Plasma Gelsolin Confers Chemoresistance in Ovarian Cancer by Resetting the Relative Abundance and Function of Macrophage Subtypes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients’ Characteristics
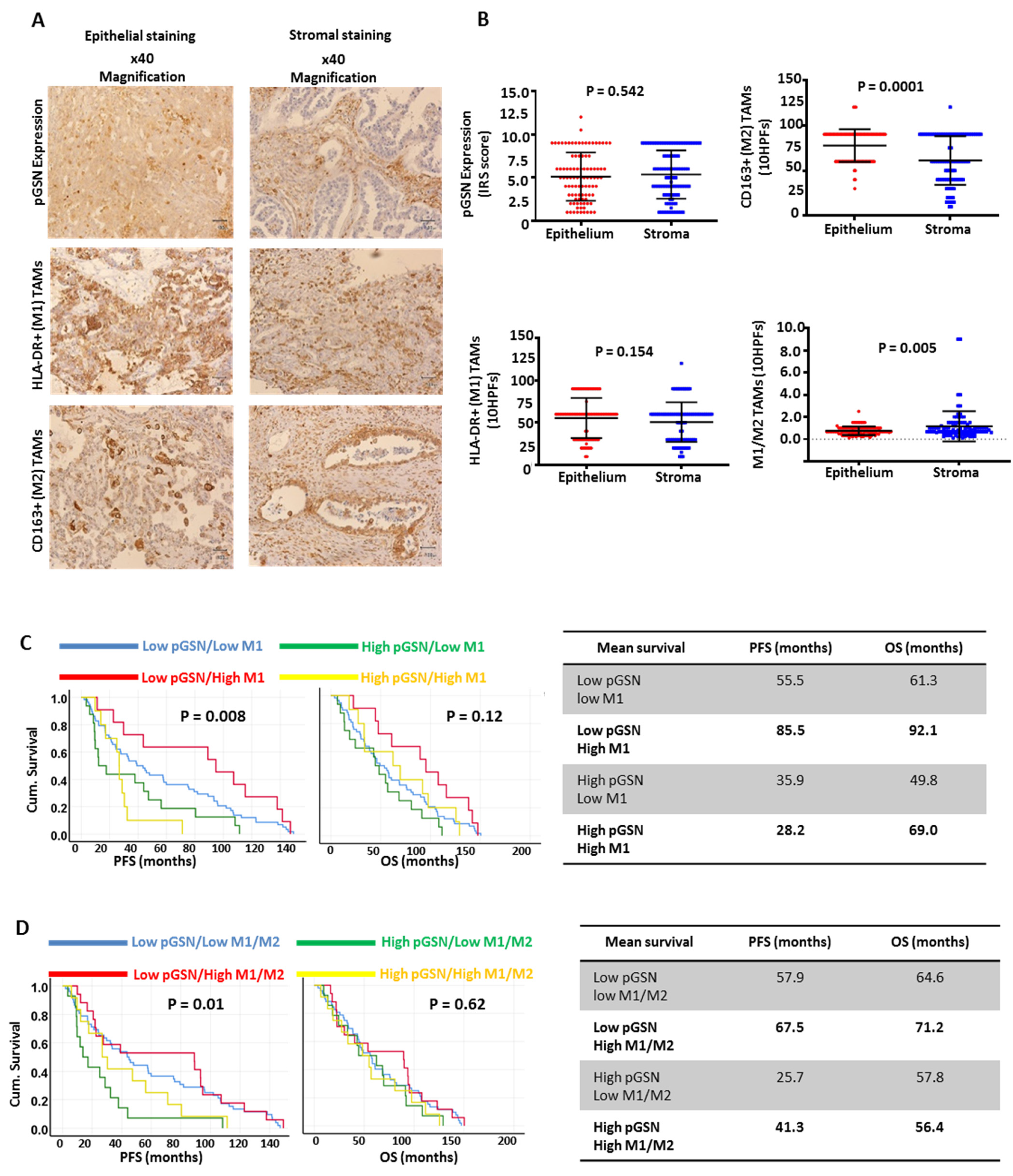
3.2. Increased Epithelial pGSN Expression Is Associated with Suppressed Survival Benefits of Infiltrated M1 Macrophages in OVCA Patients
3.3. Epithelial pGSN Expression, M1/M2 Density and Survival in OVCA Histologic Subtypes
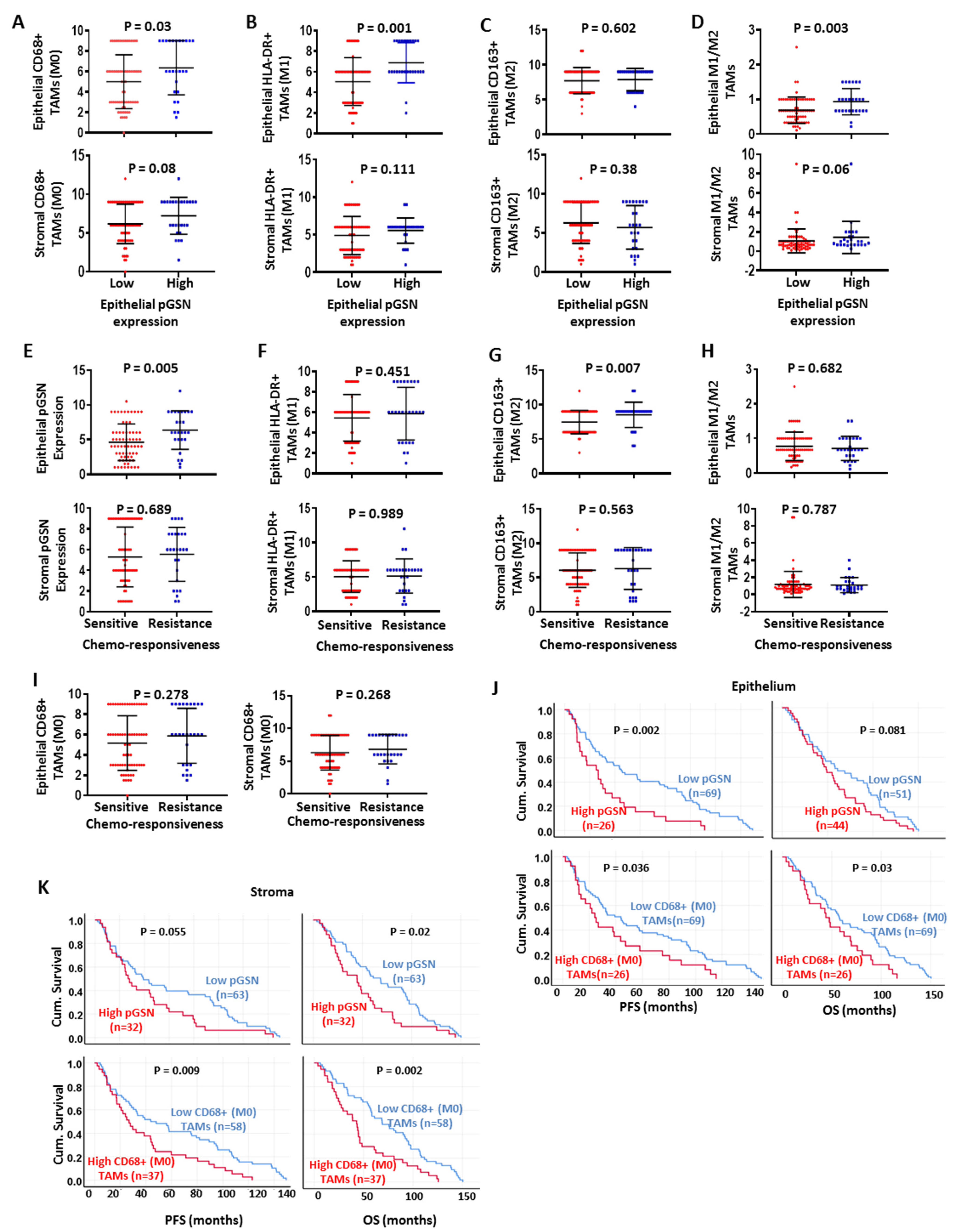
3.4. Chemoresistance Is Associated with Increased Epithelial pGSN Expression and M2 Infiltration
3.5. Increased pGSN Expression Is Associated with Poor Patient Survival, Whereas Increased Epithelial M1/M2 Macrophage Density Is Associated with Improved Patient Survival
3.6. Prognostic Impact of Epithelial pGSN, TAMs and Other Clinicopathologic Parameters
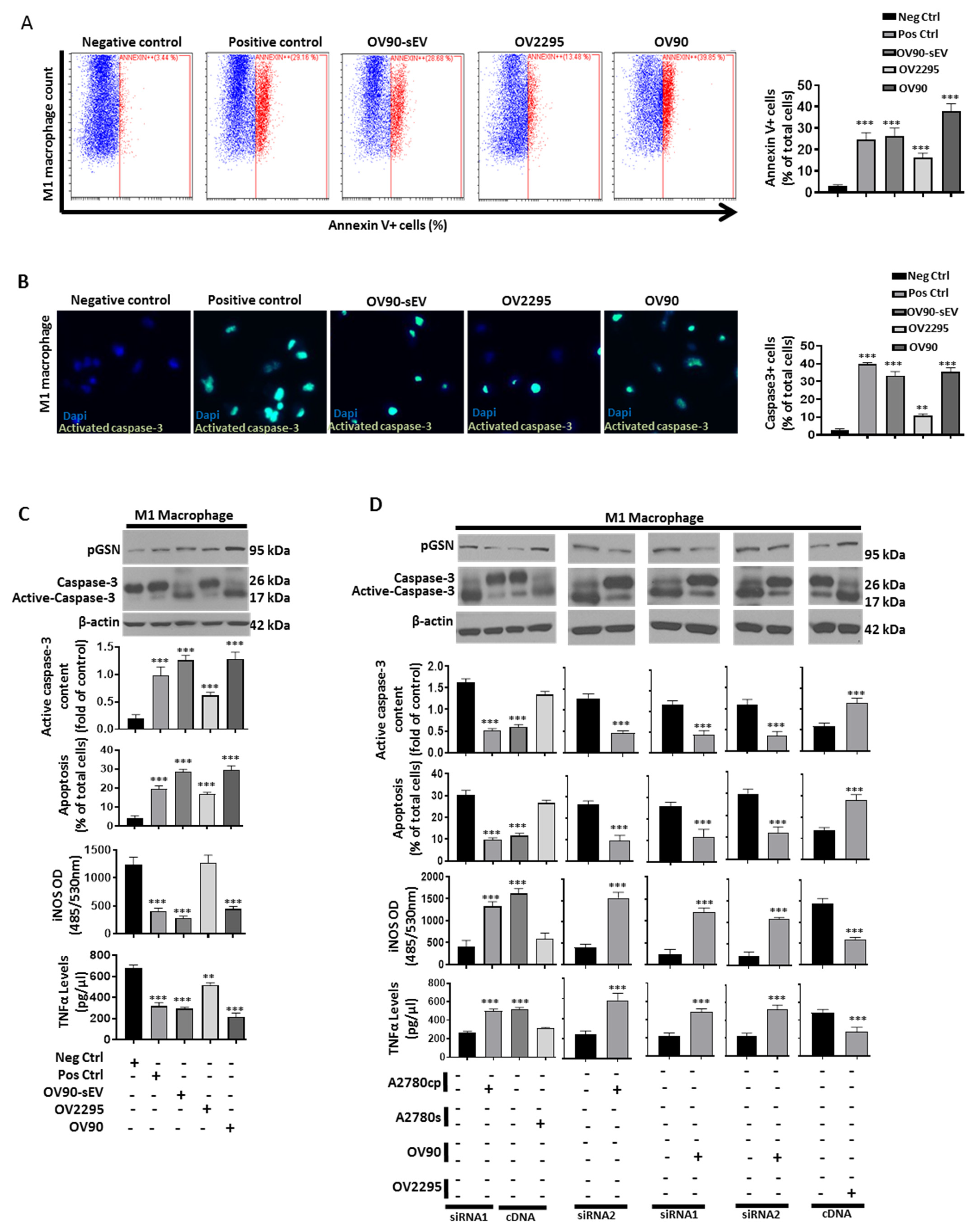
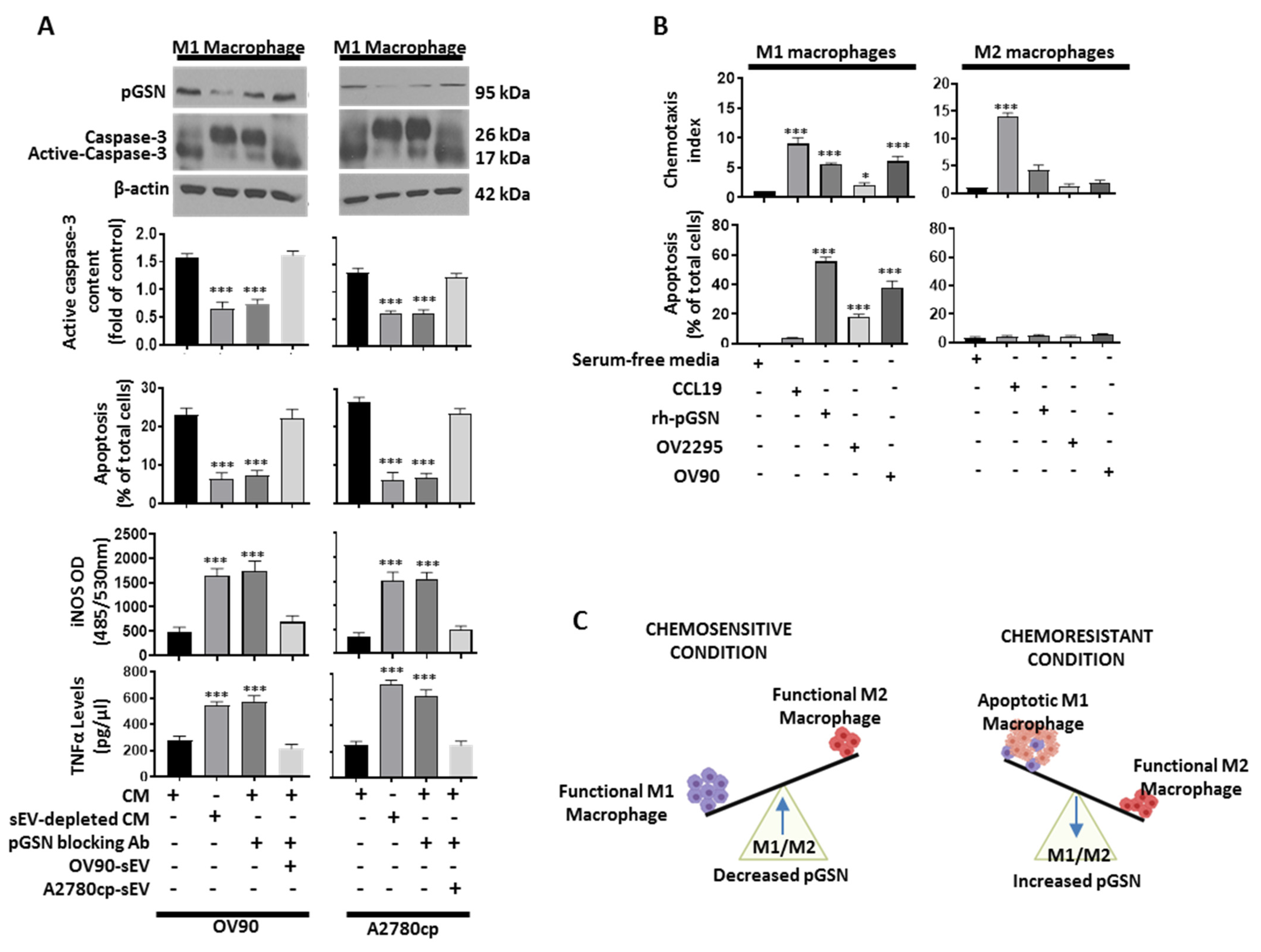
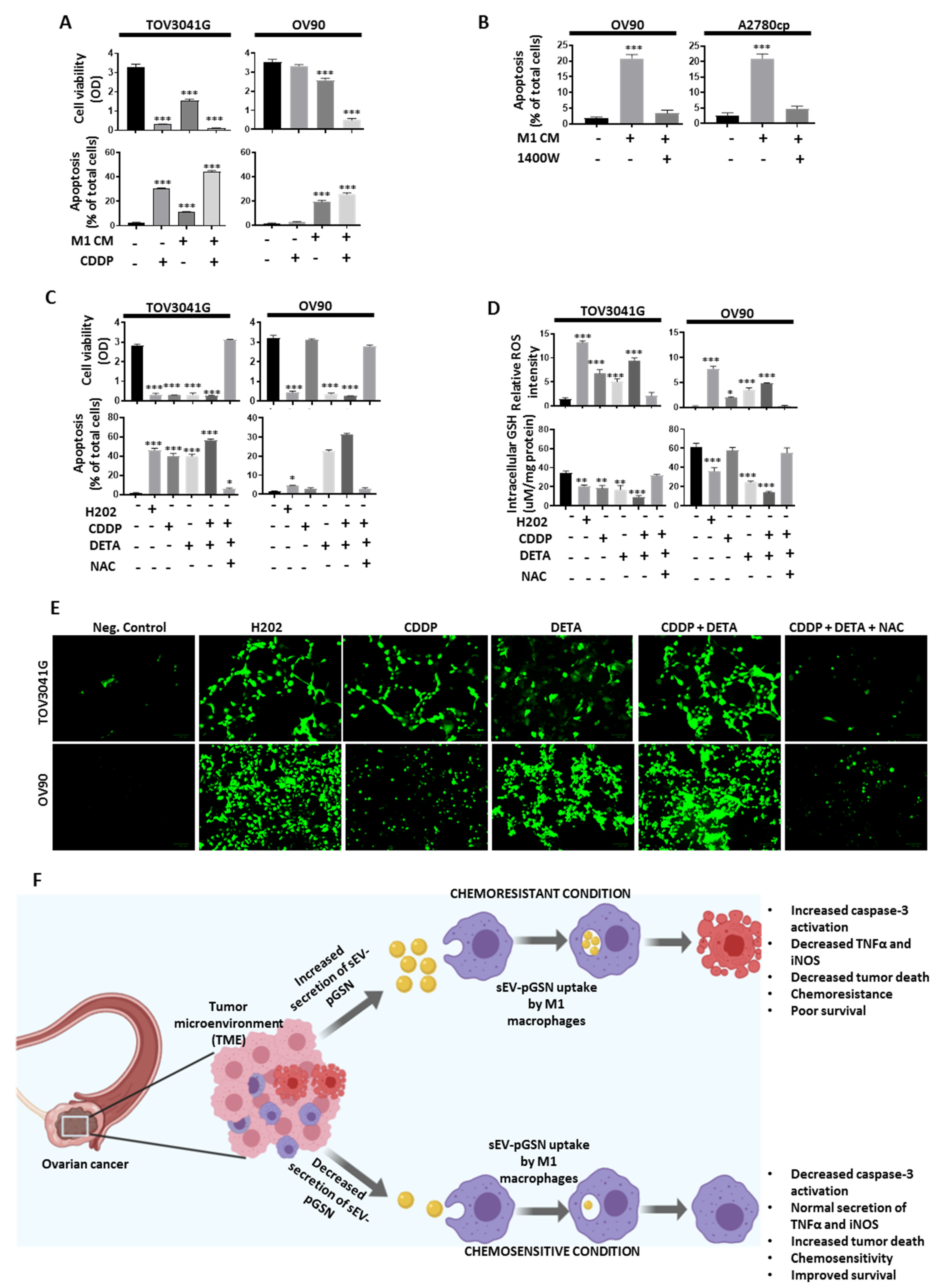
3.7. Chemoresistant-Cell-Derived sEV Attenuates M1 Macrophage’s Anti-Tumor Function by Increased Caspase-3-Dependent Apoptosis and Decreased Secretion of iNOS and TNFα
3.8. Increased pGSN Selectively Attracts and Suppresses M1 Macrophage Survival but Not M2 Macrophages
3.9. M1 Macrophage-Derived Nitric Oxide (NO) Sensitizes Chemoresistant OVCA Cells to CDDP-Induced Death via Increased Reactive Oxygen Species (ROS) Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsibulak, I.; Zeimet, A.G.; Marth, C. Hopes and failures in front-line ovarian cancer therapy. Crit. Rev. Oncol. 2019, 143, 14–19. [Google Scholar] [CrossRef]
- Public Health Agency of Canada; Statistics Canada; Canadian Cancer Society. Release notice—Canadian Cancer Statistics 2019. Health Promot. Chronic Dis. Prev. Can. 2019, 39, 255. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Doo, D.W.; Norian, L.A.; Arend, R.C. Checkpoint inhibitors in ovarian cancer: A review of preclinical data. Gynecol. Oncol. Rep. 2019, 29, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian Cancer Immunotherapy: Turning up the Heat. Int. J. Mol. Sci. 2019, 20, 2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matanes, E.; Gotlieb, W.H. Immunotherapy of gynecological cancers. Best Pr. Res. Clin. Obstet. Gynaecol. 2019, 60, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qin, B.-D.; Xiao, K.; Xu, S.; Yang, J.-S.; Zang, Y.-S.; Stebbing, J.; Xie, L.-P. A meta-analysis comparing responses of Asian versus non-Asian cancer patients to PD-1 and PD-L1 inhibitor-based therapy. OncoImmunology 2020, 9, 1781333. [Google Scholar] [CrossRef]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.-T.; Lee, W.-T.; Abedini, M.R.; Wang, P.-W.; Shieh, D.-B.; Dilworth, F.J.; Carmona, E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2020, 39, 1600–1616. [Google Scholar] [CrossRef] [Green Version]
- Asare-Werehene, M.; Communal, L.; Carmona, E.; Han, Y.; Song, Y.S.; Burger, D.; Mes-Masson, A.-M.; Tsang, B.K. Plasma Gelsolin Inhibits CD8+T-cell Function and Regulates Glutathione Production to Confer Chemoresistance in Ovarian Cancer. Cancer Res. 2020, 80, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, K.; Komohara, Y.; Tashiro, H.; Ohtake, H.; Nakagawa, T.; Katabuchi, H.; Takeya, M. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010, 101, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Drakes, M.L.; Stiff, P.J. Regulation of Ovarian Cancer Prognosis by Immune Cells in the Tumor Microenvironment. Cancers 2018, 10, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.; Yull, F.; Khabele, D. Bipolar Tumor-Associated Macrophages in Ovarian Cancer as Targets for Therapy. Cancers 2018, 10, 366. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-C.; Chen, C.-W.; Yang, C.-L.; Tsai, I.-M.; Hou, Y.-C.; Chen, C.-J.; Shan, Y.-S. Tumor-Associated Macrophages Promote Epigenetic Silencing of Gelsolin through DNA Methyltransferase 1 in Gastric Cancer Cells. Cancer Immunol. Res. 2017, 5, 885–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lolo, F.N.; Rius, C.; Casas-Tinto, S. Elimination of classically-activated macrophages in tumor-conditioned medium by alternatively-activated macrophages. Biol. Open 2017, 6, 1897–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, X.; Wu, Q.; Wu, X.; Zhu, Q.; Wang, X.; Jiang, L.; Chen, X.; Wang, X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget 2016, 7, 43076–43087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Liu, B.; Bu, X.; Zhao, P. Cross-talk between ovarian cancer cells and macrophages through periostin promotes macrophage recruitment. Cancer Sci. 2018, 109, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Feldt, J.; Schicht, M.; Garreis, F.; Welss, J.; Schneider, U.W.; Paulsen, F. Structure, regulation and related diseases of the actin-binding protein gelsolin. Expert Rev. Mol. Med. 2018, 20, e7. [Google Scholar] [CrossRef]
- Sun, H.Q.; Yamamoto, M.; Mejillano, M.; Yin, H.L. Gelsolin, a Multifunctional Actin Regulatory Protein. J. Biol. Chem. 1999, 274, 33179–33182. [Google Scholar] [CrossRef] [Green Version]
- Spinardi, L.; Witke, W. Gelsolin and Diseases. Subcell. Biochem. 2007, 45, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Fernandes, A.R.; Baptista, P.V. Exosome in Tumour Microenvironment: Overview of the Crosstalk between Normal and Cancer Cells. BioMed Res. Int. 2014, 2014, 179486. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, K.; Yin, X.; Li, H.; Li, Y.; Zhang, Q.; Wang, H.; Qiu, Y. Lower Expression of Gelsolin in Colon Cancer and Its Diagnostic Value in Colon Cancer Patients. J. Cancer 2019, 10, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chiou, S.H.; Yang, C.L.; Chow, K.C.; Lin, T.Y.; Chang, H.W.; You, W.C.; Huang, H.W.; Chen, C.M.; Chen, N.C.; et al. Secreted gelsolin desensitizes and induces apoptosis of infiltrated lymphocytes in prostate cancer. Oncotarget 2017, 8, 77152–77167. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, Y.; Wang, L.; Zhao, R.; Qiao, Y.; Han, N.; Sun, Q.; Dong, N.; Liu, Y.; Wu, D.; et al. miR-200a targets Gelsolin: A novel mechanism regulating secretion of microvesicles in hepatocellular carcinoma cells. Oncol. Rep. 2017, 37, 2711–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedini, M.R.; Wang, P.-W.; Huang, Y.-F.; Cao, M.; Chou, C.-Y.; Shieh, D.-B.; Tsang, B.K. Cell fate regulation by gelsolin in human gynecologic cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 14442–14447. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-W.; Abedini, M.R.; Yang, L.-X.; Ding, A.-A.; Figeys, D.; Chang, J.-Y.; Tsang, B.K.; Shieh, D.-B. Gelsolin regulates cisplatin sensitivity in human head-and-neck cancer. Int. J. Cancer 2014, 135, 2760–2769. [Google Scholar] [CrossRef] [Green Version]
- Asare-Werehene, M.; Communal, L.; Carmona, E.; Le, T.; Provencher, D.; Mes-Masson, A.-M.; Tsang, B.K. Pre-operative Circulating Plasma Gelsolin Predicts Residual Disease and Detects Early Stage Ovarian Cancer. Sci. Rep. 2019, 9, 13924. [Google Scholar] [CrossRef] [Green Version]
- El-Sehemy, A.; Postovit, L.-M.; Fu, Y. Nitric oxide signaling in human ovarian cancer: A potential therapeutic target. Nitric Oxide 2016, 54, 30–37. [Google Scholar] [CrossRef]
- Anttila, M.A.; Voutilainen, K.; Merivalo, S.; Saarikoski, S.; Kosma, V.-M. Prognostic significance of iNOS in epithelial ovarian cancer. Gynecol. Oncol. 2007, 105, 97–103. [Google Scholar] [CrossRef]
- Kielbik, M.; Szulc, I.; Brzezinska, M.; Bednarska, K.; Przygodzka, P.; Sulowska, Z.; Nowak, M.; Klink, M. Nitric oxide donors reduce the invasion ability of ovarian cancer cells in vitro. Anti-Cancer Drugs 2014, 25, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Brüne, B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003, 10, 864–869. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kurokawa, T.; Horiuchi, Y.; Sawamura, Y.; Shinagawa, A.; Kotsuji, F. Localisation of phosphorylated mTOR expression is critical to tumour progression and outcomes in patients with endometrial cancer. Eur. J. Cancer 2010, 46, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Provencher, D.M.; Lounis, H.; Champoux, L.; Tétrault, M.; Manderson, E.N.; Wang, J.C.; Eydoux, P.; Savoie, R.; Tonin, P.N.; Mes-Masson, A.-M. Characterization of Four Novel Epithelial Ovarian Cancer Cell Lines. In Vitr. Cell. Dev. Biol. 2000, 36, 357–361. [Google Scholar] [CrossRef]
- Létourneau, I.J.; Quinn, M.C.J.; Wang, L.-L.; Portelance, L.; Caceres, K.Y.; Cyr, L.; Delvoye, N.; Meunier, L.; De Ladurantaye, M.; Shen, Z.; et al. Derivation and characterization of matched cell lines from primary and recurrent serous ovarian cancer. BMC Cancer 2012, 12, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleury, H.; Communal, L.; Carmona, E.; Portelance, L.; Arcand, S.L.; Rahimi, K.; Tonin, P.N.; Provencher, D.; Mes-Masson, A.-M. Novel high-grade serous epithelial ovarian cancer cell lines that reflect the molecular diversity of both the sporadic and hereditary disease. Genes Cancer 2015, 6, 378–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.Y.; Abedini, M.R.; Tsang, B.K. The oncogenic phosphatase PPM1D confers cisplatin resistance in ovarian carcinoma cells by attenuating checkpoint kinase 1 and p53 activation. Oncogene 2011, 31, 2175–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedini, M.R.; Muller, E.J.; Bergeron, R.; Gray, D.; Tsang, B.K. Akt promotes chemoresistance in human ovarian cancer cells by modulating cisplatin-induced, p53-dependent ubiquitination of FLICE-like inhibitory protein. Oncogene 2009, 29, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Fraser, M.; Moll, U.M.; Basak, A.; Tsang, B.K. Akt-Mediated Cisplatin Resistance in Ovarian Cancer: Modulation of p53 Action on Caspase-Dependent Mitochondrial Death Pathway. Cancer Res. 2006, 66, 3126–3136. [Google Scholar] [CrossRef] [Green Version]
- Abedini, M.R.; Qiu, Q.; Yan, X.; Tsang, B.K. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene 2004, 23, 6997–7004. [Google Scholar] [CrossRef] [Green Version]
- Diskin, C.; Pålsson-McDermott, E.M. Metabolic Modulation in Macrophage Effector Function. Front. Immunol. 2018, 9, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, B.D.; Yu, X.; Castano, A.P.; Bouffard, A.A.; Schmidts, A.; Larson, R.C.; Bailey, S.R.; Boroughs, A.C.; Frigault, M.J.; Leick, M.B.; et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019, 37, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kryczek, I.; Dostál, L.; Lin, H.; Tan, L.; Zhao, L.; Lu, F.; Wei, S.; Maj, T.; Peng, D.; et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016, 165, 1092–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Marisetty, A.; Schrand, B.; Gabrusiewicz, K.; Hashimoto, Y.; Ott, M.; Grami, Z.; Kong, L.-Y.; Ling, X.; Caruso, H.G.; et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J. Clin. Investig. 2018, 129, 137–149. [Google Scholar] [CrossRef]
- Honkanen, T.J.; Tikkanen, A.; Karihtala, P.; Mäkinen, M.; Väyrynen, J.P.; Koivunen, J.P. Prognostic and predictive role of tumour-associated macrophages in HER2 positive breast cancer. Sci. Rep. 2019, 9, 10961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Huang, Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.; Wang, L.; Chen, W. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics 2019, 9, 1714–1727. [Google Scholar] [CrossRef]
- Waheed, S.; Cheng, R.Y.; Casablanca, Y.; Maxwell, G.L.; Wink, D.A.; Syed, V. Nitric Oxide Donor DETA/NO Inhibits the Growth of Endometrial Cancer Cells by Upregulating the Expression of RASSF1 and CDKN1A. Molecules 2019, 24, 3722. [Google Scholar] [CrossRef] [Green Version]
- Dubey, M.; Nagarkoti, S.; Awasthi, D.; Singh, A.; Chandra, T.; Kumaravelu, J.; Barthwal, M.K.; Dikshit, M. Nitric oxide-mediated apoptosis of neutrophils through caspase-8 and caspase-3-dependent mechanism. Cell Death Dis. 2016, 7, e2348. [Google Scholar] [CrossRef]
- Snyder, C.M.; Shroff, E.H.; Liu, J.; Chandel, N.S. Nitric Oxide Induces Cell Death by Regulating Anti-Apoptotic BCL-2 Family Members. PLoS ONE 2009, 4, e7059. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhang, H.; Xing, X.; Wang, L.; Zhang, J.; Yan, L.; Zheng, X.; Zhang, M. Gelsolin regulates proliferation, apoptosis and invasion in natural killer/T-cell lymphoma cells. Biol. Open 2018, 7, bio027557. [Google Scholar]
- Piktel, E.; Wnorowska, U.; Cieśluk, M.; Deptula, P.; Pogoda, K.; Misztalewska-Turkowicz, I.; Paprocka, P.; Niemirowicz-Laskowska, K.; Wilczewska, A.Z.; Janmey, P.A.; et al. Inhibition of inflammatory response in human keratinocytes by magnetic nanoparticles functionalized with PBP10 peptide derived from the PIP2-binding site of human plasma gelsolin. J. Nanobiotechnol. 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hu, X.; Liu, C.; Chen, M.; Wang, J.; Wang, M.; Gao, F.; Han, J.; Zhang, C.; Sun, D.; et al. Gelsolin Inhibits the Inflammatory Process Induced by LPS. Cell. Physiol. Biochem. 2017, 41, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Parasar, D.; Sagar, A.; Choudhary, V.; Chopra, B.S.; Garg, R.; Ashish; Khatri, N. Analgesic and Anti-Inflammatory Properties of Gelsolin in Acetic Acid Induced Writhing, Tail Immersion and Carrageenan Induced Paw Edema in Mice. PLoS ONE 2015, 10, e0135558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Univariate | ||||||
|---|---|---|---|---|---|---|
| Variable | PFS | OS | ||||
| HR * | 95% CI ^ | p-Value | HR * | 95% CI ^ | p-Value | |
| Age (years) | ||||||
| ≤56 vs. >56 | 1.046 | 1.012–1.082 | 0.008 | 1.049 | 1.002–1.098 | 0.041 |
| Stage (FIGO) | ||||||
| ≤2 vs. >2 | 4.803 | 2.153–10.717 | <0.001 | 3.682 | 1.232–11.005 | 0.020 |
| RD (cm) | ||||||
| ≤1 vs. >1 | 0.114 | 0.052–0.251 | <0.001 | 0.117 | 0.039–0.350 | <0.01 |
| pGSN epi | ||||||
| Low vs. high | 1.233 | 1.07–1.421 | 0.004 | 1.054 | 0.870–1.276 | 0.591 |
| CD68 epi | ||||||
| Low vs. high | 1.092 | 0.946–1.26 | 0.23 | 1.011 | 0.832–1.228 | 0.915 |
| HLA-DR (M1) epi | ||||||
| Low vs. high | 1.035 | 0.881–1.215 | 0.676 | 0.826 | 0.661–1.033 | 0.093 |
| CD163 (M2) epi | ||||||
| Low vs. high | 1.328 | 1.065–1.658 | 0.012 | 1.436 | 1.047–1.971 | 0.025 |
| M1/M2 epi | ||||||
| Low vs. high | 0.787 | 0.391–1.584 | 0.503 | 0.397 | 0.138–1.137 | 0.085 |
| Histologic subtype | 0.787 | 0.549–1.129 | 0.194 | 0.806 | 0.488–1.333 | 0.401 |
| Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|
| Variable | PFS | OS | ||||
| HR * | 95% CI ^ | p-Value | HR * | 95% CI ^ | p-Value | |
| Age (years) | ||||||
| ≤56 vs. >56 | 1.126 | 0.387–3.279 | 0.828 | 1.352 | 0.331–5.530 | 0.675 |
| Stage (FIGO) | ||||||
| ≤2 vs. >2 | 1.090 | 0.358–3.320 | 0.880 | 0.945 | 0.183–4.874 | 0.946 |
| RD (cm) ≤1 vs. >1 | ||||||
| 0.103 | 0.033–0.322 | <0.001 | 0.139 | 0.032–0.605 | 0.009 | |
| pGSN epi | ||||||
| Low vs. high | 1.300 | 1.096–1.541 | 0.003 | 1.019 | 0.793–1.309 | 0.884 |
| CD68 epi | ||||||
| Low vs. high | 0.939 | 0.764–1.154 | 0.549 | 0.943 | 0.706–1.261 | 0.694 |
| HLA-DR (M1) epi | ||||||
| Low vs. high | 1.030 | 0.809–1.312 | 0.808 | 0.828 | 0.552–1.242 | 0.362 |
| CD163 (M2) epi | ||||||
| Low vs. high | 1.274 | 0.969–1.674 | 0.082 | 1.481 | 0.910–2.413 | 0.114 |
| M1/M2 epi | ||||||
| Low vs. high | 0.603 | 0.198–1.836 | 0.373 | 1.173 | 0.174–7.912 | 0.870 |
| Histologic subtype | 0.547 | 0.312–0.954 | 0.035 | 0.730 | 0.354–1.503 | 0.393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asare-Werehene, M.; Tsuyoshi, H.; Zhang, H.; Salehi, R.; Chang, C.-Y.; Carmona, E.; Librach, C.L.; Mes-Masson, A.-M.; Chang, C.-C.; Burger, D.; et al. Plasma Gelsolin Confers Chemoresistance in Ovarian Cancer by Resetting the Relative Abundance and Function of Macrophage Subtypes. Cancers 2022, 14, 1039. https://doi.org/10.3390/cancers14041039
Asare-Werehene M, Tsuyoshi H, Zhang H, Salehi R, Chang C-Y, Carmona E, Librach CL, Mes-Masson A-M, Chang C-C, Burger D, et al. Plasma Gelsolin Confers Chemoresistance in Ovarian Cancer by Resetting the Relative Abundance and Function of Macrophage Subtypes. Cancers. 2022; 14(4):1039. https://doi.org/10.3390/cancers14041039
Chicago/Turabian StyleAsare-Werehene, Meshach, Hideaki Tsuyoshi, Huilin Zhang, Reza Salehi, Chia-Yu Chang, Euridice Carmona, Clifford L. Librach, Anne-Marie Mes-Masson, Chia-Ching Chang, Dylan Burger, and et al. 2022. "Plasma Gelsolin Confers Chemoresistance in Ovarian Cancer by Resetting the Relative Abundance and Function of Macrophage Subtypes" Cancers 14, no. 4: 1039. https://doi.org/10.3390/cancers14041039






