Identification of Atypical Circulating Tumor Cells with Prognostic Value in Metastatic Breast Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients’ Population
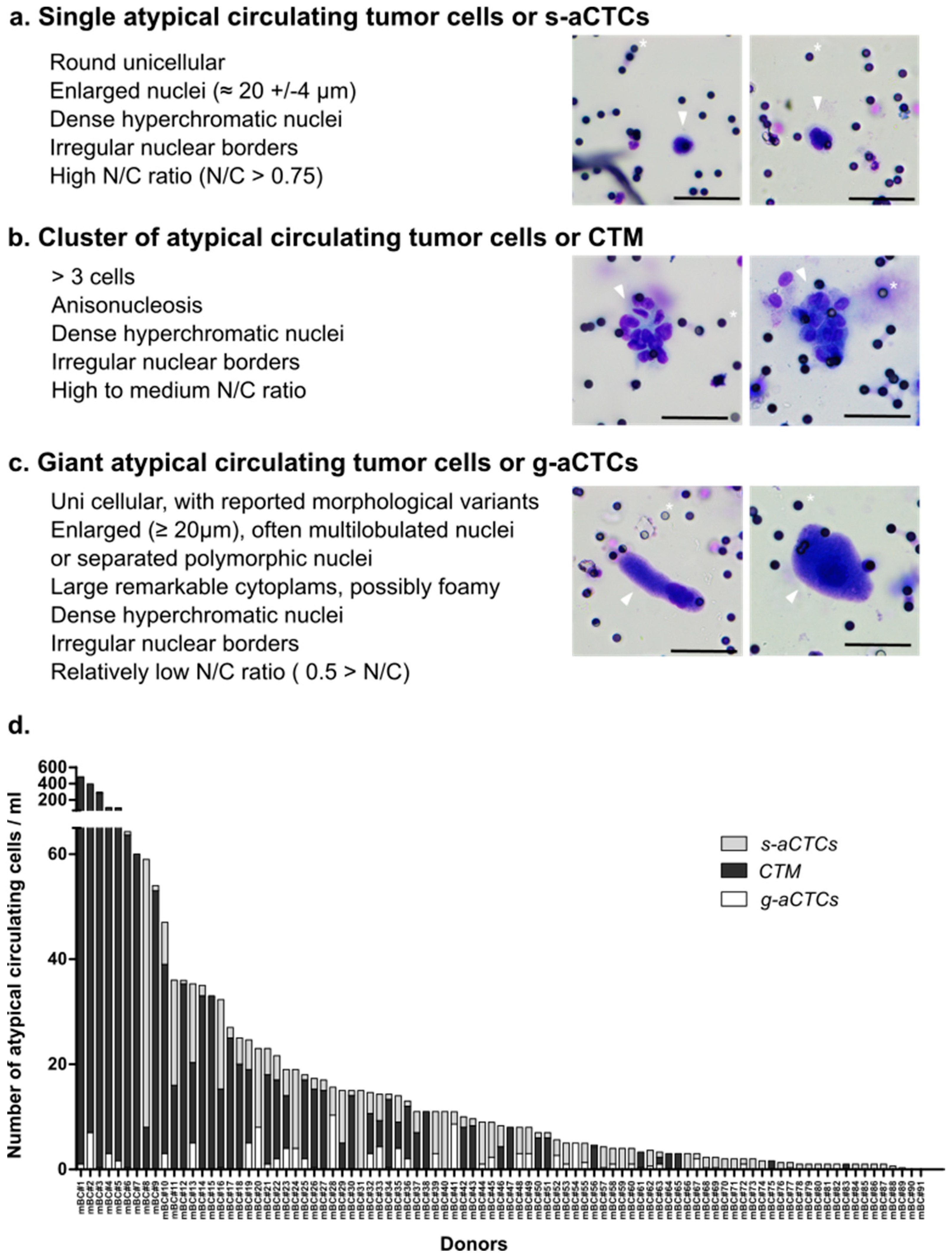
2.2. Three Subsets of aCTCs Are Found in the Blood of mBC Patients
2.3. The Count of aCTC Subsets Is Highly Variable in mBC Patients
2.4. Different aCTC Subsets Correlate with Different Clinicopathological Features
2.5. The g-aCTCs “Positive” Status Is an Independent Prognostic Factor for PFS and OS
2.6. A hybrid Epithelial–Mesenchymal Phenotype Is Associated with LGR5 and ABCB1 Markers Co-Expression
2.7. A hybrid Epithelial–Mesenchymal Phenotype Is Associated with Shorter PFS
3. Discussion
3.1. Reliability
3.2. Comparison
3.3. Limitation
3.4. Benefit and Practical Implication
4. Materials and Methods
4.1. PERMED-01 Sub-Study
4.2. Atypical Circulating Cells Enrichment Using ScreenCell®CYTO Device
4.3. Atypical Circulating Cells Staining with May Grünwald Giemsa (MGG)
4.4. Atypical Circulating Cells Subsets according to Cytological Criteria by MGG
4.5. Immunofluorescence Staining of aCTCs for Confocal Microscopic Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasgupta, A.; Lim, A.R.; Ghajar, C.M. Circulating and disseminated tumor cells: Harbingers or initiators of metastasis? Mol. Oncol. 2017, 11, 40–61. [Google Scholar] [CrossRef] [Green Version]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Plaks, V.; Koopman, C.D.; Werb, Z. Cancer. Circulating tumor cells. Science 2013, 341, 1186–1188. [Google Scholar] [CrossRef]
- Paoletti, C.; Miao, J.; Dolce, E.M.; Darga, E.P.; Repollet, M.I.; Doyle, G.V.; Gralow, J.R.; Hortobagyi, G.N.; Smerage, J.B.; Barlow, W.E.; et al. Circulating Tumor Cell Clusters in Patients with Metastatic Breast Cancer: A SWOG S0500 Translational Medicine Study. Clin. Cancer Res. 2019, 25, 6089–6097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, C.R.; Seagle, B.-L.L.; Friedl, T.W.P.; Rack, B.; Lato, K.; Fink, V.; Cristofanilli, M.; Donnelly, E.D.; Janni, W.; Shahabi, S.; et al. Association of Circulating Tumor Cell Status with Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol. 2018, 4, e180163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, S.; Stenzl, A.; Pantel, K.; Todenhöfer, T. Expression of Epithelial Mesenchymal Transition and Cancer Stem Cell Markers in Circulating Tumor Cells. Adv. Exp. Med. Biol. 2017, 994, 205–228. [Google Scholar]
- Rosenbaum, M.W.; Cauley, C.E.; Kulemann, B.; Liss, A.S.; Castillo, C.F.-D.; Warshaw, A.L.; Lillemoe, K.D.; Thayer, S.P.; Pitman, M.B. Cytologic characteristics of circulating epithelioid cells in pancreatic disease. Cancer Cytopathol. 2017, 125, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Drucker, A.; Teh, E.M.; Kostyleva, R.; Rayson, D.; Douglas, S.; Pinto, D.M. Comparative performance of different methods for circulating tumor cell enrichment in metastatic breast cancer patients. PLoS ONE 2020, 15, e0237308. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Falchini, M.; Maddau, C.; Salvianti, F.; Nistri, M.; Bertelli, E.; Sali, L.; Zuccherelli, S.; Vella, A.; Matucci, M.; et al. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J. Cancer Res. Clin. Oncol. 2016, 142, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kulemann, B.; Pitman, M.B.; Liss, A.S.; Valsangkar, N.; Castillo, C.F.-D.; Lillemoe, K.D.; Hoeppner, J.; Mino-Kenudson, M.; Warshaw, A.L.; Thayer, S.P. Circulating tumor cells found in patients with localized and advanced pancreatic cancer. Pancreas 2015, 44, 547–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A. Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers 2019, 11, 1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Fatayer, H.; Hanby, A.M.; Horgan, K.; Perry, S.L.; Valleley, E.M.; Verghese, E.T.; Williams, B.J.; Thorne, J.L.; Hughes, T.A. Neoadjuvant chemotherapy induces expression levels of breast cancer resistance protein that predict disease-free survival in breast cancer. PLoS ONE 2013, 8, e62766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC subfamily A transporters: Multifaceted players with incipient potentialities in cancer. Semin. Cancer Biol. 2020, 60, 57–71. [Google Scholar] [CrossRef]
- Hagerling, C.; Owyong, M.; Sitarama, V.; Wang, C.-Y.; Lin, C.; van den Bijgaart, R.J.E.; Koopman, C.D.; Brenot, A.; Nanjaraj, A.; Wärnberg, F.; et al. LGR5 in breast cancer and ductal carcinoma in situ: A diagnostic and prognostic biomarker and a therapeutic target. BMC Cancer 2020, 20, 542. [Google Scholar] [CrossRef]
- Fojo, T.; Coley, H.M. The role of efflux pumps in drug-resistant metastatic breast cancer: New insights and treatment strategies. Clin. Breast Cancer 2007, 7, 749–756. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Raimondi, C.; Francescangeli, F.; Ceccarelli, S.; Trenta, P.; Magri, V.; Marchese, C.; Zeuner, A.; Gradilone, A.; Gazzaniga, P. EpCAM-expressing circulating tumor cells in colorectal cancer. Int. J. Biol. Markers 2017, 32, e415–e420. [Google Scholar] [CrossRef]
- Hendricks, A.; Brandt, B.; Geisen, R.; Dall, K.; Röder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Isolation and Enumeration of CTC in Colorectal Cancer Patients: Introduction of a Novel Cell Imaging Approach and Comparison to Cellular and Molecular Detection Techniques. Cancers 2020, 12, 2643. [Google Scholar] [CrossRef]
- Lamouille, S.; Derynck, R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 2007, 178, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Krol, I.; Schwab, F.D.; Carbone, R.; Ritter, M.; Picocci, S.; De Marni, M.L.; Stepien, G.; Franchi, G.M.; Zanardi, A.; Rissoglio, M.D.; et al. Detection of clustered circulating tumour cells in early breast cancer. Br. J. Cancer 2021, 125, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Benali-Furet, N.; Uzan, G.; Znaty, A.; Ye, Z.; Paolillo, C.; Wang, C.; Austin, L.; Rossi, G.; Fortina, P.; et al. Detection and Characterization of Circulating Tumor Associated Cells in Metastatic Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1665. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, S.; Liu, Z.; Huang, J.; Pu, X.; Li, J.; Yang, D.; Deng, H.; Yang, N.; Xu, J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS ONE 2015, 10, e0123976. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Jansson, S.; Bendahl, P.-O.; Larsson, A.-M.; Aaltonen, K.E.; Rydén, L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 2016, 16, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauch, S.F.; Riethdorf, S.; Sprick, M.R.; Schütz, F.; Schönfisch, B.; Brucker, S.Y.; Deutsch, T.M.; Nees, J.; Saini, M.; Becker, L.M.; et al. Sustained prognostic impact of circulating tumor cell status and kinetics upon further progression of metastatic breast cancer. Breast Cancer Res. Treat. 2019, 173, 155–165. [Google Scholar] [CrossRef]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B.; et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef] [Green Version]
- Manjunath, Y.; Porciani, D.; Mitchem, J.B.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Burke, D.H.; Li, G.; Kaifi, J.T. Tumor-Cell-Macrophage Fusion Cells as Liquid Biomarkers and Tumor Enhancers in Cancer. Int. J. Mol. Sci. 2020, 21, 1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnan, M.; Mathur, S.R.; Mukhopadhyay, A. Fusion-derived epithelial cancer cells express hematopoietic markers and contribute to stem cell and migratory phenotype in ovarian carcinoma. Cancer Res. 2013, 73, 5360–5370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.-M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef] [Green Version]
- Augustyn, A.; Adams, D.L.; He, J.; Qiao, Y.; Verma, V.; Liao, Z.; Tang, C.-M.; Heymach, J.V.; Tsao, A.S.; Lin, S.H. Giant Circulating Cancer-Associated Macrophage-Like Cells Are Associated with Disease Recurrence and Survival in Non–Small-Cell Lung Cancer Treated with Chemoradiation and Atezolizumab. Clin. Lung Cancer 2021, 22, e451–e465. [Google Scholar] [CrossRef]
- Gironda, D.J.; Adams, D.L.; He, J.; Xu, T.; Gao, H.; Qiao, Y.; Komaki, R.; Reuben, J.M.; Liao, Z.; Blum-Murphy, M.; et al. Cancer associated macrophage-like cells and prognosis of esophageal cancer after chemoradiation therapy. J. Transl. Med. 2020, 18, 413. [Google Scholar] [CrossRef]
- Pillai, S.G.; Zhu, P.; Siddappa, C.M.; Adams, D.L.; Li, S.; Makarova, O.V.; Amstutz, P.; Nunley, R.; Tang, C.-M.; Watson, M.A.; et al. Enrichment and Molecular Analysis of Breast Cancer Disseminated Tumor Cells from Bone Marrow Using Microfiltration. PLoS ONE 2017, 12, e0170761. [Google Scholar] [CrossRef]
- Kemény, L.V.; Kurgyis, Z.; Buknicz, T.; Groma, G.; Jakab, Á.; Zänker, K.; Dittmar, T.; Németh, I.B. Melanoma Cells Can Adopt the Phenotype of Stromal Fibroblasts and Macrophages by Spontaneous Cell Fusion In Vitro. Int. J. Mol. Sci. 2016, 17, 826. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.L.; Adams, D.K.; Alpaugh, R.K.; Cristofanilli, M.; Martin, S.S.; Chumsri, S.; Tang, C.M.; Marks, J.R. Circulating Cancer-Associated Macrophage-Like Cells Differentiate Malignant Breast Cancer and Benign Breast Conditions. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1037–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karihtala, P.; Jääskeläinen, A.; Roininen, N.; Jukkola, A. Prognostic factors in metastatic breast cancer: A prospective single-centre cohort study in a Finnish University Hospital. BMJ Open 2020, 10, e038798. [Google Scholar] [CrossRef]
- Xu, L.; Mao, X.; Guo, T.; Chan, P.Y.; Shaw, G.; Hines, J.; Stankiewicz, E.; Wang, Y.; Oliver, R.T.D.; Ahmad, A.S.; et al. The Novel Association of Circulating Tumor Cells and Circulating Megakaryocytes with Prostate Cancer Prognosis. Clin. Cancer Res. 2017, 23, 5112–5122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, S.C.; Metcalf, R.L.; Trapani, F.; Mohan, S.; Antonello, J.; Abbott, B.; Leong, H.S.; Chester, C.P.E.; Simms, N.; Polanski, R.; et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016, 7, 13322. [Google Scholar] [CrossRef] [PubMed]
- Cima, I.; Kong, S.L.; Sengupta, D.; Tan, I.B.; Phyo, W.M.; Lee, D.; Hu, M.; Iliescu, C.; Alexander, I.; Goh, W.L.; et al. Tumor-derived circulating endothelial cell clusters in colorectal cancer. Sci. Transl. Med. 2016, 8, 345ra89. [Google Scholar] [CrossRef] [Green Version]
- Bertucci, F.; Gonçalves, A.; Guille, A.; Adelaïde, J.; Garnier, S.; Carbuccia, N.; Billon, E.; Finetti, P.; Sfumato, P.; Monneur, A.; et al. Prospective high-throughput genome profiling of advanced cancers: Results of the PERMED-01 clinical trial. Genome Med. 2021, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Desitter, I.; Guerrouahen, B.S.; Benali-Furet, N.; Wechsler, J.; Jänne, P.A.; Kuang, Y.; Yanagita, M.; Wang, L.; Berkowitz, J.A.; Distel, R.J.; et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011, 31, 427–441. [Google Scholar]
- Hofman, V.; Bonnetaud, C.; Ilié, M.; Vielh, P.; Vignaud, J.M.; Fléjou, J.F.; Lantuejoul, S.; Piaton, E.; Mourad, N.; Butori, C.; et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin. Cancer Res. 2011, 17, 827–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wechsler, J. Cellules Tumorales Circulantes des Cancers Solides/Circulating Tumor Cells from Solid Cancers; Sauramps Medical: Montpellier, France, 2015; pp. 1–72. [Google Scholar]
- Manjunath, Y.; Mitchem, J.B.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Deroche, C.B.; Pantel, K.; Li, G.; Kaifi, J.T. Circulating Giant Tumor-Macrophage Fusion Cells Are Independent Prognosticators in Patients With NSCLC. J. Thorac. Oncol. 2020, 15, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [Green Version]


| Patients’ Characteristics | N (%) | |
|---|---|---|
| Age at inclusion, years | 55 (27–79) | |
| Metastasis to diagnosis interval, years | 3 (0.59–23) | |
| Pathological type of primary tumor | ductal | 75 (93%) |
| lobular | 6 (7%) | |
| missing | 10 | |
| Pathological grade of primary | 1 | 5 (7%) |
| 2 | 32 (43%) | |
| 3 | 37 (50%) | |
| missing | 17 | |
| Molecular subtype of primary tumor | HR+/HER2− | 50 (56%) |
| HER2+ | 11 (12%) | |
| TN | 29 (32%) | |
| missing | 1 | |
| Molecular subtype of metastasis | HR+/HER2− | 36 (41%) |
| HER2+ | 11 (13%) | |
| TN | 40 (46%) | |
| missing | 4 | |
| Bone metastasis | no | 32 (35%) |
| yes | 59 (65%) | |
| Liver metastasis | no | 39 (43%) |
| yes | 52 (57%) | |
| Lung-pleural metastasis | no | 45 (49%) |
| yes | 46 (51%) | |
| Brain-meningeal metastasis | no | 81 (89%) |
| yes | 10 (11%) | |
| Lymph node metastasis | no | 34 (37%) |
| yes | 57 (63%) | |
| Skin metastasis | no | 73 (80%) |
| yes | 18 (20%) | |
| Peritoneum metastasis | no | 81 (89%) |
| yes | 10 (11%) | |
| Other metastatic site | no | 66 (73%) |
| yes | 25 (27%) | |
| Number of metastasic sites at inclusion, N | 3 (1–8) | |
| Number of previous lines of systemic therapy at inclusion, N | 4 (1–10) | |
| Atypical circulating cells (all subsets) | Negative | 35 (38%) |
| Positive | 56 (62%) | |
| s-aCTC | Negative | 42 (46%) |
| Positive | 49 (54%) | |
| CTM | Negative | 44 (48%) |
| Positive | 47 (52%) | |
| g-aCTC | Negative | 49 (54%) |
| Positive | 42 (46%) | |
| Follow-up median, months (range) | 12 (1–52) | |
| PFS events, N (%) | 90 (99%) | |
| Median PFS, months (min–max) | 5 (1–47) | |
| 1-year PFS, % [95% CI] | 13% (8–22) | |
| OS events, N (%) | 68 (75%) | |
| Median OS, months (min-max) | 14 (1–52) | |
| 1-year OS, % [95% CI] | 58% (49–70) | |
| PFS | OS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||||
| N | HR [95% CI] | p-Value | N | HR [95% CI] | p-Value | N | HR [95% CI] | p-Value | N | HR [95% CI] | p-Value | ||
| Age at inclusion #, years | 91 | 1.02 (1.002–1.04) | 0.0334 | 91 | 1.02 (1.00–1.04) | 0.0378 | 91 | 1.01 (0.99–1.04) | 0.286 | ||||
| Metastasis to diagnosis interval #, years | 91 | 1.02 (0.98–1.06) | 0.34 | 91 | 1.03 (0.99–1.08) | 0.173 | |||||||
| Pathological type of primary tumor | lobular vs. ductal | 81 | 1.24 (0.53–2.86) | 0.621 | 81 | 1.60 (0.57–4.49) | 0.371 | ||||||
| Pathological grade of primary | 2 vs. 1 | 74 | 0.74 (0.28–1.97) | 0.453 | 74 | 0.64 (0.19–2.18) | 0.533 | ||||||
| 3 vs. 1 | 0.59 (0.23–1.55) | 0.53 (0.16–1.80) | |||||||||||
| Molecular subtype of primary | HER2pos | 90 | 1.47 (0.74–2.91) | 0.151 | 90 | 1.50 (0.67–3.39) | 0.262 | ||||||
| TN | 1.99 (0.96–4.12) | 1.98 (0.85–4.64) | |||||||||||
| Molecular subtype of metastasis | HER2pos | 87 | 0.87 (0.43–1.76) | 0.866 | 87 | 1.26 (0.58–2.73) | 0.694 | ||||||
| TN | 1.05 (0.67–1.67) | 1.25 (0.73–2.14) | |||||||||||
| Bone metastasis | yes vs. no | 91 | 0.89 (0.57–1.38) | 0.591 | 91 | 1.04 (0.62–1.74) | 0.892 | ||||||
| Liver metastasis | yes vs. no | 91 | 0.81 (0.52–1.25) | 0.335 | 91 | 1.01 (0.62–1.66) | 0.958 | ||||||
| Lung-pleural metastasis | yes vs. no | 91 | 1.09 (0.72–1.66) | 0.679 | 91 | 1.06 (0.65–1.71) | 0.817 | ||||||
| Brain-meningeal metastasis | yes vs. no | 91 | 1.42 (0.73–2.75) | 0.304 | 91 | 3.15 (1.58–6.28) | 0.00115 | 91 | 2.74 (1.37–5.48) | 0.00454 | |||
| Lymph node metastasis | yes vs. no | 91 | 1.06 (0.68–1.65) | 0.784 | 91 | 1.14 (0.68–1.91) | 0.607 | ||||||
| Skin metastasis | yes vs. no | 91 | 1.02 (0.60–1.74) | 0.942 | 91 | 1.43 (0.81–2.51) | 0.219 | ||||||
| Peritoneum metastasis | yes vs. no | 91 | 1.20 (0.62–2.32) | 0.597 | 91 | 1.50 (0.71–3.16) | 0.287 | ||||||
| Other metastatic site | yes vs. no | 91 | 0.95 (0.59–1.53) | 0.845 | 91 | 1.34 (0.80–2.23) | 0.269 | ||||||
| Number of metastasic sites at inclusion #, N | 91 | 1.00 (0.86–1.15) | 0.948 | 91 | 1.12 (0.96–1.30) | 0.158 | |||||||
| Number of previous lines of systemic therapy at inclusion #, N | 91 | 1.10 (1.01–1.21) | 0.0348 | 91 | 1.06 (0.96–1.16) | 0.23193 | 91 | 1.11 (1.01–1.23) | 0.0396 | 91 | 1.09 (0.98–1.20) | 0.10355 | |
| all atypical circulating cells | positive vs. negative | 91 | 1.37 (0.89–2.10) | 0.158 | 91 | 1.50 (0.91–2.49) | 0.115 | ||||||
| s-aCTC | positive vs. negative | 91 | 1.51 (0.99–2.29) | 0.056 | 91 | 1.51 (0.93–2.45) | 0.093 | ||||||
| CTM | positive vs. negative | 91 | 1.16 (0.77–1.76) | 0.478 | 91 | 1.35 (0.83–2.20) | 0.221 | ||||||
| g-aCTC | positive vs. negative | 91 | 1.94 (1.25–3.01) | 0.00298 | 91 | 1.87 (1.19–2.95) | 0.00661 | 91 | 2.46 (1.47–4.12) | 0.000584 | 91 | 2.23 (1.31–3.78) | 0.00298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopresti, A.; Acquaviva, C.; Boudin, L.; Finetti, P.; Garnier, S.; Aulas, A.; Liberatoscioli, M.L.; Cabaud, O.; Guille, A.; de Nonneville, A.; et al. Identification of Atypical Circulating Tumor Cells with Prognostic Value in Metastatic Breast Cancer Patients. Cancers 2022, 14, 932. https://doi.org/10.3390/cancers14040932
Lopresti A, Acquaviva C, Boudin L, Finetti P, Garnier S, Aulas A, Liberatoscioli ML, Cabaud O, Guille A, de Nonneville A, et al. Identification of Atypical Circulating Tumor Cells with Prognostic Value in Metastatic Breast Cancer Patients. Cancers. 2022; 14(4):932. https://doi.org/10.3390/cancers14040932
Chicago/Turabian StyleLopresti, Alexia, Claire Acquaviva, Laurys Boudin, Pascal Finetti, Séverine Garnier, Anaïs Aulas, Maria Lucia Liberatoscioli, Olivier Cabaud, Arnaud Guille, Alexandre de Nonneville, and et al. 2022. "Identification of Atypical Circulating Tumor Cells with Prognostic Value in Metastatic Breast Cancer Patients" Cancers 14, no. 4: 932. https://doi.org/10.3390/cancers14040932
APA StyleLopresti, A., Acquaviva, C., Boudin, L., Finetti, P., Garnier, S., Aulas, A., Liberatoscioli, M. L., Cabaud, O., Guille, A., de Nonneville, A., Da Costa, Q., Denicolai, E., Pakradouni, J., Goncalves, A., Birnbaum, D., Bertucci, F., & Mamessier, E. (2022). Identification of Atypical Circulating Tumor Cells with Prognostic Value in Metastatic Breast Cancer Patients. Cancers, 14(4), 932. https://doi.org/10.3390/cancers14040932










