CRIF1-CDK2 Interface Inhibitors Enhance Taxol Inhibition of the Lethal Triple-Negative Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inhibitors and Chemicals
2.2. Cell Culture
2.3. Cell Proliferation Measurement: An Assay for DNA Content with Cell Proliferation Reagent WST-1
2.4. Flow Cytometric Cell Cycle Analysis
2.5. Apoptosis Test
2.6. Western Blot
2.7. Cytotoxicity Analysis
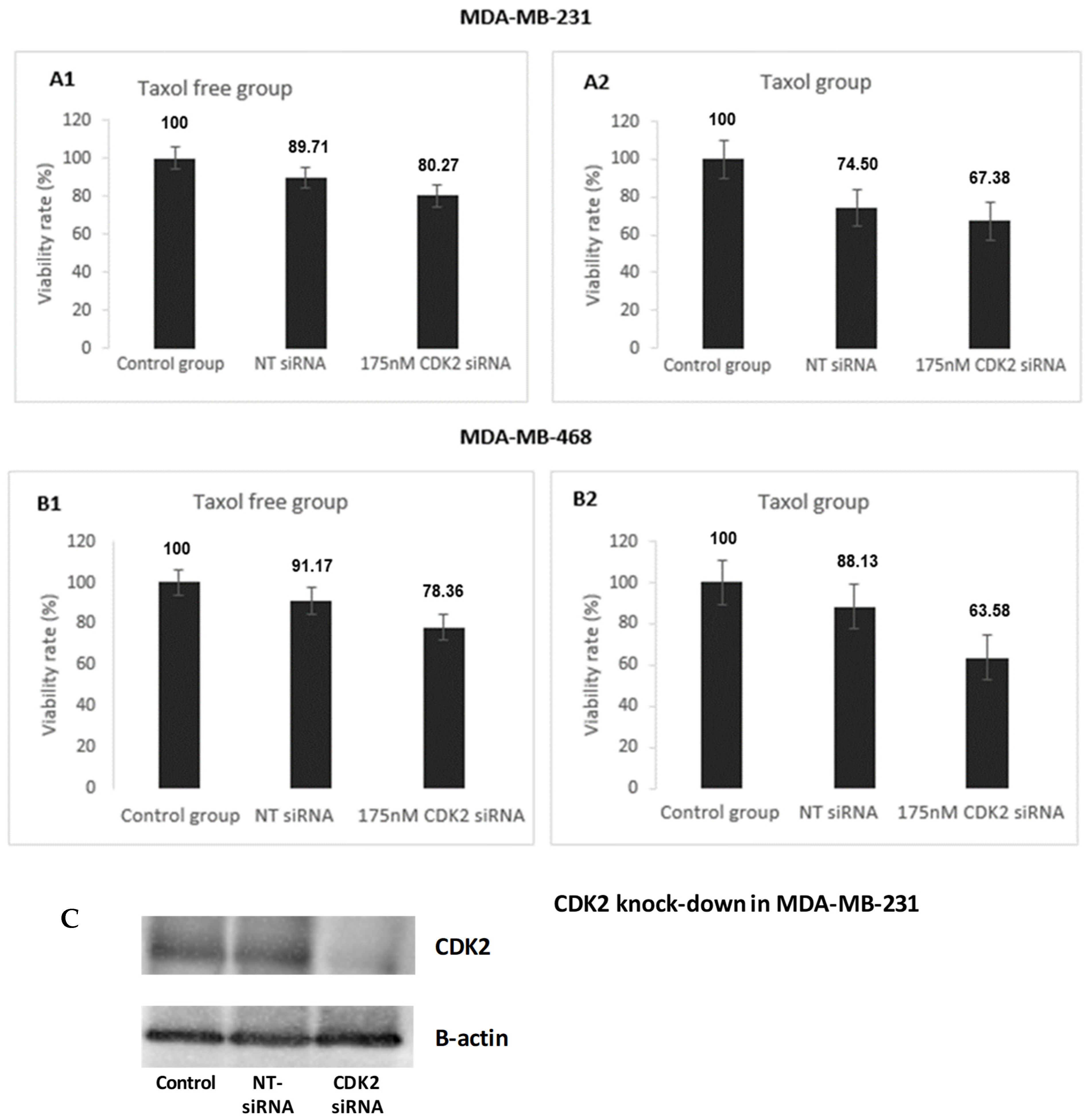
2.8. CDK2 Knockdown
2.9. Statistical Analysis
3. Results
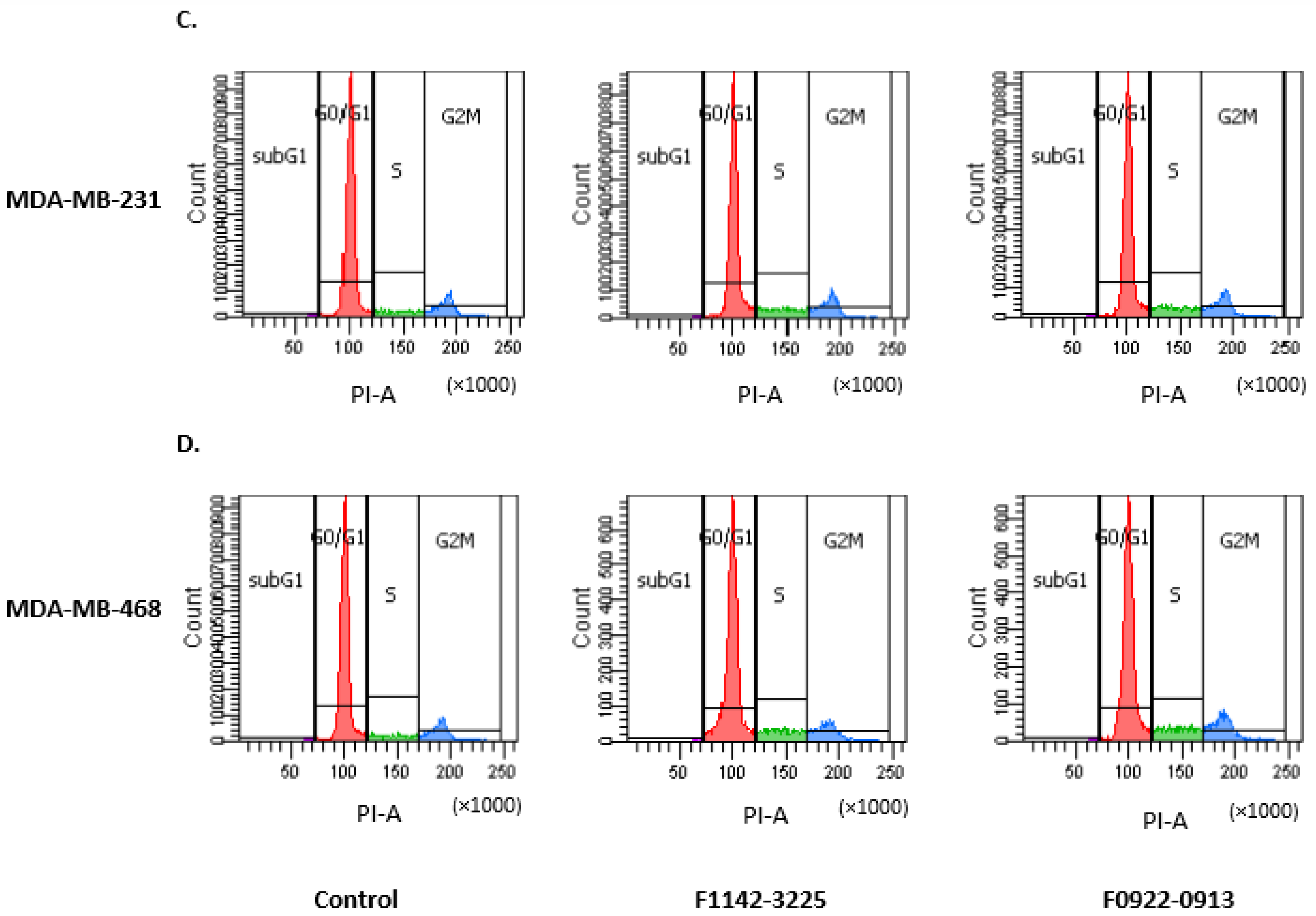
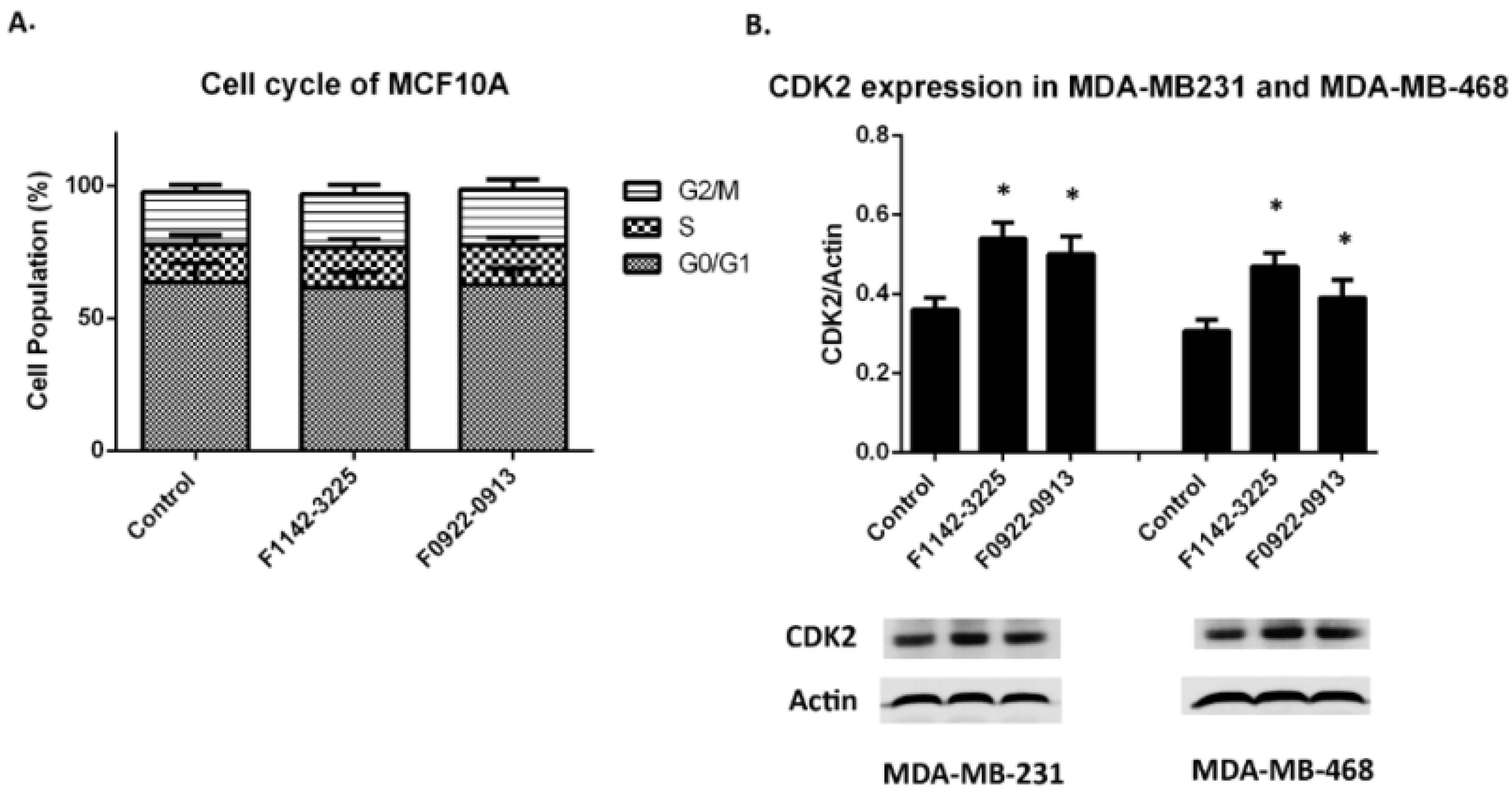
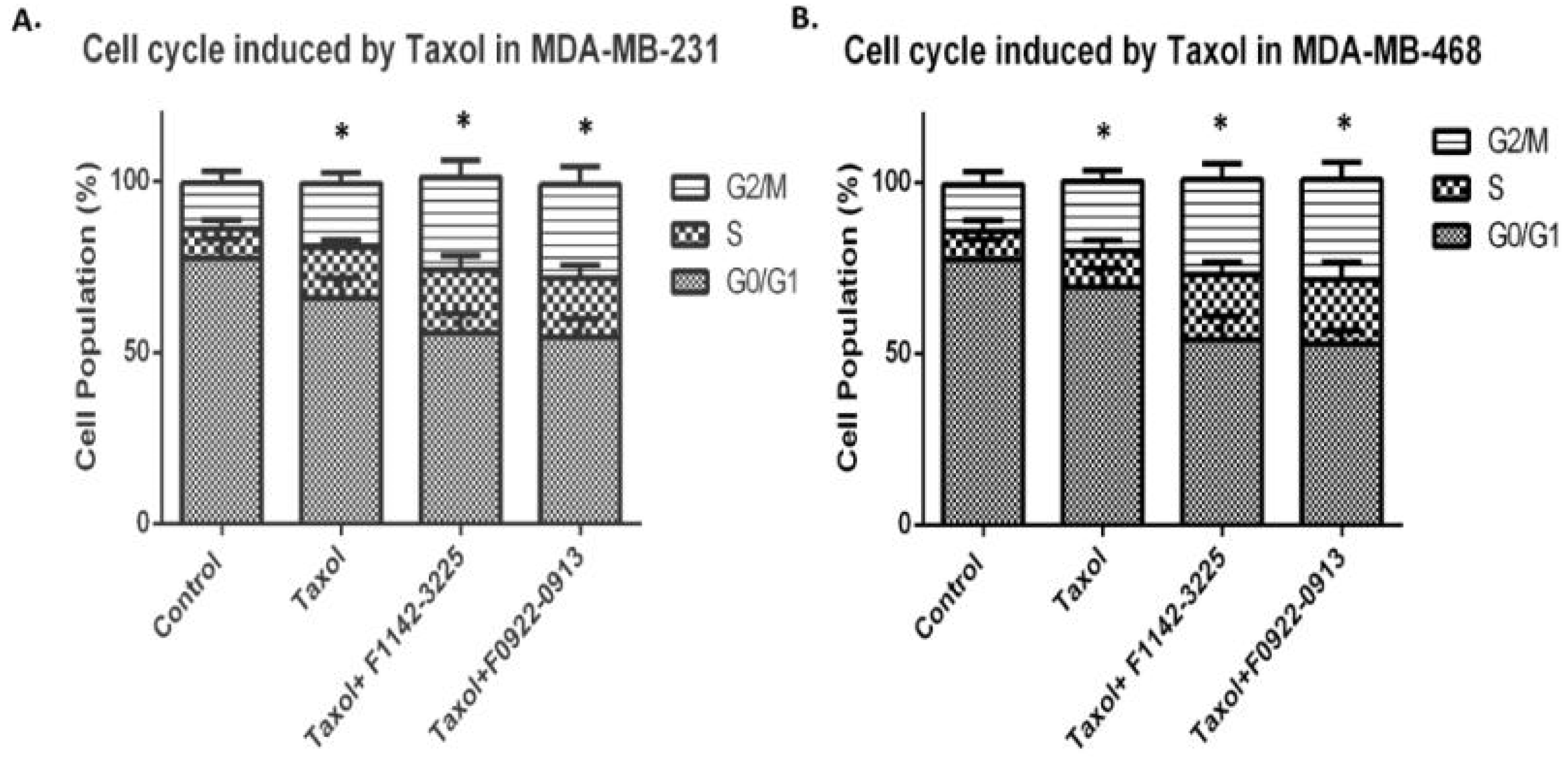
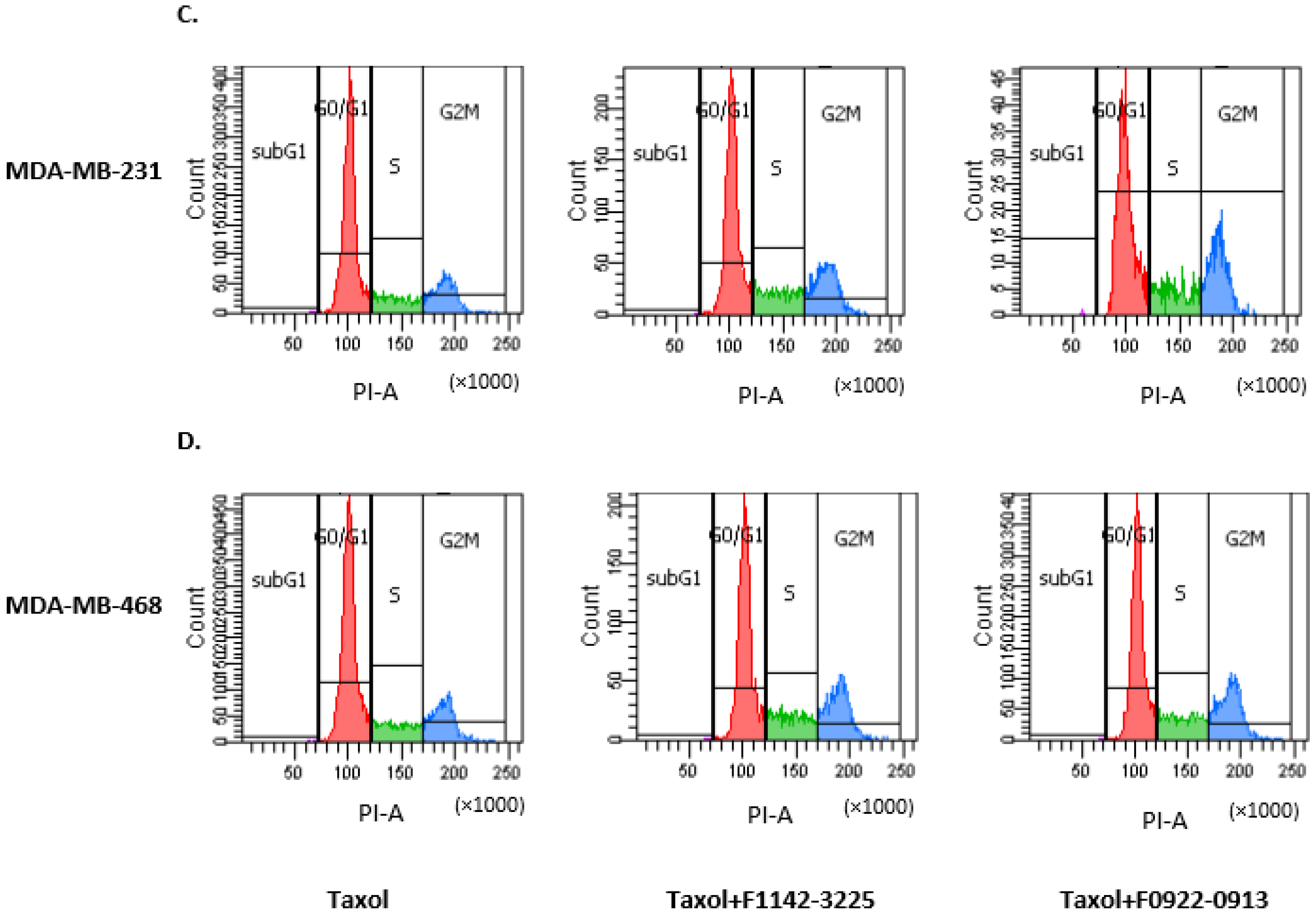
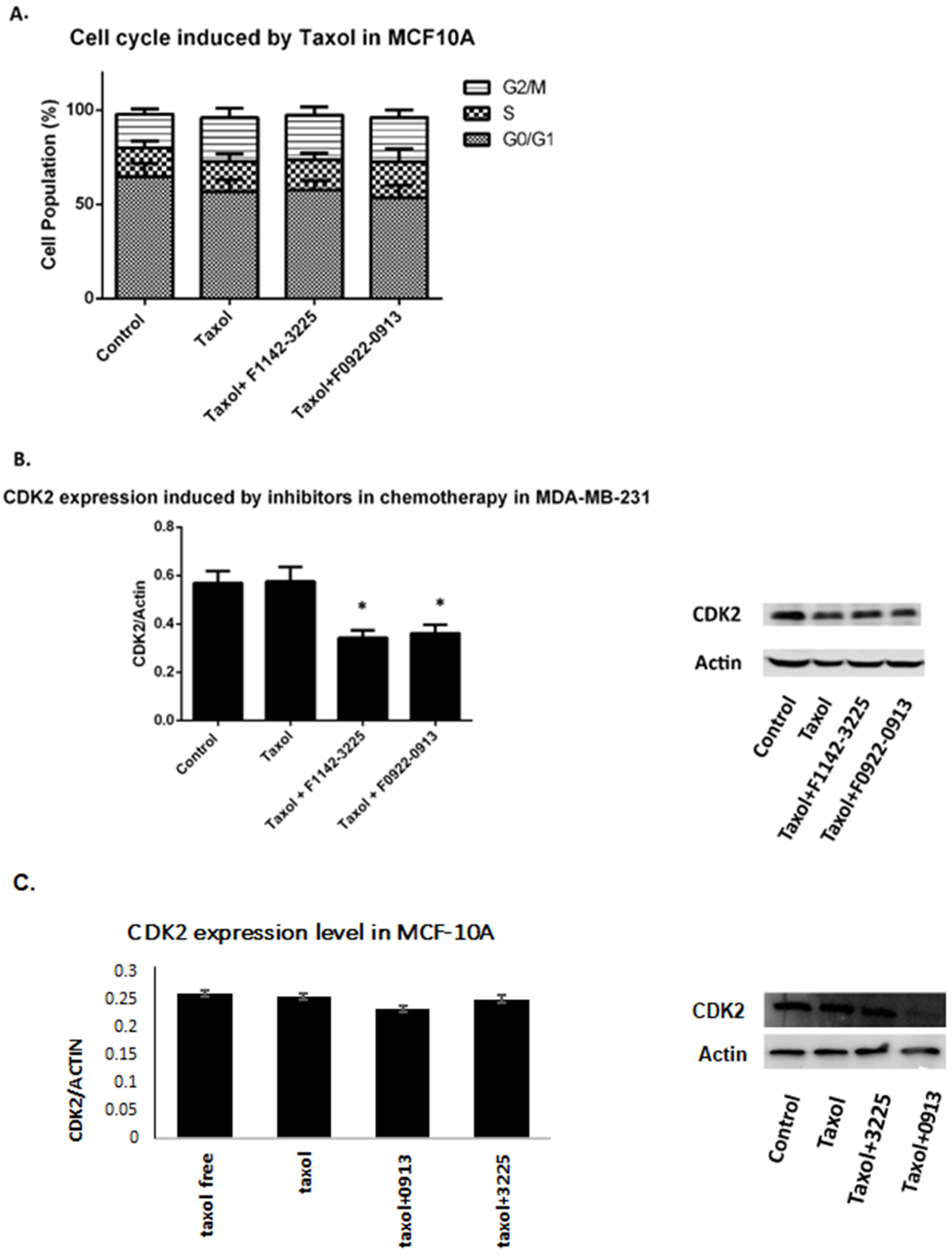
3.1. The CRIF1–CDK2 Interface Inhibitors Promoted the Transition of the Cell from G0/G1 to S Phase in TNBC Cell Lines
3.1.1. TNBC Cell Cycle Regulation
3.1.2. Effect of CDK2 Knock Down on TNBC Cell Proliferation
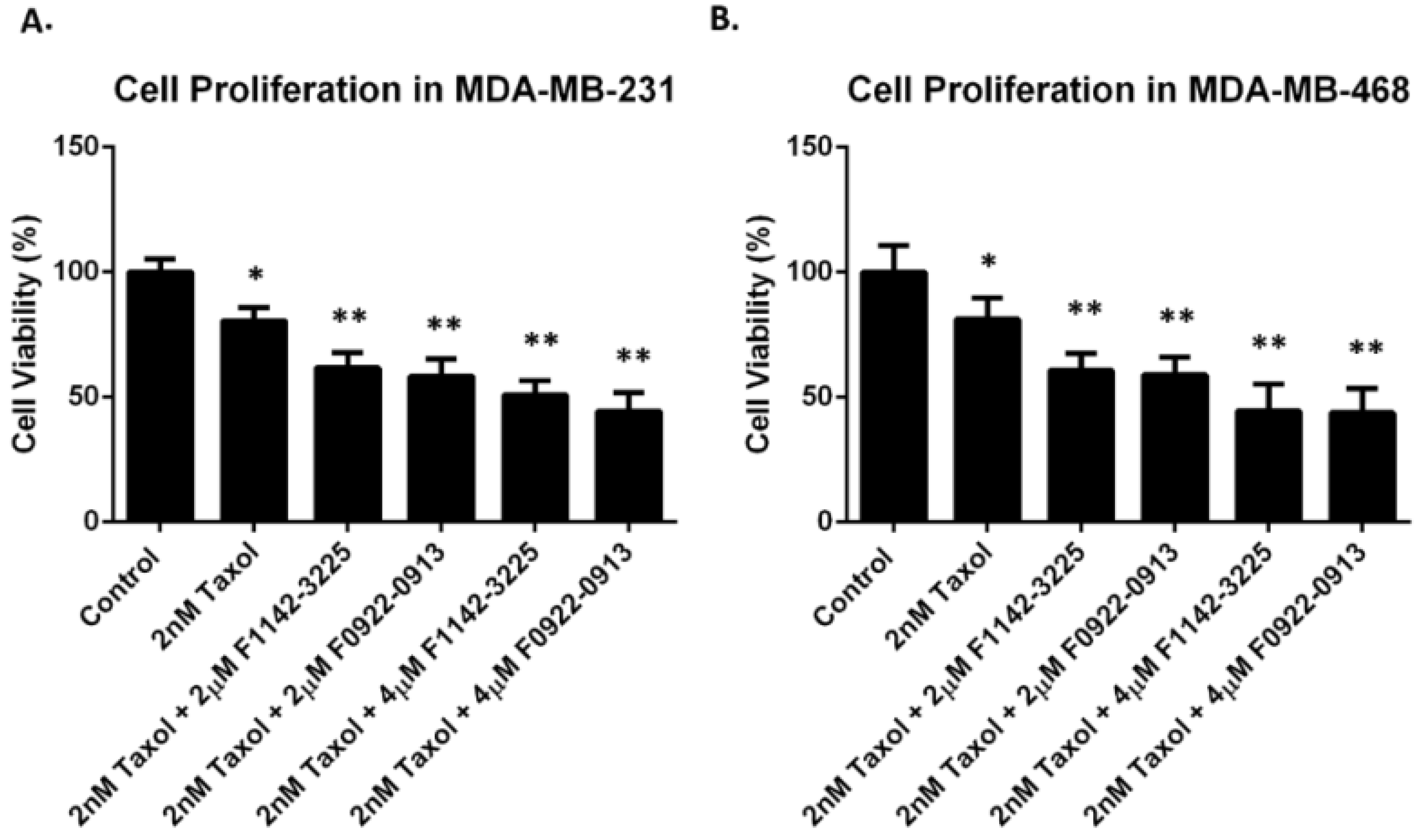
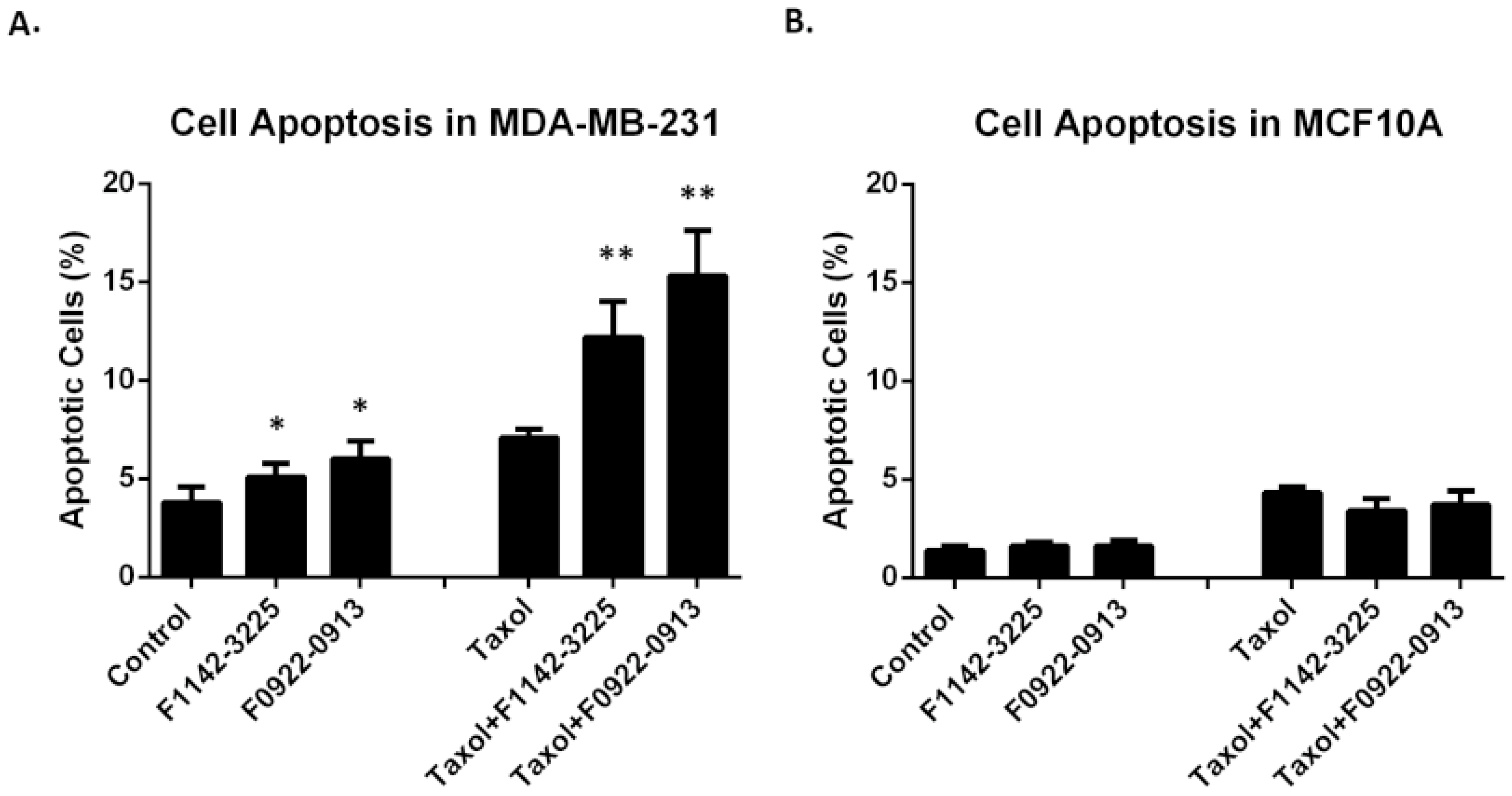
3.2. The CRIF1–CDK2 Interface Inhibitors Induced Apoptosis in TNBC Cell Line but Not in MCF10A
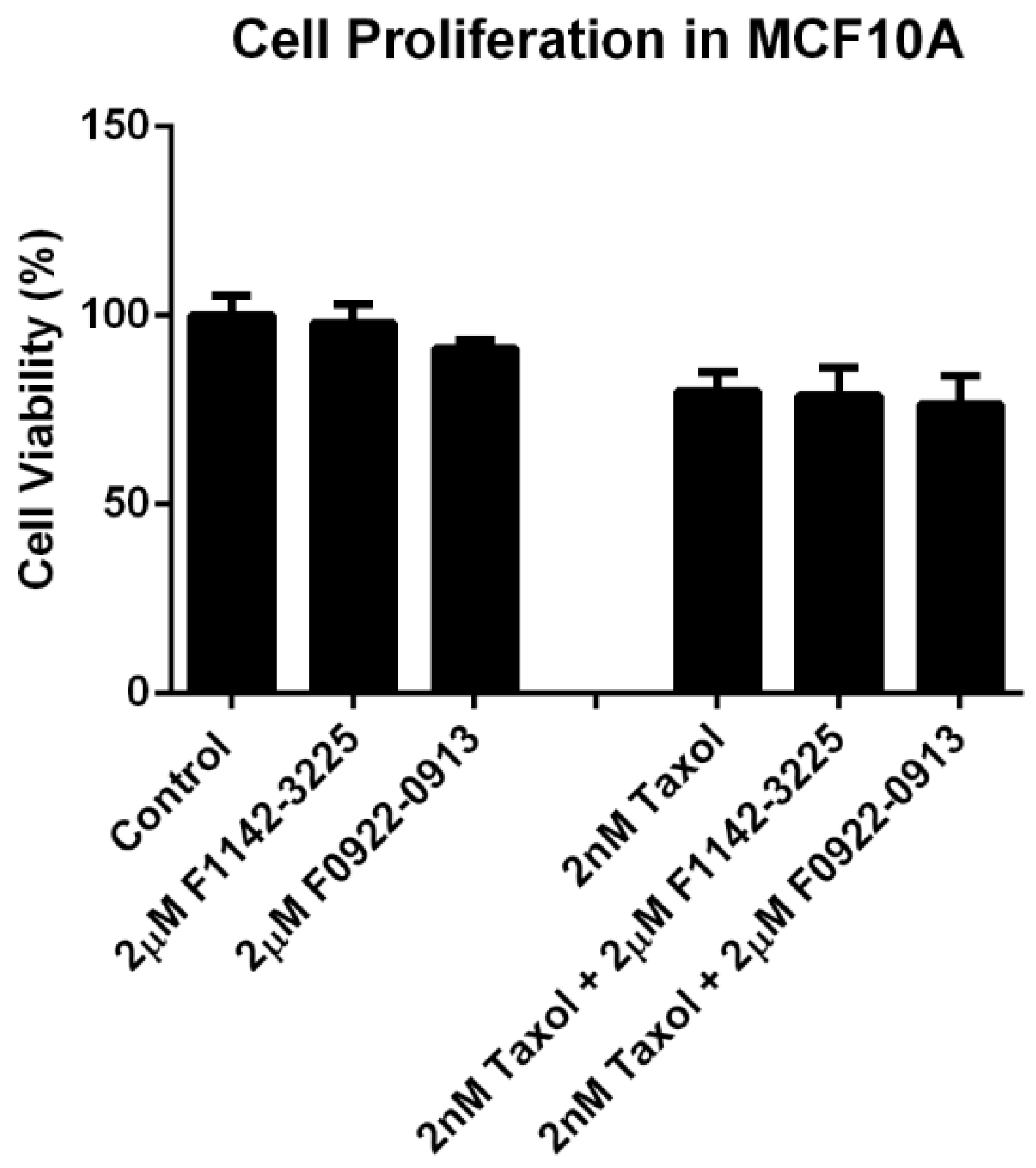
3.3. No Significant Cytotoxicity Was Observed for the CRIF1–CDK2 Interface Inhibitors within the Treatment Concentration in MCF10A Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Kalampokas, E.; Kalampokas, T.; Spartalis, E.; Daskalopoulou, A.; Valsami, S.; Kontos, M.; Nonni, A.; et al. Histone Deacetylases as New Therapeutic Targets in Triple-negative Breast Cancer: Progress and Promises. Cancer Genom. Proteom. 2017, 14, 299–313. [Google Scholar] [CrossRef][Green Version]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-negative breast cancer: Is there a treatment on the horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef]
- Jin, J.; Fang, H.; Yang, F.; Ji, W.; Guan, N.; Sun, Z.; Shi, Y.; Zhou, G.; Guan, X. Combined Inhibition of ATR and WEE1 as a Novel Therapeutic Strategy in Triple-Negative Breast Cancer. Neoplasia 2018, 20, 478–488. [Google Scholar] [CrossRef]
- Wahba, H.A.; El-Hadaad, H.A. Current approaches in treatment of triple-negative breast cancer. Cancer Biol. Med. 2015, 12, 106–116. [Google Scholar] [CrossRef]
- Oudin, M.J.; Barbier, L.; Schäfer, C.; Kosciuk, T.; Miller, M.A.; Han, S.; Jonas, O.; Lauffenburger, D.A.; Gertler, F.B. MENA Confers Resistance to Paclitaxel in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2017, 16, 143–155. [Google Scholar] [CrossRef]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Earnshaw, W.C. Induction of Apoptosis by Cancer Chemotherapy. Exp. Cell Res. 2000, 256, 42–49. [Google Scholar] [CrossRef]
- Gluz, O.; Liedtke, C.; Gottschalk, N.; Pusztai, L.; Nitz, U.; Harbeck, N. Triple-negative breast cancer—Current status and future directions. Ann. Oncol. 2009, 20, 1913–1927. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Choi, Y.J.; Anders, L. Signaling through cyclin D-dependent kinases. Oncogene 2014, 33, 1890–1903. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Sergio, C.M.; Sutherland, R.L.; Musgrove, E.A. Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells. BMC Cancer 2014, 14, 32. [Google Scholar] [CrossRef]
- Ran, Q.; Hao, P.; Xiao, Y.; Xiang, L.; Ye, X.; Deng, X.; Zhao, J.; Li, Z. CRIF1 Interacting with CDK2 Regulates Bone Marrow Microenvironment-Induced G0/G1 Arrest of Leukemia Cells. PLoS ONE 2014, 9, e85328. [Google Scholar] [CrossRef]
- Ran, Q.; Xiang, Y.; Stephen, P.; Wu, C.; Li, T.; Lin, S.-X.; Li, Z. CRIF1–CDK2 Interface Inhibitors: An Unprecedented Strategy for Modulation of Cell Radiosensitivity. J. Am. Chem. Soc. 2019, 141, 1420–1424. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef]
- Hjortoe, G.M. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood 2004, 103, 3029–3037. [Google Scholar] [CrossRef]
- Law, M.E.; Corsino, P.E.; Narayan, S.; Law, B.K. Cyclin-Dependent Kinase Inhibitors as Anticancer Therapeutics. Mol. Pharmacol. 2015, 88, 846–852. [Google Scholar] [CrossRef]
- Ran, Q.; Jin, F.; Xiang, Y.; Xiang, L.; Wang, Q.; Li, F.; Chen, L.; Zhang, Y.; Wu, C.; Zhou, L.; et al. CRIF1 as a potential target to improve the radiosensitivity of osteosarcoma. Proc. Natl. Acad. Sci. USA 2019, 116, 20511–20516. [Google Scholar] [CrossRef]
- Cui, P.H.; Rawling, T.; Gillani, T.B.; Bourget, K.; Wang, X.-S.; Zhou, F.; Murray, M. Anti-proliferative actions of N′-desmethylsorafenib in human breast cancer cells. Biochem. Pharmacol. 2013, 86, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Han, B.; Yu, Y.; Yao, W.; Bose, S.; Karlan, B.Y.; Giuliano, A.E.; Cui, X. Evaluation of MCF10A as a Reliable Model for Normal Human Mammary Epithelial Cells. PLoS ONE 2015, 10, e0131285. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; Harper, J.W. Anticancer drug targets: Cell cycle and checkpoint control. J. Clin. Investig. 1999, 104, 1645–1653. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Yvon, A.-M.C.; Wadsworth, P.; Jordan, M.A. Taxol Suppresses Dynamics of Individual Microtubules in Living Human Tumor Cells. Mol. Biol. Cell 1999, 10, 947–959. [Google Scholar] [CrossRef]
- Tin, A.S.; Sundar, S.N.; Tran, K.Q.; Park, A.H.; Poindexter, K.M.; Firestone, G.L. Antiproliferative effects of artemisinin on human breast cancer cells requires the downregulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes. Anti-Cancer Drugs 2012, 23, 370–379. [Google Scholar] [CrossRef]
- He, X.; Xiang, H.; Zong, X.; Yan, X.; Yu, Y.; Liu, G.; Zou, D.; Yang, H. CDK2-AP1 inhibits growth of breast cancer cells by regulating cell cycle and increasing docetaxel sensitivity in vivo and in vitro. Cancer Cell Int. 2014, 14, 130. [Google Scholar] [CrossRef]
- Mills, C.C.; Kolb, E.; Sampson, V.B. Development of Chemotherapy with Cell-Cycle Inhibitors for Adult and Pediatric Cancer Therapy. Cancer Res. 2018, 78, 320–325. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Robert, S. DiPaola To Arrest or Not To G2-M Cell-Cycle Arrest. Clin. Cancer Res. 2002, 8, 3311–3314. [Google Scholar]
- Cancer, A.B.; Hematopoiesis, C.; Myopathy, A.; Ozaki, A.; Tanimoto, T.; Saji, S. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 1672–1673. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, X.; Belmessabih, N.; Wang, R.; Stephen, P.; Lin, S.-X. CRIF1-CDK2 Interface Inhibitors Enhance Taxol Inhibition of the Lethal Triple-Negative Breast Cancer. Cancers 2022, 14, 989. https://doi.org/10.3390/cancers14040989
Sang X, Belmessabih N, Wang R, Stephen P, Lin S-X. CRIF1-CDK2 Interface Inhibitors Enhance Taxol Inhibition of the Lethal Triple-Negative Breast Cancer. Cancers. 2022; 14(4):989. https://doi.org/10.3390/cancers14040989
Chicago/Turabian StyleSang, Xiaoye, Nassira Belmessabih, Ruixuan Wang, Preyesh Stephen, and Sheng-Xiang Lin. 2022. "CRIF1-CDK2 Interface Inhibitors Enhance Taxol Inhibition of the Lethal Triple-Negative Breast Cancer" Cancers 14, no. 4: 989. https://doi.org/10.3390/cancers14040989
APA StyleSang, X., Belmessabih, N., Wang, R., Stephen, P., & Lin, S.-X. (2022). CRIF1-CDK2 Interface Inhibitors Enhance Taxol Inhibition of the Lethal Triple-Negative Breast Cancer. Cancers, 14(4), 989. https://doi.org/10.3390/cancers14040989





