Pelvic Exenteration for the Treatment of Locally Advanced Vulvar Cancer in South West Wales
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Procedures
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients and Outcomes of Treatment for Locally Advanced Vulvar Cancer
3.2. Pelvic Exenteration for Primary Treatment of Locally Advanced Vulvar Cancer
3.3. Pelvic Exenteration for Recurrent Locally Advanced Vulvar Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royal College of Obstetricians and Gynaecologists. Guidelines for the Diagnosis and Management of Vulval Carcinoma. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/vulvalcancerguideline.pdf (accessed on 11 September 2020).
- Hoffman, M.S. Squamous-cell carcinoma of the vulva: Locally advanced disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 635–647. [Google Scholar] [CrossRef]
- Imoto, S.; Inamine, M.; Kudaka, W.; Nagai, Y.; Wakayama, A.; Nakamoto, T.; Ooyama, T.; Aoki, Y. Prognostic factors in patients with vulvar cancer treated with primary surgery: A single-center experience. SpringerPlus 2016, 5, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudineau, A.; Weitbruch, D.; Quetin, P.; Heymann, S.; Petit, T.; Volkmar, P.; Bodin, F.; Velten, M.; Rodier, J.F. Neoadjuvant chemoradiotherapy followed by surgery in locally advanced squamous cell carcinoma of the vulva. Oncol. Lett. 2012, 4, 719–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, N.; Aoki, Y.; Fujita, K.; Tanaka, K. Stage IVa squamous cell carcinoma of the vulva managed with primary chemoradiation. Int. J. Clin. Oncol. 2005, 10, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, H.C.; Ansink, A.; Verhaar-Langereis, M.M.J.; Stalpers, L.L. Neoadjuvant chemoradiation for advanced primary vulvar cancer. Cochrane Database Syst. Rev. 2006, 3, CD003752. [Google Scholar]
- Maneo, A.; Landoni, F.; Colombo, A.; Colombo, A.; Villa, A.; Caspani, G. Randomised study between neoadjuvant chemoradiotherapy and primary surgery for the treatment of advanced vulvar cancer. Int. J. Gynecol. Cancer 2003, 13 (Suppl. 1), 6. [Google Scholar] [CrossRef]
- Landrum, L.M.; Lanneau, G.S.; Skaggs, V.J.; Gould, N.; Walker, J.L.; McMeekin, D.S.; Gold, M.A. Gynecologic Oncology Group risk groups for vulvar carcinoma: Improvement in survival in the modern era. Gynecol. Oncol. 2007, 106, 521–525. [Google Scholar] [CrossRef]
- Aragona, A.M.; Soderini, A.H.; Cuneo, N.A. Defining the concept of locally advanced squamous cell carcinoma of the vulva: A new perspective based on standardization of criteria and current evidence. J. Gynecol. Oncol. 2014, 25, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Hacker, N.F.; Eifel, P.J. Vulvar Cancer. In Berek & Hacker’s Gynecologic Oncology; Berek, J.S., Hacker, N.F., Eds.; Wolters Kluwer Health: Philadelphia, PA, USA, 2020; pp. 503–546. [Google Scholar]
- Hopkins, M.P.; Morley, G.W. Pelvic exenteration for the treatment of vulvar cancer. Cancer 1992, 70, 2835–2838. [Google Scholar] [CrossRef] [Green Version]
- Forner, D.M.; Lampe, B. Exenteration in the treatment of Stage III/IV vulvar cancer. Gynecol. Oncol. 2012, 124, 87–91. [Google Scholar] [CrossRef]
- O’Donnell, R.L.; Verleye, L.; Ratnavelu, N.; Galaal, K.; Fisher, A.; Naik, R. Locally advanced vulva cancer: A single centre review of anovulvectomy and a systematic review of surgical, chemotherapy and radiotherapy alternatives. Is an international collaborative RCT destined for the “too difficult to do” box? Gynecol. Oncol. 2017, 144, 438–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, J.A.; Eiriksson, L.; Dean, E.; Sebastianelli, A.; Bahoric, B.; Salvador, S. No. 370-Management of Squamous Cell Cancer of the Vulva. J. Obstet. Gynaecol. Can. 2019, 41, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Morris, M.; Levenback, C.; Burke, T.W.; Gershenson, D.M. Pelvic exenteration for primary and recurrent vulvar cancer. Gynecol. Oncol. 1995, 58, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Collarino, A.; Garganese, G.; Valdés Olmos, R.A.; Stefanelli, A.; Perotti, G.; Mirk, P.; Fragomeni, S.M.; Ieria, F.P.; Scambia, G.; Giordano, A.; et al. Evaluation of Dual-Timepoint 18F-FDG PET/CT Imaging for Lymph Node Staging in Vulvar Cancer. J. Nucl. Med. 2017, 58, 1913–1918. [Google Scholar] [CrossRef] [Green Version]
- Robertson, N.L.; Hricak, H.; Sonoda, Y.; Sosa, R.E.; Benz, M.; Lyons, G.; Abu-Rustum, N.R.; Sala, E.; Vargas, H.A. The impact of FDG-PET/CT in the management of patients with vulvar and vaginal cancer. Gynecol. Oncol. 2016, 140, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Rodriguwz-Bigas, M.A.; Petrelli, N.J. Pelvic exenteration and its modifications. Am. J. Surg. 1996, 171, 293–298. [Google Scholar] [CrossRef]
- Schiessel, R.; Novi, G.; Holzer, B.; Rosen, H.R.; Renner, K.; Hölbling, N.; Feil, W.; Urban, M. Technique and long-term results of intersphincteric resection for low rectal cancer. Dis. Colon. Rectum. 2005, 48, 1858–1865. [Google Scholar] [CrossRef]
- Brady, R.R.; Collie, M.H.; Ho, G.T.; Bartolo, D.C.; Wilson, R.G.; Dunlop, M.G. Outcomes of the rectal remnant following colectomy for ulcerative colitis. Colorectal Dis. 2008, 10, 144–150. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Imesch, P.; Fink, D.; Egger, H. Indications and long-term clinical outcomes in 282 patients with pelvic exenteration for advanced or recurrent cervical cancer. Gynecol. Oncol. 2012, 125, 604–609. [Google Scholar] [CrossRef]
- Westin, S.N.; Rallapalli, V.; Fellman, B.; Urbauer, D.L.; Pal, N.; Frumovitz, M.M.; Ramondetta, L.M.; Bodurka, D.C.; Ramirez, P.T.; Soliman, P.T. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecol. Oncol. 2014, 134, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ma, S.Q.; Tan, X.J.; Zhong, S.; Wu, M. Pelvic Exenteration for recurrent and persistent cervical cancer. Chin. Med. J. 2018, 131, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, M.P.; Sood, A.K.; Dos Reis, R.; Ramalingam, P.; Chen, C.; Frumovitz, M.; Jhingran, A.; Pitcher, B.; Ramirez, P.T.; Schmeler, K.M. Perineural invasion (PNI) in vulvar carcinoma: A review of 421 cases. Gynecol. Oncol. 2019, 152, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Forte, S.; Ardighieri, L.; Bonetti, E.; Fernando, B.; Sartori, E.; Odicino, F. Multivariate analysis of prognostic factors in primary squamous cell vulvar cancer: The role of perineural invasion in recurrence and survival. Eur. J. Surg. Oncol. 2019, 45, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Wydra, D.; Emerich, J.; Sawicki, S.; Ciach, K.; Marciniak, A. Major complications following exenteration in cases of pelvic malignancy: A 10-year experience. World J. Gastroenterol. 2006, 12, 1115–1119. [Google Scholar] [CrossRef]
- Beller, U.; Benedet, J.L.; Creasman, W.T.; Ngan, H.Y.; Quinn, M.A.; Maisonneuve, P.; Pecorelli, S.; Odicino, F.; Heintz, A.P. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S7–S27. [Google Scholar] [CrossRef]
- Koh, W.J.; Wallace, H.J., 3rd; Greer, B.E.; Cain, J.; Stelzer, K.J.; Russell, K.J.; Tamimi, H.K.; Figge, D.C.; Russell, A.H.; Griffin, T.W. Combined radiotherapy and chemotherapy in the management of local-regionally advanced vulvar cancer. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 809–816. [Google Scholar] [CrossRef]
- Rogers, L.J.; Howard, B.; Van Wijk, L.; Wei, W.; Dehaeck, K.; Soeters, R.; Denny, L.A. Chemoradiation in advanced vulval carcinoma. Int. J. Gynecol. Cancer. 2009, 19, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Mukai, Y.; Koike, I.; Matsunaga, T.; Yokota, N.R.; Kaizu, H.; Takano, S.; Sugiura, M.; Ito, E.; Miyagi, E.; Hata, M. Outcome of Radiation Therapy for Locally Advanced Vulvar Carcinoma: Analysis of Inguinal Lymph Node. In Vivo 2020, 34, 307–313. [Google Scholar] [CrossRef]
- Shylasree, T.S.; Bryant, A.; Howells, R.E. Chemoradiation for advanced primary vulval cancer. Cochrane Database Syst. Rev. 2011, 2011, CD003752. [Google Scholar] [CrossRef]
- Mulayim, N.; Foster Silver, D.; Schwartz, P.E.; Higgins, S. Chemoradiation with 5-fluorouracil and mitomycin C in the treatment of vulvar squamous cell carcinoma. Gynecol. Oncol. 2004, 93, 659–666. [Google Scholar] [CrossRef] [PubMed]
| All | Primary | Recurrence | |
|---|---|---|---|
| Patient Demographics | |||
| Number of patients (n) | 19 | 14 | 5 |
| Mean age (years, range) | 65.2 (47–81) | 62.2 (47–81) | 73.6 (70–78) |
| Mean BMI (n = 12, range) | 30.3 (19.9–40) | 30.4 (19.9–40) | 29.8 (24.5–37) |
| American Society of Anesthesiologists (ASA) Physical Status (n/%) | |||
| I | 1 (5.3) | 1 (7.1) | 0 (0) |
| II | 6 (31.6) | 5 (35.7) | 1 (20) |
| III | 5 (26.3) | 3 (21.4) | 2 (40) |
| Missing | 7 (36.8) | 5 (35.7) | 2 (40) |
| Surgical Outcomes | |||
| Figo Stage (n,%) | |||
| Stage II | 2 (14.2) | 2 (14.2) | N/A |
| Stage III | 2 (14.2) | 2 (14.2) | N/A |
| Stage IV | 10 (71.4) | 10 (71.4) | N/A |
| Histology (n/%) | |||
| Squamous cell carcinoma | 19 (100) | 14 (100) | 5 (100) |
| Differentiation (n/%) | |||
| Well | 1 (5.2) | 0 (0) | 1 (20) |
| Moderate | 12 (63.2) | 10 (71.4) | 2 (40) |
| Poorly | 6 (31.6) | 4 (28.6) | 2 (40) |
| MEAN TUMOUR DIAMETER (range, mm) | 52.8 (18–100) | 54.7 (18–100) | 47.6 (32–60) |
| Resection Margin (n, %) | |||
| Surgical R0 (microscopically negative) | 18 (94.7) | 14 (100) | 4 (80) |
| Surgical R1 (microscopically remnant) | 1 (5.33) | 0 (0) | 1 (20) |
| Surgical R2 (macroscopic remnant) | 0 (0) | 0 (0) | 0 (0) |
| Nodal Status | |||
| Inguinal lymph node metastases (n, %) | 10 (71.4) | 8 (66.7) | 2 (100) |
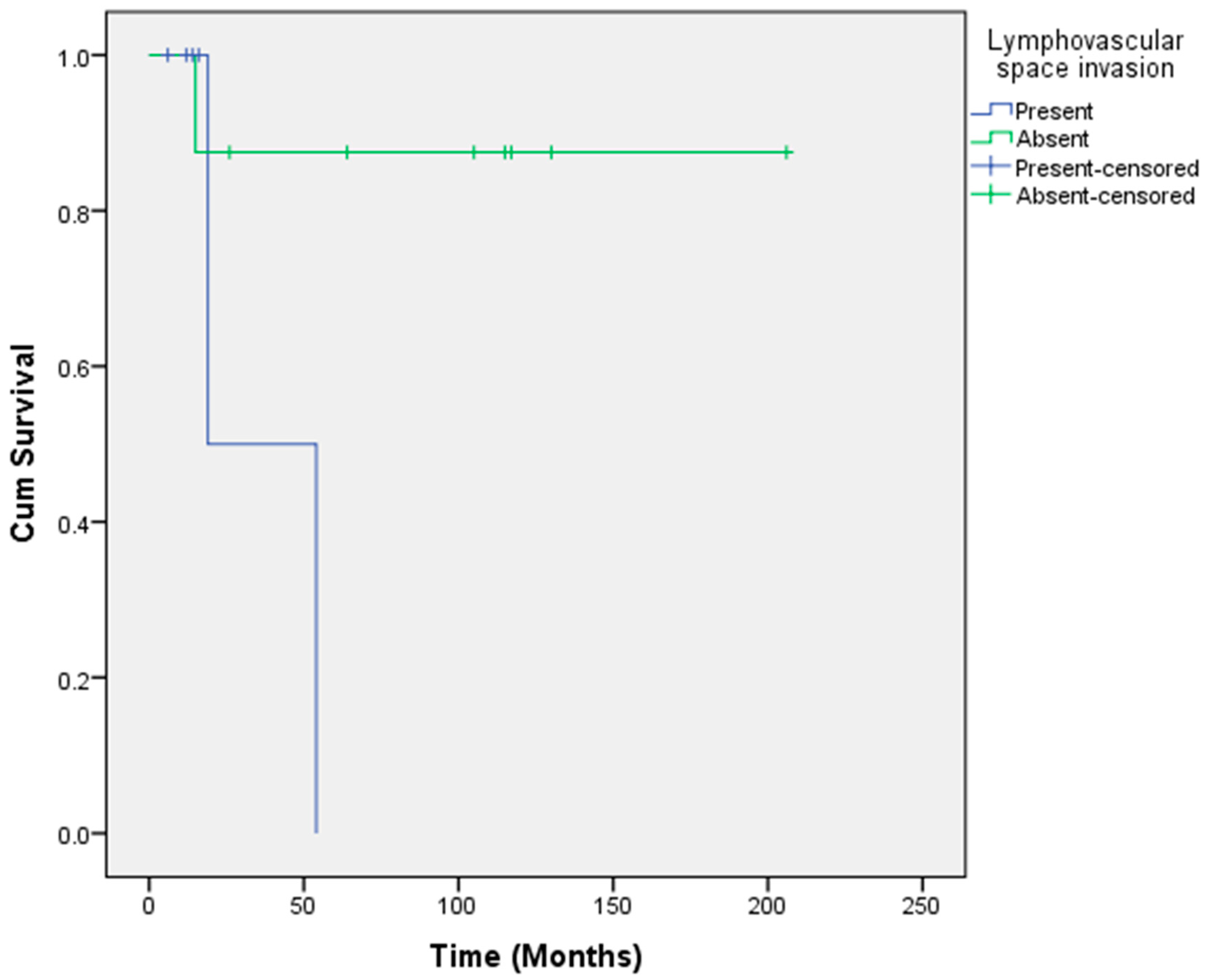
| Lymphovascular space invasion present (n, %) | 9 (47.4) | 6 (42.9) | 3 (60) |
| Perineural invasion present (n, %) | 8 (42.1) | 5 (35.7) | 3 (60) |
| Lymphovascular space invasion + Perineural invasion present (n, %) | 4 (21.1) | 2 (14.3) | 2 (40) |
| Node positive without extracapsular spread | 8 | 7 | 1 |
| Node positive with extracapsular spread | 2 | 1 | 1 |
| Node negative | 4 | 4 | 0 |
| Surgical Procedure (n, %) | |||
| Posterior exenteration | 14 (73.7) | 11 (78.6) | 3 (60) |
| Total exenteration | 5 (26.3) | 3 (21.4) | 2 (40) |
| Ileal conduit | 5 (26.3) | 3 (21.4) | 2 (40) |
| Reconstruction (n, %): | |||
| Primary closure | 6 (31.6) | 4 (28.6) | 2 (40) |
| Vertical Rectus Abdominis Myocutaneous Flap (VRAM) | 5 (26.3) | 3 (21.4) | 2 (40) |
| Bilateral gracilis myocutaneous flap | 2 (10.5) | 2 (14.3) | 0 (0) |
| VRAM + gracilis | 3 (15.8) | 2 (14.3) | 1 (20) |
| Inferior gluteal artery myocutaneous (IGAM) | 1 (5.3) | 1 (7.1) | 0 (0) |
| Fasciocutaneous flap | 2 (10.5) | 2 (14.3) | 0 (0) |
| Perioperative Features | |||
| Mean blood loss (mL, range) | 667 (150–2180) | 798 (200–2180) | 338 (150–500) |
| Major morbidity {Clavien–Dindo Grade 3 and above, n (%)} | 9 (47.4) | 6 (42.9) | 3 (60) |
| Mean length of stay (days) | 20 (9–39) | 19 (9–39) | 24 (14–30) |
| 30-day major morbidity rate (n, %) | 8/19 (42.1%) | 6/14 (42.9%) | 2/5 (40%) |
| 30-day mortality rate | 0 | 0 | 0 |
| Survival | |||
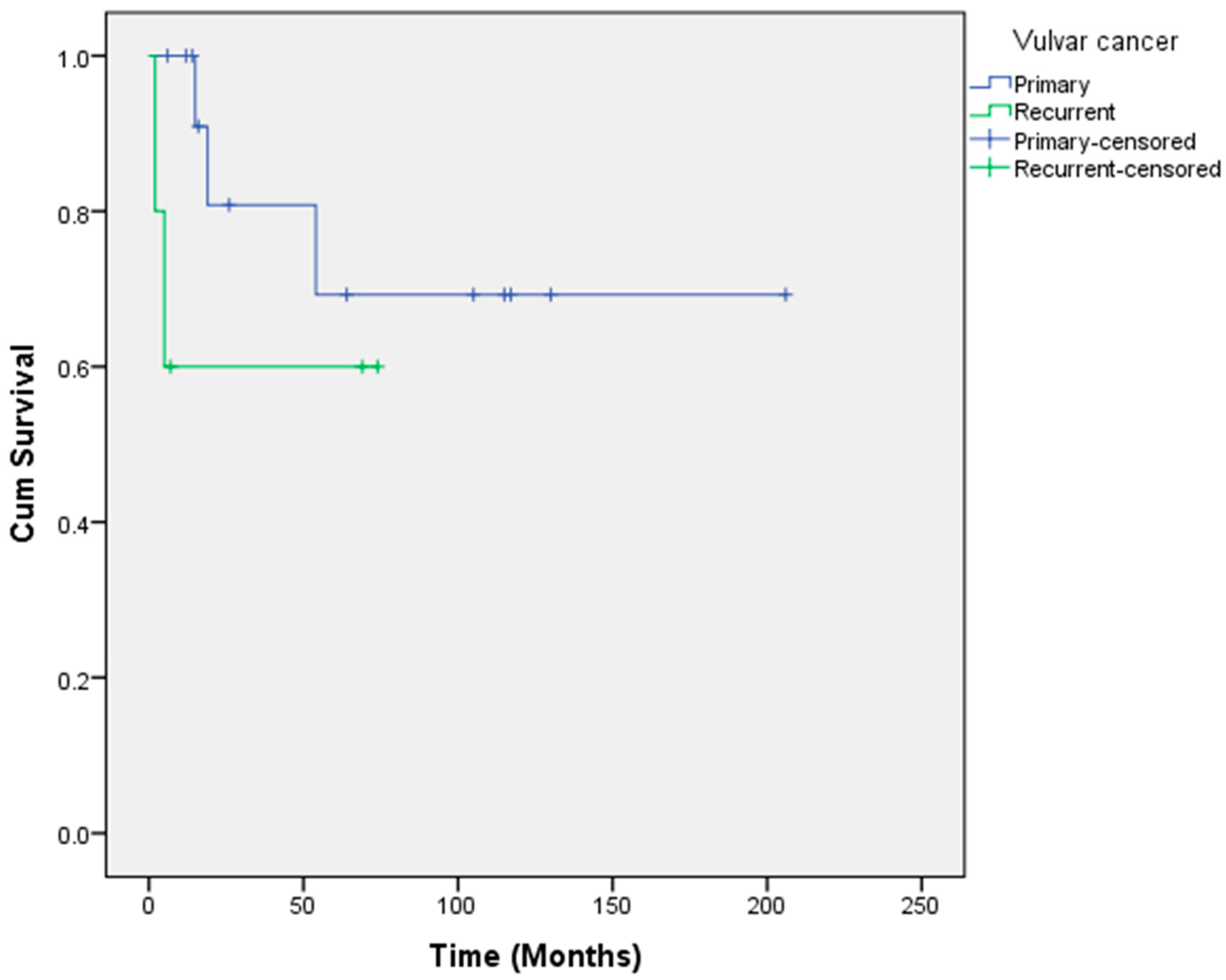
| Overall survival (months, range) | 144.8 (2–206) | 152.2 (6–206) | 45.8 (2–74) |
| % 1-year survival | 89.5 | 100 | 60 |
| % 5-year survival | 66.7 | 69.3 | 60 |
| % 10-year survival | 66.7 | 69.3 | Not reached yet |
| Overall survival when lymphovascular space invasion present (months) | 44.1 | 36.5 | 51 |
| Overall survival when lymphovascular space invasion absent (months) | 166.5 | 182.1 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulrahman, G.O.; Das, N.; Chandrasekaran, T.V.; Khot, U.; Drew, P.J.; Bose, P.; Vet, J.N.; Tofazzal, N.; Roberts, S.; Lutchman Singh, K. Pelvic Exenteration for the Treatment of Locally Advanced Vulvar Cancer in South West Wales. Cancers 2022, 14, 1767. https://doi.org/10.3390/cancers14071767
Abdulrahman GO, Das N, Chandrasekaran TV, Khot U, Drew PJ, Bose P, Vet JN, Tofazzal N, Roberts S, Lutchman Singh K. Pelvic Exenteration for the Treatment of Locally Advanced Vulvar Cancer in South West Wales. Cancers. 2022; 14(7):1767. https://doi.org/10.3390/cancers14071767
Chicago/Turabian StyleAbdulrahman, Ganiy Opeyemi, Nagindra Das, Thipparajapura V. Chandrasekaran, Umesh Khot, Peter J. Drew, Pradeep Bose, Jessica N. Vet, Nasima Tofazzal, Shaun Roberts, and Kerryn Lutchman Singh. 2022. "Pelvic Exenteration for the Treatment of Locally Advanced Vulvar Cancer in South West Wales" Cancers 14, no. 7: 1767. https://doi.org/10.3390/cancers14071767
APA StyleAbdulrahman, G. O., Das, N., Chandrasekaran, T. V., Khot, U., Drew, P. J., Bose, P., Vet, J. N., Tofazzal, N., Roberts, S., & Lutchman Singh, K. (2022). Pelvic Exenteration for the Treatment of Locally Advanced Vulvar Cancer in South West Wales. Cancers, 14(7), 1767. https://doi.org/10.3390/cancers14071767





