Virtual Clinical Trials in 2D and 3D X-ray Breast Imaging and Dosimetry: Comparison of CPU-Based and GPU-Based Monte Carlo Codes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Monte Carlo Platforms
2.1.1. Geant4—Agata (U Naples)
2.1.2. XRMC (U Cagliari)
2.1.3. gCTD (U Texas)
2.2. Case Studies
2.2.1. Flat Field
2.2.2. Breast Object—Planar Imaging and Dosimetry
2.2.3. Uniform Cylinder—CT Imaging and Dosimetry
2.2.4. Virtual Clinical Phantom—Dosimetry
3. Results
3.1. Flat Field
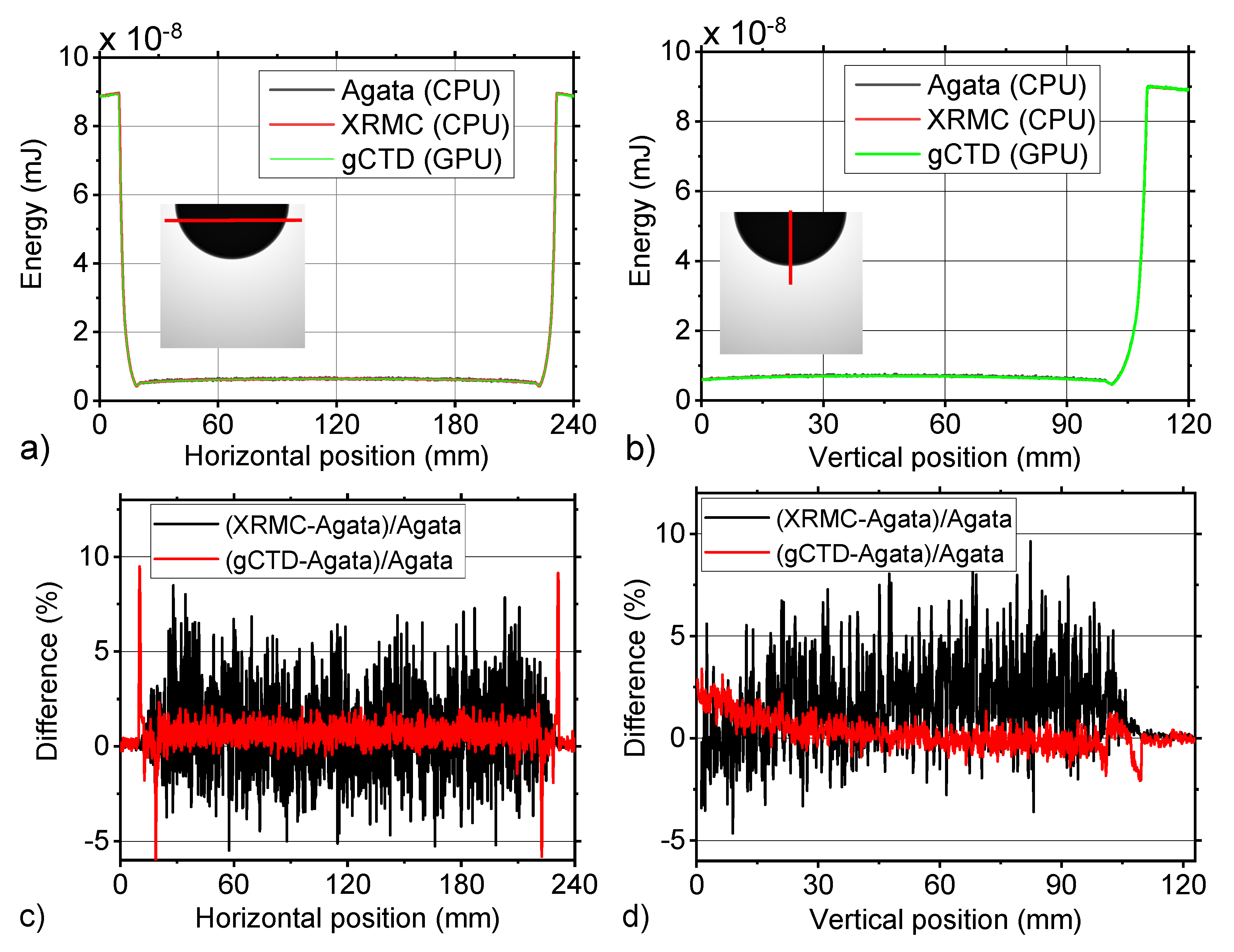
3.2. Breast Object
3.3. Uniform Cylinder—CT Dose
3.4. Anthropomorphic 3D Digital Breast Phantom
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Franco, F.; Sarno, A.; Mettivier, G.; Hernandez, A.; Bliznakova, K.; Boone, J.; Russo, P. GEANT4 Monte Carlo simulations for virtual clinical trials in breast X-ray imaging: Proof of concept. Phys. Med. 2020, 74, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sarno, A.; Mettivier, G.; di Franco, F.; Varallo, A.; Bliznakova, K.; Hernandez, A.M.; Boone, J.M.; Russo, P. Dataset of patient-derived digital breast phantoms for in-silico studies in breast computed tomography, digital breast tomosynthesis, and digital mammography. Med Phys. 2021, 48, 2682–2693. [Google Scholar] [CrossRef] [PubMed]
- Boita, J.; Mackenzie, A.; Sechopoulos, I. Validation of a Method to Simulate the Acquisition of Mammographic Images with Different Techniques. In Proceedings of the Medical Imaging 2019: Physics of Medical Imaging, San Diego, CA, USA, 16–21 February 2019; Volume 10948. [Google Scholar]
- Bakic, P.R.; Barufaldi, B.; Higginbotham, D.; Weinstein, S.P.; Avanaki, A.N.; Espig, K.S.; Xthona, A.; Kimpe, T.R.L.; Maidment, A.D.A. Virtual clinical trial of lesion detection in digital mammography and digital breast tomosynthesis. In Proceedings of the Medical Imaging 2018: Physics of Medical Imaging, Houston, TX, USA, 10–15 February 2018; Volume 10573, p. 1057306. [Google Scholar]
- Baneva, Y.; Bliznakova, K.; Cockmartin, L.; Marinov, S.; Buliev, I.; Mettivier, G.; Bosmans, H.; Russo, P.; Marshall, N.; Bliznakov, Z. Evaluation of a breast software model for 2D and 3D X-ray imaging studies of the breast. Phys. Med. 2017, 41, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, P.; Warren, L.M.; Mackenzie, A.; Rashidnasab, A.; Diaz, O.; Dance, D.R.; Young, K.C.; Bosmans, H.; Strudley, C.J.; Wells, K. Development and validation of a modelling framework for simulating 2D-mammography and breast tomosynthesis images. Phys. Med. Biol. 2014, 59, 4275–4293. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bourland, J.D. Analytical Calculation of the Compton Single Scatter Component of Pencil Beam Scatter Kernel for Scatter Correction in kV Cone Beam CT (kV-CBCT). Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2018, 7, 214–230. [Google Scholar] [CrossRef][Green Version]
- Badano, A.; Graff, C.G.; Badal, A.; Sharma, D.; Zeng, R.; Samuelson, F.W.; Glick, S.J.; Myers, K.J. Evaluation of Digital Breast Tomosynthesis as Replacement of Full-Field Digital Mammography Using an In-silico Imaging Trial. JAMA Netw. Open 2018, 1, e185474. [Google Scholar] [CrossRef]
- Sarno, A.; Mettivier, G.; Tucciariello, R.M.; Bliznakova, K.; Boone, J.M.; Sechopoulos, I.; di Lillo, F.; Russo, P. Monte Carlo evaluation of glandular dose in cone-beam X-ray computed tomography dedicated to the breast: Homogeneous and heterogeneous breast models. Phys. Med. 2018, 51, 99–107. [Google Scholar] [CrossRef]
- Barufaldi, B.; Bakic, P.R.; Higginbotham, D.; Maidment, A.D.A. OpenVCT: A GPU-accelerated virtual clinical trial pipeline for mammography and digital breast tomosynthesis. In Proceedings of the Medical Imaging 2018: Physics of Medical Imaging, Houston, TX, USA, 10–15 February 2018; Volume 10573, p. 1057358. [Google Scholar]
- Jia, X.; Yan, H.; Gu, X.; Jiang, S.B. Fast Monte Carlo simulation for patient-specific CT/CBCT imaging dose calculation. Phys. Med. Biol. 2012, 57, 577–590. [Google Scholar] [CrossRef]
- Jia, X.; Yan, H.; Cervino, L.; Folkerts, M.; Jiang, S.B. A GPU tool for efficient, accurate, and realistic simulation of cone beam CT projections. Med. Phys. 2012, 39, 7368–7378. [Google Scholar] [CrossRef]
- Massera, R.T.; Thomson, R.M.; Tomal, A. Technical note: MC-GPU breast dosimetry validations with other Monte Carlo codes and phase space file implementation. Med. Phys. 2021, 49, 244–253. [Google Scholar] [CrossRef]
- Sharma, D.; Badal, A.; Badano, A. HybridMANTIS: A CPU–GPU Monte Carlo method for modeling indirect X-ray detectors with columnar scintillators. Phys. Med. Biol. 2012, 57, 2357–2372. [Google Scholar] [CrossRef] [PubMed]
- Badal, A.; Badano, A. Accelerating Monte Carlo simulations of photon transport in a voxelized geometry using a massively parallel graphics processing unit. Med. Phys. 2009, 36, 4878–4880. [Google Scholar] [CrossRef] [PubMed]
- Sisniega, A.; Zbijewski, W.; Badal, A.; Kyprianou, I.S.; Stayman, J.W.; Vaquero, J.J.; Siewerdsen, J.H. Monte Carlo study of the effects of system geometry and antiscatter grids on cone-beam CT scatter distributions. Med. Phys. 2013, 40, 051915. [Google Scholar] [CrossRef] [PubMed]
- Fedon, C.; Longo, F.M.; Mettivier, G.; Longo, R. GEANT4 for breast dosimetry: Parameters optimization study. Phys. Med. Biol. 2015, 60, N311–N323. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. Geant4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Hadjipanteli, A.; Elangovan, P.; Mackenzie, A.; Looney, P.T.; Wells, K.; Dance, D.R.; Young, K.C. The effect of system geometry and dose on the threshold detectable calcification diameter in 2D-mammography and digital breast tomosynthesis. Phys. Med. Biol. 2017, 62, 858–877. [Google Scholar] [CrossRef]
- Sarno, A.; Mettivier, G.; di Lillo, F.; Russo, P. A Monte Carlo study of monoenergetic and polyenergetic normalized glandular dose (DgN) coefficients in mammography. Phys. Med. Biol. 2017, 62, 306. [Google Scholar] [CrossRef]
- Masi, M.; di Lillo, F.; Sarno, A.; Mettivier, G.; Castriconi, R.; Russo, P. Volume Dose Distribution in Digital Breast Tomosyn-thesis: A Phantom Study. In Proceedings of the 2016 IEEE Nuclear Science Symposium, Medical Imaging Conference and Room-Temperature Semiconductor Detector Workshop NSS/MIC/RTSD, Strasbourg, France, 29 October–6 November 2016. [Google Scholar]
- Sarno, A.; Tucciariello, R.M.; Mettivier, G.; di Franco, F.; Russo, P. Monte Carlo calculation of monoenergetic and polyenergetic DgN coefficients for mean glandular dose estimates in mammography using a homogeneous breast model. Phys. Med. Biol. 2019, 64, 125012. [Google Scholar] [CrossRef]
- Sarno, A.; Mettivier, G.; di Lillo, F.; Russo, P. Monte Carlo Evaluation of Normalized Glandular Dose Coefficients in Mammography. Hybrid Learn. Educ. 2016, 9699, 190–196. [Google Scholar]
- Golosio, B.; Schoonjans, T.; Brunetti, A.; Oliva, P.; Masala, G.L. Monte Carlo simulation of X-ray imaging and spectroscopy experiments using quadric geometry and variance reduction techniques. Comput. Phys. Commun. 2014, 185, 1044–1052. [Google Scholar] [CrossRef]
- Golosio, B.; Delogu, P.; Zanette, I.; Oliva, P.; Stefanini, A.; Stegel, G.; Carpinelli, M. Visibility of tumor-like details in inline phase contrast mammography using quasimonochromatic X-ray sources. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 608, S66–S69. [Google Scholar] [CrossRef]
- Oliva, P.; Golosio, B.; Stumbo, S.; Carpinelli, M. Advantages of quasi-monochromatic X-ray sources in absorption mammography. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 608, S106–S108. [Google Scholar] [CrossRef]
- Oliva, P.; Golosio, B.; Stumbo, S.; Bravin, A.; Tomassini, P. Compact X-ray sources for mammographic applications: Monte Carlo simulations of image quality. Med. Phys. 2009, 36, 5149–5161. [Google Scholar] [CrossRef] [PubMed]
- Oliva, P.; Bacci, A.; Bottigli, U.; Carpinelli, M.; Delogu, P.; Ferrario, M.; Giulietti, D.; Golosio, B.; Petrillo, V.; Serafini, L.; et al. Start-to-end simulation of a Thomson source for mammography. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 615, 93–99. [Google Scholar] [CrossRef]
- Bottigli, U.; Golosio, B.; Masala, G.; Oliva, P.; Stumbo, S.; Bravin, A.; Bacci, A.; Serafini, L.; Maroli, C.; Petrillo, V.; et al. Effect of different spectral distributions to image a contrast detail phantom in the mammography energy range. Nuovo Cim. Soc. Ital. Fis. C 2006, 29, 215–228. [Google Scholar]
- Golosio, B.; Endrizzi, M.; Oliva, P.; Delogu, P.; Carpinelli, M.; Pogorelsky, I.; Yakimenko, V. Measurement of an inverse Compton scattering source local spectrum using k-edge filters. Appl. Phys. Lett. 2012, 100, 164104. [Google Scholar] [CrossRef]
- Oliva, P.; Carpinelli, M.; Golosio, B.; Delogu, P.; Endrizzi, M.; Park, J.; Pogorelsky, I.; Yakimenko, V.; Williams, O.; Rosenzweig, J. Quantitative evaluation of single-shot inline phase contrast imaging using an inverse compton X-ray source. Appl. Phys. Lett. 2010, 97, 134104. [Google Scholar] [CrossRef]
- Vincze, L.; Janssens, K.; Adams, F. A general Monte Carlo simulation of energy-dispersive X-ray fluorescence spectrometers—I: Unpolarized radiation, homogeneous samples. Spectrochim. Acta Part B At. Spectrosc. 1993, 48, 553–573. [Google Scholar] [CrossRef]
- Vincze, L.; Janssens, K.; Vekemans, B.; Adams, F. Monte Carlo simulation of X-ray fluorescence spectra: Part 4. Photon scattering at high X-ray energies. Spectrochim. Acta Part B At. Spectrosc. 1999, 54, 1711–1722. [Google Scholar] [CrossRef]
- Vincze, L.; Janssens, K.; Adams, F.; Rivers, M.; Jones, K. A general Monte Carlo simulation of ED-XRF spectrometers. II: Polarized monochromatic radiation, homogeneous samples. Spectrochim. Acta Part B At. Spectrosc. 1995, 50, 127–147. [Google Scholar] [CrossRef]
- Bottigli, U.; Brunetti, A.; Golosio, B.; Oliva, P.; Stumbo, S.; Vincze, L.; Randaccio, P.; Bleuet, P.; Simionovici, A.; Somogyi, A. Voxel-based Monte Carlo simulation of X-ray imaging and spectroscopy experiments. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 1747–1754. [Google Scholar] [CrossRef]
- Boone, J.M.; Nelson, T.R.; Lindfors, K.K.; Seibert, J.A. Dedicated Breast CT: Radiation Dose and Image Quality Evaluation. Radiology 2001, 221, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, E.; Murphy, T.; Hemmings, P.; Longworth, S. Techniques Used in the GEM Code for Monte Carlo Neutronics Calculation. In Applications of Computing Methods to Reactors Problems; Argonne National Laboratory: Argonne, IL, USA, 1965; pp. 557–579. [Google Scholar]
- Hernandez, A.M.; Seibert, J.A.; Nosratieh, A.; Boone, J.M. Generation and analysis of clinically relevant breast imaging X-ray spectra. Med. Phys. 2017, 44, 2148–2160. [Google Scholar] [CrossRef]
- Low, D.A.; Harms, W.B.; Mutic, S.; Purdy, J.A. A technique for the quantitative evaluation of dose distributions. Med. Phys. 1998, 25, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.M. Glandular Breast Dose for Monoenergetic and High-Energy X-ray Beams: Monte Carlo Assessment. Radiology 1999, 213, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Van Aarle, W.; Palenstijn, W.J.; Cant, J.; Janssens, E.; Bleichrodt, F.; Dabravolski, A.; de Beenhouwer, J.; Batenburg, K.J.; Sijbers, J. Fast and flexible X-ray tomography using the ASTRA toolbox. Opt. Express 2016, 24, 25129–25147. [Google Scholar] [CrossRef]
- Badano, A.; Sempau, J. MANTIS: Combined X-ray, electron and optical Monte Carlo simulations of indirect radiation imaging systems. Phys. Med. Biol. 2006, 51, 1545–1561. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.A.; Dance, D.R.; Bakic, P.R.; Maidment, A.D.A.; Sandborg, M.; Ullman, G.; Carlsson, G.A. Calculation of the properties of digital mammograms using a computer simulation. Radiat. Prot. Dosim. 2005, 114, 395–398. [Google Scholar] [CrossRef]
- Delis, H.; Spyrou, G.; Panayiotakis, G.; Tzanakos, G. DOSIS: A Monte Carlo simulation program for dose related studies in mammography. Eur. J. Radiol. 2005, 54, 371–376. [Google Scholar] [CrossRef]
- Ullman, G.; Dance, D.R.; Sandborg, M.; Carlsson, G.A.; Svalkvist, A.; Båth, M. A Monte Carlo-based model for simulation of digital chest tomosynthesis. Radiat. Prot. Dosim. 2010, 139, 159–163. [Google Scholar] [CrossRef]
- Svalkvist, A.; Månsson, L.G.; Båth, M. Monte Carlo simulations of the dosimetry of chest tomosynthesis. Radiat. Prot. Dosim. 2010, 139, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sabol, J.M. A Monte Carlo estimation of effective dose in chest tomosynthesis. Med. Phys. 2009, 36, 5480–5487. [Google Scholar] [CrossRef]
- Sechopoulos, I.; Suryanarayanan, S.; Vedantham, S.; D’Orsi, C.; Karellas, A. Computation of the glandular radiation dose in digital tomosynthesis of the breast. Med. Phys. 2006, 34, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Bakic, P.R.; Barufaldi, B.; Pokrajac, D.D.; Lago, M.A.; Maidment, A.D.A. Developing populations of software breast phantoms for virtual clinical trials. In Proceedings of the 14th International Workshop on Breast Imaging (IWBI 2018), Atlanta, GA, USA, 8–11 July 2018; Volume 10718, p. 107181U. [Google Scholar]
- Borges, L.R.; Barufaldi, B.; Caron, R.F.; Bakic, P.R.; Foi, A.; Maidment, A.D.A.; Vieira, M.A.C. Technical Note: Noise models for virtual clinical trials of digital breast tomosynthesis. Med. Phys. 2019, 46, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanteli, A.; Elangovan, P.; Mackenzie, A.; Wells, K.; Dance, D.R.; Young, K.C. The threshold detectable mass diameter for 2D-mammography and digital breast tomosynthesis. Phys. Med. 2019, 57, 25–32. [Google Scholar] [CrossRef]
- Segars, W.P.; Mahesh, M.; Beck, T.J.; Frey, E.C.; Tsui, B.M.W. Realistic CT simulation using the 4D XCAT phantom. Med. Phys. 2008, 35, 3800–3808. [Google Scholar] [CrossRef]
- Fung, G.S.K.; Stierstorfer, K.; Segars, W.P.; Taguchi, K.; Flohr, T.G.; Tsui, B.M.W. XCAT/DRASIM: A realistic CT/human-model simulation package. In Proceedings of the Medical Imaging 2011: Physics of Medical Imaging, Lake Buena Vista, FL, USA, 12–17 February 2011; p. 79613D. [Google Scholar]
- Jia, X.; Gu, X.; Graves, Y.J.; Folkerts, M.; Jiang, S.B. GPU-based fast Monte Carlo simulation for radiotherapy dose calculation. Phys. Med. Biol. 2011, 56, 7017–7031. [Google Scholar] [CrossRef]








| Software Code | Computer Hardware | Computing Time (s/109 photons) | Computing Time (s/109 photons/mm3) |
|---|---|---|---|
| Agata (Geant4 v10.6 patch 01) (CPU-based) | 2 x AMD EPYC 7281, 2.2 GHz, 32-Core Processors, 256 GB RAM | 2300 | 2.816 × 10−3 |
| XRMC (CPU-based) | Intel Core i9-9700K 8-Core Processors, 3.6 GHz | 1100 | 1.347 × 10−3 |
| gCTD (GPU-based) | NVIDIA GeForce RTXTM 3090 | 1 | 1.224 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mettivier, G.; Sarno, A.; Lai, Y.; Golosio, B.; Fanti, V.; Italiano, M.E.; Jia, X.; Russo, P. Virtual Clinical Trials in 2D and 3D X-ray Breast Imaging and Dosimetry: Comparison of CPU-Based and GPU-Based Monte Carlo Codes. Cancers 2022, 14, 1027. https://doi.org/10.3390/cancers14041027
Mettivier G, Sarno A, Lai Y, Golosio B, Fanti V, Italiano ME, Jia X, Russo P. Virtual Clinical Trials in 2D and 3D X-ray Breast Imaging and Dosimetry: Comparison of CPU-Based and GPU-Based Monte Carlo Codes. Cancers. 2022; 14(4):1027. https://doi.org/10.3390/cancers14041027
Chicago/Turabian StyleMettivier, Giovanni, Antonio Sarno, Youfang Lai, Bruno Golosio, Viviana Fanti, Maria Elena Italiano, Xun Jia, and Paolo Russo. 2022. "Virtual Clinical Trials in 2D and 3D X-ray Breast Imaging and Dosimetry: Comparison of CPU-Based and GPU-Based Monte Carlo Codes" Cancers 14, no. 4: 1027. https://doi.org/10.3390/cancers14041027
APA StyleMettivier, G., Sarno, A., Lai, Y., Golosio, B., Fanti, V., Italiano, M. E., Jia, X., & Russo, P. (2022). Virtual Clinical Trials in 2D and 3D X-ray Breast Imaging and Dosimetry: Comparison of CPU-Based and GPU-Based Monte Carlo Codes. Cancers, 14(4), 1027. https://doi.org/10.3390/cancers14041027








