | [165] |
| CD98 | CCA | Increased | OS | [166] |
| Signaling Molecules, Growth Pathways, Angiogenesis | ||||
| IL-6 | iCCA | Increased | OS, DFS | [47] |
| IL-17 | iCCA | Increased peritumoral expression | OS, DFS | [47] |
| SOCS3 | CCA | Low intratumoral expression | OS, lymph node metastasis, postoperative disease recurrence | [80] |
| Tumor necrosis factor α-induced protein 3 (TNFAIP3 or A20) | CCA | Increased intratumoral expression | OS, lymph node metastasis, postoperative disease recurrence | [80] |
| RNF43 | iCCA | Low intratumoral expression | OS | [81] |
| LIM and SH3 protein 1 (LASP-1) | CCA | Increased intratumoral expression | OS, tumor size, histological differentiation, lymph node metastasis, TNM stage | [82] |
| B7-H4 | CCA | Increased | OS, histological differentiation, lymph node metastasis, staging, early recurrence of tumor | [83] |
| Hepatoma-derived growth factor (HDGF) | iCCA | Increased | OS, lymph node metastasis, TNM stage | [84] |
| Ki-67, p73 | pCCA | Increased | OS, TNM stage | [85] |
| Sex-determining region Y-box 4 (SOX4) | iCCA | Increased | OS | [86] |
| Sex-determining region Y-box 9 (SOX9) | iCCA | Increased | OS | [86] |
| KRAS | CCA | Increased | OS | [167] |
| TP53 | CCA | Increased | OS | [167] |
| ARID1A | CCA, mostly fluke-associated iCCA | Decreased | OS | [71,72] |
| iCCA | Increased | OS, recurrence rate | [73] | |
| EGFR, MUC1, MUC4, fascin | CCA | Increased | OS | Metanalysis by [74] |
| VEGF, COX-2, GLUT-1, cyclin D1, Ki67 | eCCA | Increased | OS | Metanalysis by [75] |
| p16, p27, E-cadherin | eCCA | Increased | OS | Metanalysis by [75] |
| c-MET | CCA | Increased | OS, DFS | [168] |
| DKK1 | iCCA, pCCA | Increased | OS, lymph-node metastasis | [169,170] |
| BAP1 | CCA | Retained expression | OS, DFS | [25,169,170] |
| Loss of expression | Trend towards improved OS, histological differentiation, lymph-node metastasis | |||
| PBRM1 | CCA | Retained expression | OS, DFS | [29,171] |
| Mucins | ||||
| MUC5AC | Liver fluke-associated iCCA, iCCA | Increased | OS, lymph node metastasis, TNM stage, tumor size | [88,89] |
| MUC4 | CCA | Increased | OS | [172] |
| MUC16 | iCCA | Increased | OS | [173] |
| Tumor Stroma and Microenvironment | ||||
| Epithelial cell adhesion molecule (EpCAM) | iCCA | Increased expression in peritumoral stroma | OS, DFS | [90] |
| Lysil oxidase-like 2 (LOXL2) | iCCA | Increased expression in peritumoral stroma | OS, DFS | [91] |
| Matrix metalloproteinase -9 (MMP-9) | pCCA | Increased tissue expression | OS | [92] |
| Matrix metalloproteinase -11 (MMP-11) | CCA | Increased tissue expression | OS | [93] |
| Non-Coding RNA | ||||
| lncRNA H19 | CCA | Increased tissue expression | OS, DFS, tumor size, TNM stage | [95] |
| lncRNA-PANDRA | CCA | Increased tissue expression | OS, DFS, lymph node metastasis, TNM stage | [96] |
| Panel (miR-675-5p, miR-652-3p and miR-338-3p) | iCCA | Overexpression | OS, DFS | [97] |
| miR-29a | CCA | Overexpression | OS, lymph node metastasis, histological differentiation, clinical staging | [98] |
| miR-21 | Liver fluke-associated iCCA | Overexpression | OS, lymph-node metastasis | [174] |
| miR-92b | CCA | Overexpression | OS | [175] |
| miR-34a | eCCA | Decreased expression | OS, lymph-node metastasis, clinical stage | [176] |
| miR-181a | CCA | Overexpression | OS | [177] |
| miR-191 | iCCA | Overexpression | OS, DFS | [178] |
| miR-203, miR-373 | CCA | Decreased expression | OS, DFS | [178,179] |
| miR-221 | eCCA | Overexpression | DFS | [180] |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Park, R.; Al-Jumayli, M.; Al-Rajabi, R.; Sun, W. Biologics, Immunotherapy, and Future Directions in the Treatment of Advanced Cholangiocarcinoma. Clin. Color. Cancer 2019, 18, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kendall, T.; Verheij, J.; Gaudio, E.; Evert, M.; Guido, M.; Goeppert, B.; Carpino, G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 7–18. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Özdirik, B.; Müller, T.; Wree, A.; Tacke, F.; Sigal, M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci. 2021, 22, 6975. [Google Scholar] [CrossRef] [PubMed]
- Lurje, G.; Bednarsch, J.; Czigany, Z.; Lurje, I.; Schlebusch, I.K.; Boecker, J.; Meister, F.A.; Tacke, F.; Roderburg, C.; Dulk, M.D.; et al. The prognostic role of lymphovascular invasion and lymph node metastasis in perihilar and intrahepatic cholangiocarcinoma. Eur. J. Surg. Oncol. 2019, 45, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Tacke, F.; Strnad, P.; Ulmer, T.F.; Gaisa, N.T.; Bruners, P.; Neumann, U.P.; Lurje, G. Left- versus right-sided hepatectomy with hilar en-bloc resection in perihilar cholangiocarcinoma. HPB 2020, 22, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moustafa, M.; Linecker, M.; Lurje, G.; Capobianco, I.; Baumgart, J.; Ratti, F.; Rauchfuss, F.; Balci, D.; Fernandes, E.; et al. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-Center Study. Ann. Surg. Oncol. 2020, 27, 1372–1384. [Google Scholar] [CrossRef]
- Haber, P.K.; Wabitsch, S.; Kästner, A.; Andreou, A.; Krenzien, F.; Schöning, W.; Pratschke, J.; Schmelzle, M. Laparoscopic Liver Resection for Intrahepatic Cholangiocarcinoma: A Single-Center Experience. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 1354–1359. [Google Scholar] [CrossRef]
- Jonas, S.; Krenzien, F.; Atanasov, G.; Hau, H.-M.; Gawlitza, M.; Moche, M.; Wiltberger, G.; Pratschke, J.; Schmelzle, M. Hilar en bloc resection for hilar cholangiocarcinoma in patients with limited liver capacities—Preserving parts of liver segment 4. Eur. Surg. 2018, 50, 22–29. [Google Scholar] [CrossRef]
- Krenzien, F.; Nevermann, N.; Krombholz, A.; Benzing, C.; Haber, P.; Fehrenbach, U.; Lurje, G.; Pelzer, U.; Pratschke, J.; Schmelzle, M.; et al. Treatment of Intrahepatic Cholangiocarcinoma—A Multidisciplinary Approach. Cancers 2022, 14, 362. [Google Scholar] [CrossRef]
- Lurje, G.; Bednarsch, J.; Roderburg, C.; Trautwein, C.; Neumann, U.P. Aktueller Therapiealgorithmus des intrahepatischen cholangiozellulären Karzinoms. Der Chir. 2018, 89, 858–864. [Google Scholar] [CrossRef]
- Bednarsch, J.; Neumann, U.P.; Lurje, G. Reply to: Does lymphovascular invasion really associate with decreased overall survival for patients with resected cholangiocarcinoma? Eur. J. Surg. Oncol. 2019, 45, 1513–1514. [Google Scholar] [CrossRef]
- Schmelzle, M.; Schöning, W.; Pratschke, J. Chirurgische Therapie maligner Gallengangserkrankungen. Der Chirurg 2019, 91, 3–10. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Amygdalos, I.; Strnad, P.; Halm, P.; Wiltberger, G.; Ulmer, T.F.; Schulze-Hagen, M.; Bruners, P.; et al. Insufficient future liver remnant and preoperative cholangitis predict perioperative outcome in perihilar cholangiocarcinoma. HPB 2020, 23, 99–108. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Strnad, P.; Bruners, P.; Ulmer, T.F.; den Dulk, M.; Lurje, G.; Neumann, U.P. The role of ALPPS in intrahepatic cholangiocarcinoma. Langenbeck’s Arch. Surg. 2019, 404, 885–894. [Google Scholar] [CrossRef]
- Czigany, Z.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation from Donation After Brain Death. Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef]
- Pavicevic, S.; Uluk, D.; Reichelt, S.; Fikatas, P.; Globke, B.; Raschzok, N.; Schmelzle, M.; Öllinger, R.; Schöning, W.; Eurich, D.; et al. Hypothermic oxygenated machine perfusion for extended criteria donor allografts—Preliminary experience with extended organ preservation times in the setting of organ reallocation. Artif. Organs 2021, 46, 306–311. [Google Scholar] [CrossRef]
- Lauterio, A.; De Carlis, R.; Centonze, L.; Buscemi, V.; Incarbone, N.; Vella, I.; De Carlis, L. Current Surgical Management of Peri-Hilar and Intra-Hepatic Cholangiocarcinoma. Cancers 2021, 13, 3657. [Google Scholar] [CrossRef]
- Vibert, E.; Boleslawski, E. Transplantation Versus Resection for Hilar Cholangiocarcinoma. Ann. Surg. 2019, 269, e5–e6. [Google Scholar] [CrossRef]
- Ballman, K.V. Biomarker: Predictive or Prognostic? J. Clin. Oncol. 2015, 33, 3968–3971. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource [Internet]; Food and Drug Administration (US): Silver Spring, MD, USA; National Institutes of Health (US): Bethesda, MD, USA, 2016.
- Czigany, Z.; Lurje, I.; Schmelzle, M.; Schöning, W.; Öllinger, R.; Raschzok, N.; Sauer, I.M.; Tacke, F.; Strnad, P.; Trautwein, C.; et al. Ischemia-Reperfusion Injury in Marginal Liver Grafts and the Role of Hypothermic Machine Perfusion: Molecular Mechanisms and Clinical Implications. J. Clin. Med. 2020, 9, 846. [Google Scholar] [CrossRef]
- Hayes, D.F.; Bast, R.; Desch, C.E.; Fritsche, H.; Kemeny, N.E.; Jessup, J.M.; Locker, G.Y.; Macdonald, J.S.; Mennel, R.G.; Norton, L.; et al. Tumor Marker Utility Grading System: A Framework to Evaluate Clinical Utility of Tumor Markers. J. Natl. Cancer Inst. 1996, 88, 1456–1466. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; ElZawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Lowery, M.; Ptashkin, R.N.; Jordan, E.J.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Nault, J.; Villanueva, A. Biomarkers for Hepatobiliary Cancers. Hepatology 2020, 73, 115–127. [Google Scholar] [CrossRef]
- Churi, C.R.; Shroff, R.; Wang, Y.; Rashid, A.; Kang, H.; Weatherly, J.; Zuo, M.; Zinner, R.; Hong, D.; Meric-Bernstam, F.; et al. Mutation Profiling in Cholangiocarcinoma: Prognostic and Therapeutic Implications. PLoS ONE 2014, 9, e115383. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Bolm, L.; Petrova, E.; Weitz, J.; Rückert, F.; Wittel, U.A.; Makowiec, F.; Lapshyn, H.; Bronsert, P.; Rau, B.M.; Khatkov, I.E.; et al. Prognostic relevance of preoperative bilirubin-adjusted serum carbohydrate antigen 19-9 in a multicenter subset analysis of 179 patients with distal cholangiocarcinoma. HPB 2019, 21, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-K.; Lin, J.-J.; He, G.-H.; Wang, H.; Lu, J.-H.; Yang, G.-S. Preoperative serum CA19-9 levels is an independent prognostic factor in patients with resected hilar cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 7890–7898. [Google Scholar] [PubMed]
- Lee, B.S.; Lee, S.H.; Son, J.H.; Jang, D.K.; Chung, K.H.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T. Prognostic value of CA 19-9 kinetics during gemcitabine-based chemotherapy in patients with advanced cholangiocarcinoma. J. Gastroenterol. Hepatol. 2016, 31, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, L.; He, Q.; Wang, S.; Pan, Z.; Wang, T.; Zhao, Y. Diagnostic Accuracy of Serum CA19-9 in Patients with Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2015, 21, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Koch, A.; Vucur, M.; Schneider, A.T.; Binnebösel, M.; Ulmer, T.F.; Lurje, G.; Schoening, W.; et al. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Sci. Rep. 2017, 7, 16975. [Google Scholar] [CrossRef]
- Fang, T.; Wang, H.; Wang, Y.; Lin, X.; Cui, Y.; Wang, Z. Clinical Significance of Preoperative Serum CEA, CA125, and CA19-9 Levels in Predicting the Resectability of Cholangiocarcinoma. Dis. Markers 2019, 2019, e6016931. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.-J.; Chen, J.; Liu, W.; Li, J.-W.; Jiang, P.; Zhao, X.; Guo, F.; Li, X.-W.; Wang, S.-G.; et al. Application of Joint Detection of AFP, CA19-9, CA125 and CEA in Identification and Diagnosis of Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 3451–3455. [Google Scholar] [CrossRef]
- Qiu, Y.; He, J.; Chen, X.; Huang, P.; Hu, K.; Yan, H. The diagnostic value of five serum tumor markers for patients with cholangiocarcinoma. Clin. Chim. Acta 2018, 480, 186–192. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, L.; Wang, Y.; Ge, R.; Sun, Y.; Wei, G. Survival Outcomes and Prognostic Factors of Surgical Therapy for All Potentially Resectable Intrahepatic Cholangiocarcinoma: A Large Single-Center Cohort Study. J. Gastrointest. Surg. 2014, 18, 562–572. [Google Scholar] [CrossRef]
- Qiang, Z.-Y.; Zhang, W.-H.; Jin, S.; Dai, K.-F.; He, Y.-T.; Tao, L.-Y.; Yu, H.-B. Carcinoembryonic antigen, α-fetoprotein, and Ki67 as biomarkers and prognostic factors in intrahepatic cholangiocarcinoma: A retrospective cohort study. Ann. Hepatol. 2020, 20, 100242. [Google Scholar] [CrossRef]
- Moro, A.; Mehta, R.; Sahara, K.; Tsilimigras, D.I.; Paredes, A.Z.; Farooq, A.; Hyer, J.M.; Endo, I.; Shen, F.; Guglielmi, A.; et al. The Impact of Preoperative CA19-9 and CEA on Outcomes of Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 2888–2901. [Google Scholar] [CrossRef]
- He, C.; Zhang, Y.; Song, Y.; Wang, J.; Xing, K.; Lin, X.; Li, S. Preoperative CEA levels are supplementary to CA19-9 levels in predicting prognosis in patients with resectable intrahepatic cholangiocarcinoma. J. Cancer 2018, 9, 3117–3128. [Google Scholar] [CrossRef]
- Huang, L.; Chen, W.; Liang, P.; Hu, W.; Zhang, K.; Shen, S.; Chen, J.; Zhang, Z.; Chen, B.; Han, Y.; et al. Serum CYFRA 21-1 in Biliary Tract Cancers: A Reliable Biomarker for Gallbladder Carcinoma and Intrahepatic Cholangiocarcinoma. Am. J. Dig. Dis. 2014, 60, 1273–1283. [Google Scholar] [CrossRef]
- Itatsu, K.; Zen, Y.; Ohira, S.; Ishikawa, A.; Sato, Y.; Harada, K.; Ikeda, H.; Sasaki, M.; Nimura, Y.; Nakanuma, Y. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007, 27, 1174–1184. [Google Scholar] [CrossRef]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Pombeiro, I.; Leyh, C.; Benz, F.; Vucur, M.; Longerich, T.; Koch, A.; Braunschweig, T.; et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J. Hepatol. 2017, 67, 749–757. [Google Scholar] [CrossRef]
- Zhou, K.-Q.; Liu, W.-F.; Yang, L.-X.; Sun, Y.-F.; Hu, J.; Chen, F.-Y.; Zhou, C.; Zhang, X.-Y.; Peng, Y.-F.; Yu, L.; et al. Circulating osteopontin per tumor volume as a prognostic biomarker for resectable intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2019, 8, 582–596. [Google Scholar] [CrossRef]
- Asukai, K.; Kawamoto, K.; Eguchi, H.; Konno, M.; Nishida, N.; Koseki, J.; Noguchi, K.; Hasegawa, S.; Ogawa, H.; Yamada, D.; et al. Prognostic Impact of Peritumoral IL-17-Positive Cells and IL-17 Axis in Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2015, 22, 1524–1531. [Google Scholar] [CrossRef]
- Loosen, S.H.; Breuer, A.; Tacke, F.; Kather, J.N.; Gorgulho, J.; Alizai, P.H.; Bednarsch, J.; Roeth, A.A.; Lurje, G.; Schmitz, S.M.; et al. Circulating levels of soluble urokinase plasminogen activator receptor predict outcome after resection of biliary tract cancer. JHEP Rep. 2020, 2, 100080. [Google Scholar] [CrossRef]
- Thummarati, P.; Wijitburaphat, S.; Prasopthum, A.; Menakongka, A.; Sripa, B.; Tohtong, R.; Suthiphongchai, T. High level of urokinase plasminogen activator contributes to cholangiocarcinoma invasion and metastasis. World J. Gastroenterol. 2012, 18, 244–250. [Google Scholar] [CrossRef]
- Grunnet, M.; Christensen, I.; Lassen, U.; Jensen, L.; Lydolph, M.; Lund, I.; Thurison, T.; Høyer-Hansen, G.; Mau-Sørensen, M. Prognostic significance of circulating intact and cleaved forms of urokinase plasminogen activator receptor in inoperable chemotherapy treated cholangiocarcinoma patients. Clin. Biochem. 2014, 47, 599–604. [Google Scholar] [CrossRef]
- Lang, S.A.; Bednarsch, J.; Joechle, K.; Amygdalos, I.; Czigany, Z.; Heij, L.; Ulmer, T.F.; Neumann, U.P. Prognostic biomarkers for cholangiocarcinoma (CCA): State of the art. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 497–510. [Google Scholar] [CrossRef]
- Jing, C.-Y.; Fu, Y.-P.; Shen, H.-J.; Zheng, S.-S.; Lin, J.-J.; Yi, Y.; Huang, J.-L.; Xu, X.; Zhang, J.; Zhou, J.; et al. Albumin to gamma-glutamyltransferase ratio as a prognostic indicator in intrahepatic cholangiocarcinoma after curative resection. Oncotarget 2016, 8, 13293–13303. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, J.; Wu, B.; Chen, J.; Zhu, Z.; Cai, P.; Guo, W.; Gu, Z.; Wang, J.; Huang, S. Diagnostic and prognostic value of microRNAs in cholangiocarcinoma: A systematic review and meta-analysis. Cancer Manag. Res. 2018, 10, 2125–2139. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Yang, S.; Li, X. Identification of microRNAs as biomarkers for cholangiocarcinoma detection: A diagnostic meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 156–162. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, X.; Zhang, Q.; Wang, C.; Zhao, Y. Diagnostic value of microRNAs as biomarkers for cholangiocarcinoma. Dig. Liver Dis. 2016, 48, 1227–1232. [Google Scholar] [CrossRef]
- Loosen, S.H.; Lurje, G.; Wiltberger, G.; Vucur, M.; Koch, A.; Kather, J.N.; Paffenholz, P.; Tacke, F.; Ulmer, F.T.; Trautwein, C.; et al. Serum levels of miR-29, miR-122, miR-155 and miR-192 are elevated in patients with cholangiocarcinoma. PLoS ONE 2019, 14, e0210944. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Salem, P.E.S.; Ghazala, R.A.; El Gendi, A.M.; Emara, D.M.; Ahmed, N.M. The association between circulating MicroRNA-150 level and cholangiocarcinoma. J. Clin. Lab. Anal. 2020, 34, e23397. [Google Scholar] [CrossRef]
- Andersen, R.F.; Jakobsen, A. Screening for circulating RAS/RAF mutations by multiplex digital PCR. Clin. Chim. Acta 2016, 458, 138–143. [Google Scholar] [CrossRef]
- Lurje, G.; Nagashima, F.; Zhang, W.; Yang, D.; Chang, H.M.; Gordon, M.A.; El-Khoueiry, A.; Husain, H.; Wilson, P.M.; Ladner, R.D.; et al. Polymorphisms in cyclooxygenase-2 and epidermal growth factor receptor are associated with progression-free survival independent of K-ras in metastatic colorectal cancer patients treated with single-agent cetuximab. Clin. Cancer Res. 2008, 14, 7884–7895. [Google Scholar] [CrossRef][Green Version]
- Lurje, G.; Zhang, W.; Schultheis, A.M.; Yang, D.; Groshen, S.; Hendifar, A.E.; Husain, H.; Gordon, M.A.; Nagashima, F.; Chang, H.M.; et al. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann. Oncol. 2008, 19, 1734–1741. [Google Scholar] [CrossRef]
- Lurje, G.; Husain, H.; Power, D.; Yang, D.; Groshen, S.; Pohl, A.; Zhang, W.; Ning, Y.; Manegold, P.; El-Khoueiry, A.; et al. Genetic variations in angiogenesis pathway genes associated with clinical outcome in localized gastric adenocarcinoma. Ann. Oncol. 2010, 21, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Lurje, G.; Leers, J.M.; Pohl, A.; Oezcelik, A.; Zhang, W.; Ayazi, S.; Winder, T.; Ning, Y.; Yang, D.; Klipfel, N.E.; et al. Genetic Variations in Angiogenesis Pathway Genes Predict Tumor Recurrence in Localized Adenocarcinoma of the Esophagus. Ann. Surg. 2010, 251, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.V.; Hamesch, K.; Gross, A.; Mandorfer, M.; Moeller, L.S.; Pereira, V.; Pons, M.; Kuca, P.; Reichert, M.C.; Benini, F.; et al. Liver Phenotypes of European Adults Heterozygous or Homozygous for Pi∗Z Variant of AAT (Pi∗MZ vs. Pi∗ZZ genotype) and Noncarriers. Gastroenterology 2020, 159, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Gaisa, N.T.; Dahl, E.; Knüchel, R.; Miller, H.; Ulmer, T.F.; Strnad, P.; Trautwein, C.; et al. Genetic variant of CXCR1 (rs2234671) associates with clinical outcome in perihilar cholangiocarcinoma. Liver Cancer 2022, in press. [CrossRef]
- Fingas, C.D.; Katsounas, A.; Kahraman, A.; Siffert, W.; Jochum, C.; Gerken, G.; Nückel, H.; Canbay, A. Prognostic Assessment of Three Single-Nucleotide Polymorphisms (GNB3825C>T,BCL2-938C>A,MCL1-386C>G) in Extrahepatic Cholangiocarcinoma. Cancer Investig. 2009, 28, 472–478. [Google Scholar] [CrossRef]
- Braconi, C.; Roessler, S.; Kruk, B.; Lammert, F.; Krawczyk, M.; Andersen, J.B. Molecular perturbations in cholangiocarcinoma: Is it time for precision medicine? Liver Int. 2019, 39 (Suppl. S1), 32–42. [Google Scholar] [CrossRef]
- Rhodes, K.; Zhang, W.; Yang, D.; Press, O.; Gordon, M.; Vallbohmer, D.; Schultheis, A.; Lurje, G.; Ladner, R.; Fazzone, W.; et al. ABCB1, SLCO1B1 and UGT1A1 gene polymorphisms are associated with toxicity in metastatic colorectal cancer patients treated with first-line irinotecan. Drug Metab. Lett. 2007, 1, 23–30. [Google Scholar] [CrossRef]
- Lurje, G.; Schiesser, M.; Hoffmann, A.C.; Schneider, P.M. Circulating Tumor Cells in Gastrointestinal Malignancies: Current Techniques and Clinical Implications. J. Oncol. 2009, 2010, e392652. [Google Scholar] [CrossRef]
- Andersen, J.B.; Spee, B.; Blechacz, B.R.; Avital, I.; Komuta, M.; Barbour, A.; Conner, E.A.; Gillen, M.C.; Roskams, T.; Roberts, L.; et al. Genomic and Genetic Characterization of Cholangiocarcinoma Identifies Therapeutic Targets for Tyrosine Kinase Inhibitors. Gastroenterology 2012, 142, 1021–1031.e15. [Google Scholar] [CrossRef]
- Namjan, A.; Techasen, A.; Loilome, W.; Sa-Ngaimwibool, P.; Jusakul, A. ARID1A alterations and their clinical significance in cholangiocarcinoma. PeerJ 2020, 8, e10464. [Google Scholar] [CrossRef]
- Sasaki, M.; Nitta, T.; Sato, Y.; Nakanuma, Y. Loss of ARID1A Expression Presents a Novel Pathway of Carcinogenesis in Biliary Carcinomas. Am. J. Clin. Pathol. 2016, 145, 815–825. [Google Scholar] [CrossRef]
- Bi, C.; Liu, M.; Rong, W.; Wu, F.; Zhang, Y.; Lin, S.; Liu, Y.; Wu, J.; Wang, L. High Beclin-1 and ARID1A expression corelates with poor survival and high recurrence in intrahepatic cholangiocarcinoma: A histopathological retrospective study. BMC Cancer 2019, 19, 213. [Google Scholar] [CrossRef]
- Ruys, A.T.; Koerkamp, B.G.; Wiggers, J.K.; Klümpen, H.-J.; Kate, F.J.T.; Van Gulik, T.M. Prognostic Biomarkers in Patients with Resected Cholangiocarcinoma: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2013, 21, 487–500. [Google Scholar] [CrossRef]
- Jones, R.P.; Bird, N.T.; Smith, R.A.; Palmer, D.H.; Fenwick, S.W.; Poston, G.J.; Malik, H.Z. Prognostic molecular markers in resected extrahepatic biliary tract cancers: A systematic review and meta-analysis of immunohistochemically detected biomarkers. Biomarkers Med. 2015, 9, 763–775. [Google Scholar] [CrossRef]
- Ghidini, M.; Cascione, L.; Carotenuto, P.; Lampis, A.; Trevisani, F.; Previdi, M.C.; Hahne, J.C.; Said-Huntingford, I.; Raj, M.; Zerbi, A.; et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. Eur. J. Cancer 2017, 86, 158–165. [Google Scholar] [CrossRef]
- Huang, D.-W.; Huang, M.; Lin, X.-S.; Huang, Q. CD155 expression and its correlation with clinicopathologic characteristics, angiogenesis, and prognosis in human cholangiocarcinoma. OncoTargets Ther. 2017, ume 10, 3817–3825. [Google Scholar] [CrossRef]
- Thanee, M.; Loilome, W.; Techasen, A.; Sugihara, E.; Okazaki, S.; Abe, S.; Ueda, S.; Masuko, T.; Namwat, N.; Khuntikeo, N.; et al. CD44 variant-dependent redox status regulation in liver fluke-associated cholangiocarcinoma: A target for cholangiocarcinoma treatment. Cancer Sci. 2016, 107, 991–1000. [Google Scholar] [CrossRef]
- Saengboonmee, C.; Sawanyawisuth, K.; Chamgramol, Y.; Wongkham, S. Prognostic biomarkers for cholangiocarcinoma and their clinical implications. Expert Rev. Anticancer Ther. 2018, 18, 579–592. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, M.; Zhou, Q.; Wang, H.; Wang, Z.; Zhong, X.; Zhang, L.; Tai, S.; Cui, Y. The Prognostic Role of SOCS3 and A20 in Human Cholangiocarcinoma. PLoS ONE 2015, 10, e0141165. [Google Scholar] [CrossRef]
- Talabnin, C.; Janthavon, P.; Thongsom, S.; Suginta, W.; Talabnin, K.; Wongkham, S. Ring finger protein 43 expression is associated with genetic alteration status and poor prognosis among patients with intrahepatic cholangiocarcinoma. Hum. Pathol. 2016, 52, 47–54. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Chu, B.; Zhang, F.; Zhang, Y.; Ke, F.; Chen, Y.; Xu, Y.; Liu, S.; Zhao, S.; et al. Upregulated LASP-1 correlates with a malignant phenotype and its potential therapeutic role in human cholangiocarcinoma. Tumor Biol. 2016, 37, 8305–8315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, F.; Li, Z.; Jiang, P.; Deng, X.; Tian, F.; Li, X.; Wang, S. Aberrant expression of B7-H4 correlates with poor prognosis and suppresses tumor-infiltration of CD8+ T lymphocytes in human cholangiocarcinoma. Oncol. Rep. 2016, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, H.-D.; Liu, Y.-F.; Liu, L.; Sun, Q.; Cui, X.-J. Hepatoma-derived growth factor: A novel prognostic biomarker in intrahepatic cholangiocarcinoma. Tumor Biol. 2014, 36, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, B.; Guo, X.; Zhang, X.; Hu, J.; Hu, X.; Lu, Y. Expression of Ki-67, Bax and p73 in patients with hilar cholangiocarcinoma. Cancer Biomark. 2014, 14, 197–202. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Zhan, X.; Lin, T.; Yang, M.; Hu, J.; Han, B.; Hu, S. SOX4 is associated with poor prognosis in cholangiocarcinoma. Biochem. Biophys. Res. Commun. 2014, 452, 614–621. [Google Scholar] [CrossRef]
- Yoh, T.; Hatano, E.; Kasai, Y.; Fuji, H.; Nishi, K.; Toriguchi, K.; Sueoka, H.; Ohno, M.; Seo, S.; Iwaisako, K.; et al. Serum Nardilysin, a Surrogate Marker for Epithelial–Mesenchymal Transition, Predicts Prognosis of Intrahepatic Cholangiocarcinoma after Surgical Resection. Clin. Cancer Res. 2018, 25, 619–628. [Google Scholar] [CrossRef]
- Boonla, C.; Sripa, B.; Thuwajit, P.; Cha-On, U.; Puapairoj, A.; Miwa, M.; Wongkham, S. MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2005, 11, 4939–4946. [Google Scholar] [CrossRef]
- Abe, T.; Amano, H.; Shimamoto, F.; Hattori, M.; Kuroda, S.; Kobayashi, T.; Tashiro, H.; Ohdan, H. Prognostic evaluation of mucin-5AC expression in intrahepatic cholangiocarcinoma, mass-forming type, following hepatectomy. Eur. J. Surg. Oncol. 2015, 41, 1515–1521. [Google Scholar] [CrossRef]
- Sulpice, L.; Rayar, M.; Turlin, B.; Boucher, E.; Bellaud, P.; Desille, M.; Meunier, B.; Clément, B.; Boudjema, K.; Coulouarn, C. Epithelial cell adhesion molecule is a prognosis marker for intrahepatic cholangiocarcinoma. J. Surg. Res. 2014, 192, 117–123. [Google Scholar] [CrossRef]
- Bergeat, D.; Fautrel, A.; Turlin, B.; Merdrignac, A.; Rayar, M.; Boudjema, K.; Coulouarn, C.; Sulpice, L. Impact of stroma LOXL2 overexpression on the prognosis of intrahepatic cholangiocarcinoma. J. Surg. Res. 2016, 203, 441–450. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, C.; Xia, L.; He, Z.; Lu, Z.; Liu, C.; Jia, M.; Wang, J.; Niu, J. High expression of matrix metalloproteinase-9 indicates poor prognosis in human hilar cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 6157–6164. [Google Scholar]
- Tongtawee, T.; Kaewpitoon, S.J.; Loyd, R.; Chanvitan, S.; Leelawat, K.; Praditpol, N.; Jujinda, S.; Kaewpitoon, N. High Expression of Matrix Metalloproteinase-11 indicates Poor Prognosis in Human Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 3697–3701. [Google Scholar] [CrossRef][Green Version]
- Sun, Q.; Li, F.; Sun, F.; Niu, J. Interleukin-8 is a prognostic indicator in human hilar cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 8376–8384. [Google Scholar]
- Xu, Y.; Wang, Z.; Jiang, X.; Cui, Y. Overexpression of long noncoding RNA H19 indicates a poor prognosis for cholangiocarcinoma and promotes cell migration and invasion by affecting epithelial-mesenchymal transition. Biomed. Pharmacother. 2017, 92, 17–23. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, X.; Cui, Y. Upregulated long noncoding RNA PANDAR predicts an unfavorable prognosis and promotes tumorigenesis in cholangiocarcinoma. OncoTargets Ther. 2017, 10, 2873–2883. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Li, S.-H.; Huang, G.-L.; Lin, G.-H.; Shuang, Z.-Y.; Lao, X.-M.; Xu, L.; Lin, X.-J.; Wang, H.-Y.; Li, S.-P. Identification of a novel microRNA signature associated with intrahepatic cholangiocarcinoma (ICC) patient prognosis. BMC Cancer 2015, 15, 64. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, Y. Increased Expression of miR-29a and Its Prognostic Significance in Patients with Cholangiocarcinoma. Oncol. Res. Treat. 2017, 40, 128–132. [Google Scholar] [CrossRef]
- Ikeno, Y.; Seo, S.; Iwaisako, K.; Yoh, T.; Nakamoto, Y.; Fuji, H.; Taura, K.; Okajima, H.; Kaido, T.; Sakaguchi, S.; et al. Preoperative metabolic tumor volume of intrahepatic cholangiocarcinoma measured by 18F-FDG-PET is associated with the KRAS mutation status and prognosis. J. Transl. Med. 2018, 16, 95. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Stein, A.; Arnold, D.; Bridgewater, J.; Goldstein, D.; Jensen, L.H.; Klümpen, H.-J.; Lohse, A.W.; Nashan, B.; Primrose, J.; Schrum, S.; et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial)—A randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015, 15, 564. [Google Scholar] [CrossRef]
- Valle, J.W.; Furuse, J.; Jitlal, M.; Beare, S.; Mizuno, N.; Wasan, H.; Bridgewater, J.; Okusaka, T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2013, 25, 391–398. [Google Scholar] [CrossRef]
- Voss, M.H.; Hierro, C.; Heist, R.S.; Cleary, J.M.; Meric-Bernstam, F.; Tabernero, J.; Janku, F.; Gandhi, L.; Iafrate, A.J.; Borger, D.R.; et al. A Phase I, Open-Label, Multicenter, Dose-escalation Study of the Oral Selective FGFR Inhibitor Debio 1347 in Patients with Advanced Solid Tumors Harboring FGFR Gene Alterations. Clin. Cancer Res. 2019, 25, 2699–2707. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion–Positive Cholangiocarcinoma. Cancer Discov. 2016, 7, 252–263. [Google Scholar] [CrossRef]
- Mertens, J.C.; Rizvi, S.; Gores, G.J. Targeting cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1864, 1454–1460. [Google Scholar] [CrossRef]
- Borad, M.J.; Gores, G.J.; Roberts, L. Fibroblast growth factor receptor 2 fusions as a target for treating cholangiocarcinoma. Curr. Opin. Gastroenterol. 2015, 31, 264–268. [Google Scholar] [CrossRef]
- Saka, H.; Kitagawa, C.; Kogure, Y.; Takahashi, Y.; Fujikawa, K.; Sagawa, T.; Iwasa, S.; Takahashi, N.; Fukao, T.; Tchinou, C.; et al. Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: A Phase I study. Investig. New Drugs 2017, 35, 451–462. [Google Scholar] [CrossRef]
- Bahleda, R.; Italiano, A.; Hierro, C.; Mita, A.; Cervantes, A.; Chan, N.; Awad, M.; Calvo, E.; Moreno, V.; Govindan, R.; et al. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors. Clin. Cancer Res. 2019, 25, 4888–4897. [Google Scholar] [CrossRef]
- Kurzrock, R.; Ball, D.W.; Zahurak, M.L.; Nelkin, B.D.; Subbiah, V.; Ahmed, S.; O’Connor, A.; Karunsena, E.; Parkinson, R.M.; Bishop, J.A.; et al. A Phase I Trial of the VEGF Receptor Tyrosine Kinase Inhibitor Pazopanib in Combination with the MEK Inhibitor Trametinib in Advanced Solid Tumors and Differentiated Thyroid Cancers. Clin. Cancer Res. 2019, 25, 5475–5484. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; He, Y.; Soni, N.; et al. FOENIX-CCA2: A phase II, open-label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J. Clin. Oncol. 2020, 38, 108. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Busset, M.D.D.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2018, 120, 165–171. [Google Scholar] [CrossRef]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients with FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39, 265. [Google Scholar] [CrossRef]
- Javle, M.; Kelley, R.; Roychowdhury, S.; Weiss, K.; Abou-Alfa, G.; Macarulla, T.; Sadeghi, S.; Waldschmidt, D.; Zhu, A.; Goyal, L.; et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann. Oncol. 2018, 29, viii720. [Google Scholar] [CrossRef]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.-Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Futur. Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef]
- Borad, M.J.; Bridgewater, J.A.; Morizane, C.; Shroff, R.T.; Oh, D.-Y.; Moehler, M.H.; Furuse, J.; Benhadji, K.A.; He, H.; Valle, J.W. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). J. Clin. Oncol. 2020, 38, TPS600. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Futur. Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef]
- Terada, T.; Nakanuma, Y.; Sirica, A.E. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum. Pathol. 1998, 29, 175–180. [Google Scholar] [CrossRef]
- Pant, S.; Saleh, M.; Bendell, J.; Infante, J.; Jones, S.; Kurkjian, C.; Moore, K.; Kazakin, J.; Abbadessa, G.; Wang, Y.; et al. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann. Oncol. 2014, 25, 1416–1421. [Google Scholar] [CrossRef]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef]
- Sirica, A.-E. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2008, 14, 7033–7058. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2007, 98, 418–425. [Google Scholar] [CrossRef]
- Lurje, G.; Lenz, H.-J. EGFR Signaling and Drug Discovery. Oncology 2009, 77, 400–410. [Google Scholar] [CrossRef]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.-N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef]
- Lubner, S.J.; Mahoney, M.R.; Kolesar, J.L.; LoConte, N.K.; Kim, G.P.; Pitot, H.C.; Philip, P.A.; Picus, J.; Yong, W.-P.; Horvath, L.; et al. Report of a Multicenter Phase II Trial Testing a Combination of Biweekly Bevacizumab and Daily Erlotinib in Patients with Unresectable Biliary Cancer: A Phase II Consortium Study. J. Clin. Oncol. 2010, 28, 3491–3497. [Google Scholar] [CrossRef]
- Philip, P.A.; Mahoney, M.R.; Allmer, C.; Thomas, J.; Pitot, H.C.; Kim, G.; Donehower, R.C.; Fitch, T.; Picus, J.; Erlichman, C. Phase II Study of Erlotinib in Patients with Advanced Biliary Cancer. J. Clin. Oncol. 2006, 24, 3069–3074. [Google Scholar] [CrossRef]
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Belani, C.; Singh, D.A.; Tanaka, M.; Lenz, H.-J.; Yen, Y.; Kindler, H.L.; Iqbal, S.; Longmate, J.; Mack, P.C.; et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother. Pharmacol. 2009, 64, 777–783. [Google Scholar] [CrossRef]
- Peck, J.; Wei, L.; Zalupski, M.; O’Neil, B.; Calero, M.V.; Bekaii-Saab, T. HER2/neu May Not Be an Interesting Target in Biliary Cancers: Results of an Early Phase II Study with Lapatinib. Oncology 2012, 82, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.H.; Lindebjerg, J.; Ploen, J.; Hansen, T.; Jakobsen, A. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann. Oncol. 2012, 23, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Marino, D.; Cereda, S.; Filippi, R.; Belli, C.; Spadi, R.; Nasti, G.; Montano, M.; Amatu, A.; Aprile, G.; et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-typeKRASadvanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2015, 122, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Ole Larsen, F.; Taksony Solyom Hoegdall, D.; Hoegdall, E.; Nielsen, D. Gemcitabine, capecitabine and oxaliplatin with or without cetuximab in advanced biliary tract carcinoma. Acta Oncol. 2015, 55, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Jang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; Mcnamara, M.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2016, 36, 141–157. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Javle, M.; Churi, C.; Kang, H.; Shroff, R.T.; Janku, F.; Surapaneni, R.; Zuo, M.; Barrera, C.; Alshamsi, H.O.; Krishnan, S.; et al. HER2/neu-directed therapy for biliary tract cancer. J. Hematol. Oncol. 2015, 8, 58. [Google Scholar] [CrossRef]
- Larsen, F.O.; Markussen, A.; Diness, L.V.; Nielsen, D. Efficacy and Safety of Capecitabine, Irinotecan, Gemcitabine, and Bevacizumab as Second-Line Treatment in Advanced Biliary Tract Cancer: A Phase II Study. Oncology 2017, 94, 19–24. [Google Scholar] [CrossRef]
- Zhu, A.X.; Meyerhardt, J.A.; Blaszkowsky, L.S.; Kambadakone, A.R.; Muzikansky, A.; Zheng, H.; Clark, J.W.; Abrams, T.A.; A Chan, J.; Enzinger, P.C.; et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: A phase 2 study. Lancet Oncol. 2009, 11, 48–54. [Google Scholar] [CrossRef]
- Iyer, R.V.; Pokuri, V.K.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.M.; Grande, C.; Saab, T.B. A Multicenter Phase II Study of Gemcitabine, Capecitabine, and Bevacizumab for Locally Advanced or Metastatic Biliary Tract Cancer. Am. J. Clin. Oncol. 2018, 41, 649–655. [Google Scholar] [CrossRef]
- Bengala, C.; Bertolini, F.; Malavasi, N.; Boni, C.; Aitini, E.; Dealis, C.; Zironi, S.; Depenni, R.; Fontana, A.; Del Giovane, C.; et al. Sorafenib in patients with advanced biliary tract carcinoma: A phase II trial. Br. J. Cancer 2009, 102, 68–72. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Rankin, C.J.; Ben-Josef, E.; Lenz, H.-J.; Gold, P.J.; Hamilton, R.D.; Govindarajan, R.; Eng, C.; Blanke, C.D. SWOG 0514: A phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Investig. New Drugs 2011, 30, 1646–1651. [Google Scholar] [CrossRef]
- Lee, J.K.; Capanu, M.; O’Reilly, E.M.; Ma, J.; Chou, J.F.; Shia, J.; Katz, S.; Gansukh, B.; Reidylagunes, D.; Segal, N.H.; et al. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br. J. Cancer 2013, 109, 915–919. [Google Scholar] [CrossRef]
- Moehler, M.; Maderer, A.; Schimanski, C.; Kanzler, S.; Denzer, U.; Kolligs, F.; Ebert, M.; Distelrath, A.; Geissler, M.; Trojan, J.; et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: A double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur. J. Cancer 2014, 50, 3125–3135. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2018, 125, 902–909. [Google Scholar] [CrossRef]
- Kim, R.D.; Sanoff, H.K.; Poklepovic, A.S.; Soares, H.; Kim, J.; Lyu, J.; Liu, Y.; Nixon, A.B.; Kim, D.W. A multi-institutional phase 2 trial of regorafenib in refractory advanced biliary tract cancer. Cancer 2020, 126, 3464–3470. [Google Scholar] [CrossRef]
- Demols, A.; Borbath, I.; Eynde, M.V.D.; Houbiers, G.; Peeters, M.; Marechal, R.; Delaunoit, T.; Goemine, J.-C.; Laurent, S.; Holbrechts, S.; et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN, a randomized, double-blind, phase II trial. Ann. Oncol. 2020, 31, 1169–1177. [Google Scholar] [CrossRef]
- Golub, D.; Iyengar, N.; Dogra, S.; Wong, T.; Bready, D.; Tang, K.; Modrek, A.S.; Placantonakis, D.G. Mutant Isocitrate Dehydrogenase Inhibitors as Targeted Cancer Therapeutics. Front. Oncol. 2019, 9, 417. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Borger, D.R.; Goyal, L.; Yau, T.C.C.; Poon, R.T.; Ancukiewicz, M.; Deshpande, V.; Christiani, D.C.; Liebman, H.M.; Yang, H.; Kim, H.; et al. Circulating Oncometabolite 2-Hydroxyglutarate Is a Potential Surrogate Biomarker in Patients with Isocitrate Dehydrogenase-Mutant Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. 2014, 20, 1884–1890. [Google Scholar] [CrossRef]
- Kim, R.D.; McDonough, S.; El-Khoueiry, A.B.; Bekaii-Saab, T.S.; Stein, S.M.; Sahai, V.; Keogh, G.P.; Kim, E.J.; Baron, A.D.; Siegel, A.B.; et al. Randomised phase II trial (SWOG S1310) of single agent MEK inhibitor trametinib Versus 5-fluorouracil or capecitabine in refractory advanced biliary cancer. Eur. J. Cancer 2020, 130, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.W.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-Institutional Phase II Study of Selumetinib in Patients with Metastatic Biliary Cancers. J. Clin. Oncol. 2011, 29, 2357–2363. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Jinawath, A.; Akiyama, Y.; Sripa, B.; Yuasa, Y. Dual blockade of the Hedgehog and ERK1/2 pathways coordinately decreases proliferation and survival of cholangiocarcinoma cells. J. Cancer Res. Clin. Oncol. 2006, 133, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, T.; Yamazaki, O.; Tanaka, H.; Takemura, S.; Yamamoto, T.; Tanaka, S.; Nishiguchi, S.; Kubo, S. Serum Cytokeratin 19 Fragment (CYFRA21-1) as a Prognostic Factor in Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2007, 15, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Huang, Q.; Jin, Z.-Y.; Xie, F.; Zhu, C.-L.; Liu, Z.; Wang, C. Circulating microRNA-21 as a prognostic, biological marker in cholangiocarcinoma. J. Cancer Res. Ther. 2018, 14, 220. [Google Scholar] [CrossRef]
- Silakit, R.; Loilome, W.; Yongvanit, P.; Chusorn, P.; Techasen, A.; Boonmars, T.; Khuntikeo, N.; Chamadol, N.; Pairojkul, C.; Namwat, N. Circulating miR-192 in liver fluke-associated cholangiocarcinoma patients: A prospective prognostic indicator. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 864–872. [Google Scholar] [CrossRef]
- Cheng, Q.; Feng, F.; Zhu, L.; Zheng, Y.; Luo, X.; Liu, C.; Yi, B.; Jiang, X. Circulating miR-106a is a Novel Prognostic and Lymph Node Metastasis Indicator for Cholangiocarcinoma. Sci. Rep. 2015, 5, 16103. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhang, K.-L.; Zhang, N.; Ma, X.-W.; Yan, S.-W.; Cao, D.-H.; Shi, S.-J. Serum miR-26a as a diagnostic and prognostic biomarker in cholangiocarcinoma. Oncotarget 2015, 6, 18631–18640. [Google Scholar] [CrossRef] [PubMed]
- Paolicchi, E.; Pacetti, P.; Giovannetti, E.; Mambrini, A.; Orlandi, M.; Crea, F.; Romani, A.A.; Tartarini, R.; Danesi, R.; Peters, G.J.; et al. A single nucleotide polymorphism in EZH2 predicts overall survival rate in patients with cholangiocarcinoma. Oncol. Lett. 2013, 6, 1487–1491. [Google Scholar] [CrossRef]
- Khunluck, T.; Kukongviriyapan, V.; Puapairoj, A.; Khuntikeo, N.; Senggunprai, L.; Zeekpudsa, P.; Prawan, A. Association of NRF2 polymorphism with cholangiocarcinoma prognosis in Thai patients. Asian Pac. J. Cancer Prev. 2014, 15, 299–304. [Google Scholar] [CrossRef]
- Meng, Z.-W.; Liu, M.-C.; Hong, H.-J.; Du, Q.; Chen, Y.-L. Expression and prognostic value of soluble CD97 and its ligand CD55 in intrahepatic cholangiocarcinoma. Tumor Biol. 2017, 39, 1010428317694319. [Google Scholar] [CrossRef]
- Kaira, K.; Sunose, Y.; Oriuchi, N.; Kanai, Y.; Takeyoshi, I. CD98 is a promising prognostic biomarker in biliary tract cancer. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 654–657. [Google Scholar] [CrossRef]
- Nepal, C.; O’Rourke, C.J.; Oliveira, D.N.P.; Taranta, A.; Shema, S.; Gautam, P.; Calderaro, J.; Barbour, A.; Raggi, C.; Wennerberg, K.; et al. Genomic perturbations reveal distinct regulatory networks in intrahepatic cholangiocarcinoma. Hepatology 2018, 68, 949–963. [Google Scholar] [CrossRef]
- Miyamoto, M.; Ojima, H.; Iwasaki, M.; Shimizu, H.; Kokubu, A.; Hiraoka, N.; Kosuge, T.; Yoshikawa, D.; Kono, T.; Furukawa, H.; et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br. J. Cancer 2011, 105, 131–138. [Google Scholar] [CrossRef]
- Shi, X.-D.; Yu, X.-H.; Wu, W.-R.; Xu, X.-L.; Wang, J.-Y.; Xu, L.-B.; Zhang, R.; Liu, C. Dickkopf-1 expression is associated with tumorigenity and lymphatic metastasis in human hilar cholangiocarcinoma. Oncotarget 2016, 7, 70378–70387. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Yang, X.-R.; Shen, Q.-J.; Yang, L.-X.; Xu, Y.; Qiu, S.-J.; Sun, Y.-F.; Zhang, X.; Wang, Z.; Zhu, K.; et al. High expression of Dickkopf-related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer 2012, 119, 993–1003. [Google Scholar] [CrossRef]
- Sarcognato, S.; Gringeri, E.; Fassan, M.; Di Giunta, M.; Maffeis, V.; Guzzardo, V.; Cillo, U.; Guido, M. Prognostic role of BAP-1 and PBRM-1 expression in intrahepatic cholangiocarcinoma. Virchows Arch. 2018, 474, 29–37. [Google Scholar] [CrossRef]
- Li, B.; Tang, H.; Zhang, A.; Dong, J. Prognostic Role of Mucin Antigen MUC4 for Cholangiocarcinoma: A Meta-Analysis. PLoS ONE 2016, 11, e0157878. [Google Scholar] [CrossRef]
- Higashi, M.; Yamada, N.; Yokoyama, S.; Kitamoto, S.; Tabata, K.; Koriyama, C.; Batra, S.K.; Yonezawa, S. Pathobiological Implications of MUC16/CA125 Expression in Intrahepatic Cholangiocarcinoma-Mass Forming Type. Pathobiology 2012, 79, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Chusorn, P.; Namwat, N.; Loilome, W.; Techasen, A.; Pairojkul, C.; Khuntikeo, N.; Dechakhamphu, A.; Talabnin, C.; Chan-On, W.; Ong, C.K.; et al. Overexpression of microRNA-21 regulating PDCD4 during tumorigenesis of liver fluke-associated cholangiocarcinoma contributes to tumor growth and metastasis. Tumor Biol. 2013, 34, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.-H.; Zhou, H.-W.; Liu, M.; Sun, J.-Z. The role of miR-92b in cholangiocarcinoma patients. Int. J. Biol. Markers 2018, 33, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Li, G.; Bi, W.; Yang, L.; Yao, L.; Wu, D. microRNA-34a inhibits epithelial mesenchymal transition in human cholangiocarcinoma by targeting Smad4 through transforming growth factor-beta/Smad pathway. BMC Cancer 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, C.; Pan, S.; Liang, Y.; Han, J.; Lan, Y.; Sun, J.; Li, K.; Sun, B.; Yang, G. N-myc downstream-regulated gene 2 inhibits human cholangiocarcinoma progression and is regulated by leukemia inhibitory factor/MicroRNA-181c negative feedback pathway. Hepatology 2016, 64, 1606–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Z.-Q.; Yang, Z.-R.; Tong, D.-N.; Guan, J.; Shi, B.-J.; Nie, J.; Ding, X.-T.; Li, B.; Zhou, G.-W.; et al. MicroRNA-191 acts as a tumor promoter by modulating the TET1-p53 pathway in intrahepatic cholangiocarcinoma. Hepatology 2017, 66, 136–151. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Tian, R.; Sun, H.; Zou, S. miR-373 Negatively Regulates Methyl-CpG-Binding Domain Protein 2 (MBD2) in Hilar Cholangiocarcinoma. Am. J. Dig. Dis. 2010, 56, 1693–1701. [Google Scholar] [CrossRef]
- Li, J.; Yao, L.; Li, G.; Ma, D.; Sun, C.; Gao, S.; Zhang, P.; Gao, F. miR-221 Promotes Epithelial-Mesenchymal Transition through Targeting PTEN and Forms a Positive Feedback Loop with β-catenin/c-Jun Signaling Pathway in Extra-Hepatic Cholangiocarcinoma. PLoS ONE 2015, 10, e0141168. [Google Scholar] [CrossRef]
- Bridgewater, J.; Lopes, A.; Beare, S.; Duggan, M.; Lee, D.; Ricamara, M.; McEntee, D.; Sukumaran, A.; Wasan, H.; Valle, J.W. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: The ABC-04 study. BMC Cancer 2016, 16, 153. [Google Scholar] [CrossRef]
- Ikeda, M.; Ioka, T.; Fukutomi, A.; Morizane, C.; Kasuga, A.; Takahashi, H.; Todaka, A.; Okusaka, T.; Creasy, C.L.; Gorman, S.; et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci. 2017, 109, 215–224. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Goff, L.W.; Cardin, D.B.; Whisenant, J.G.; Du, L.; Koyama, T.; Dahlman, K.B.; Salaria, S.N.; Young, R.T.; Ciombor, K.K.; Gilbert, J.; et al. A phase I trial investigating pulsatile erlotinib in combination with gemcitabine and oxaliplatin in advanced biliary tract cancers. Investig. New Drugs 2016, 35, 95–104. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Ramasubbaiah, R.; Yu, M.; Picus, J.; Bufill, J.A.; Tong, Y.; Coleman, N.; Johnston, E.L.; Currie, C.; Loehrer, P.J. Phase II Trial of Erlotinib and Docetaxel in Advanced and Refractory Hepatocellular and Biliary Cancers: Hoosier Oncology Group GI06-101. Oncologist 2012, 17, 13. [Google Scholar] [CrossRef][Green Version]
- Kim, S.T.; Jang, K.-T.; Lee, S.J.; Jang, H.-L.; Lee, J.; Park, S.H.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Park, J.O. Tumour shrinkage at 6 weeks predicts favorable clinical outcomes in a phase III study of gemcitabine and oxaliplatin with or without erlotinib for advanced biliary tract cancer. BMC Cancer 2015, 15, 530. [Google Scholar] [CrossRef][Green Version]
- Moehler, M.; Maderer, A.; Ehrlich, A.; Foerster, F.; Schad, A.; Nickolay, T.; Ruckes, C.; Weinmann, A.; Sivanathan, V.; Marquardt, J.U.; et al. Safety and efficacy of afatinib as add-on to standard therapy of gemcitabine/cisplatin in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, phase I trial with an extensive biomarker program. BMC Cancer 2019, 19, 55. [Google Scholar] [CrossRef]
- Jeong, H.; Jeong, J.; Kim, K.-P.; Lee, S.; Oh, D.; Park, D.; Song, T.; Park, Y.; Hong, S.-M.; Ryoo, B.-Y.; et al. Feasibility of HER2-Targeted Therapy in Advanced Biliary Tract Cancer: A Prospective Pilot Study of Trastuzumab Biosimilar in Combination with Gemcitabine Plus Cisplatin. Cancers 2021, 13, 161. [Google Scholar] [CrossRef]
- Arkenau, H.-T.; Martin-Liberal, J.; Calvo, E.; Penel, N.; Krebs, M.G.; Herbst, R.S.; Walgren, R.A.; Widau, R.C.; Mi, G.; Jin, J.; et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist 2018, 23, 1407-e136. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, H.; Hao, M.; Zhou, Y.; Chen, Q.; Chen, Z. Efficacy and Safety of Apatinib in Treatment of Unresectable Intrahepatic Cholangiocarcinoma: An Observational Study. Cancer Manag. Res. 2020, 12, 5345–5351. [Google Scholar] [CrossRef]
- Zhang, G.; Gong, S.; Pang, L.; Hou, L.; He, W. Efficacy and Safety of Apatinib Treatment for Advanced Cholangiocarcinoma After Failed Gemcitabine-Based Chemotherapy: An Open-Label Phase II Prospective Study. Front. Oncol. 2021, 11, 3965. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Long, J.; Lin, J.; Mao, J.; Xie, F.; Wang, Y.; Wang, Y.; Xun, Z.; Bai, Y.; et al. The Efficacy and Safety of Apatinib Plus Camrelizumab in Patients with Previously Treated Advanced Biliary Tract Cancer: A Prospective Clinical Study. Front. Oncol. 2021, 11, e646979. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, J.; Aravantinos, G.; Koliou, G.-A.; Pentheroudakis, G.; Zagouri, F.; Psyrri, A.; Lampropoulou, D.I.; Demiri, S.; Pectasides, D.; Razis, E.; et al. First Line Gemcitabine/Pazopanib in Locally Advanced and/or Metastatic Biliary Tract Carcinoma. A Hellenic Cooperative Oncology Group Phase II Study. Anticancer Res. 2020, 40, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Gebbia, V.; Pressiani, T.; Testa, A.; Personeni, N.; Bajardi, E.A.; Foa, P.; Buonadonna, A.; Bencardino, K.; Barone, C.; et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: The VanGogh study. Ann. Oncol. 2014, 26, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Assenat, E.; Portales, F.; Ychou, M. A multicenter phase ib-IIR trial assessing activity of regorafenib in combination with modified gemcitabine-oxaliplatin (mGEMOX) in patients with advanced biliary tract cancer (aBTC). J. Clin. Oncol. 2018, 36, 427. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Sun, H.; Bai, C.; Jia, R.; Li, Y.; Zhang, W.; Liu, L.; Huang, C.; Guan, M.; et al. A single-arm, multicenter, open-label phase 2 trial of surufatinib in patients with unresectable or metastatic biliary tract cancer. Cancer 2021, 127, 3975–3984. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Sasaki, T.; Nagashima, F.; Mizuno, N.; Shimizu, S.; Ikezawa, H.; Hayata, N.; Nakajima, R.; Morizane, C. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: Primary analysis results. BMC Cancer 2020, 20, v246. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Yamamoto, N.; Naito, Y.; Kuboki, Y.; Koyama, T.; Piao, Y.; Tsujimoto, N.; Asou, H.; Inoue, K.; Kondo, S. Merestinib monotherapy or in combination for japanese patients with advanced and/or metastatic cancer: A phase 1 study. Cancer Med. 2021, 10, 6579–6589. [Google Scholar] [CrossRef] [PubMed]
- Hack, S.P.; Verret, W.; Mulla, S.; Liu, B.; Wang, Y.; Macarulla, T.; Ren, Z.; El-Khoueiry, A.B.; Zhu, A.X. IMbrave 151: A randomized phase II trial of atezolizumab combined with bevacizumab and chemotherapy in patients with advanced biliary tract cancer. Ther. Adv. Med Oncol. 2021, 13, 17588359211036544. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavicevic, S.; Reichelt, S.; Uluk, D.; Lurje, I.; Engelmann, C.; Modest, D.P.; Pelzer, U.; Krenzien, F.; Raschzok, N.; Benzing, C.; et al. Prognostic and Predictive Molecular Markers in Cholangiocarcinoma. Cancers 2022, 14, 1026. https://doi.org/10.3390/cancers14041026
Pavicevic S, Reichelt S, Uluk D, Lurje I, Engelmann C, Modest DP, Pelzer U, Krenzien F, Raschzok N, Benzing C, et al. Prognostic and Predictive Molecular Markers in Cholangiocarcinoma. Cancers. 2022; 14(4):1026. https://doi.org/10.3390/cancers14041026
Chicago/Turabian StylePavicevic, Sandra, Sophie Reichelt, Deniz Uluk, Isabella Lurje, Cornelius Engelmann, Dominik P. Modest, Uwe Pelzer, Felix Krenzien, Nathanael Raschzok, Christian Benzing, and et al. 2022. "Prognostic and Predictive Molecular Markers in Cholangiocarcinoma" Cancers 14, no. 4: 1026. https://doi.org/10.3390/cancers14041026
APA StylePavicevic, S., Reichelt, S., Uluk, D., Lurje, I., Engelmann, C., Modest, D. P., Pelzer, U., Krenzien, F., Raschzok, N., Benzing, C., Sauer, I. M., Stintzing, S., Tacke, F., Schöning, W., Schmelzle, M., Pratschke, J., & Lurje, G. (2022). Prognostic and Predictive Molecular Markers in Cholangiocarcinoma. Cancers, 14(4), 1026. https://doi.org/10.3390/cancers14041026









