Targeted Therapies for Perihilar Cholangiocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
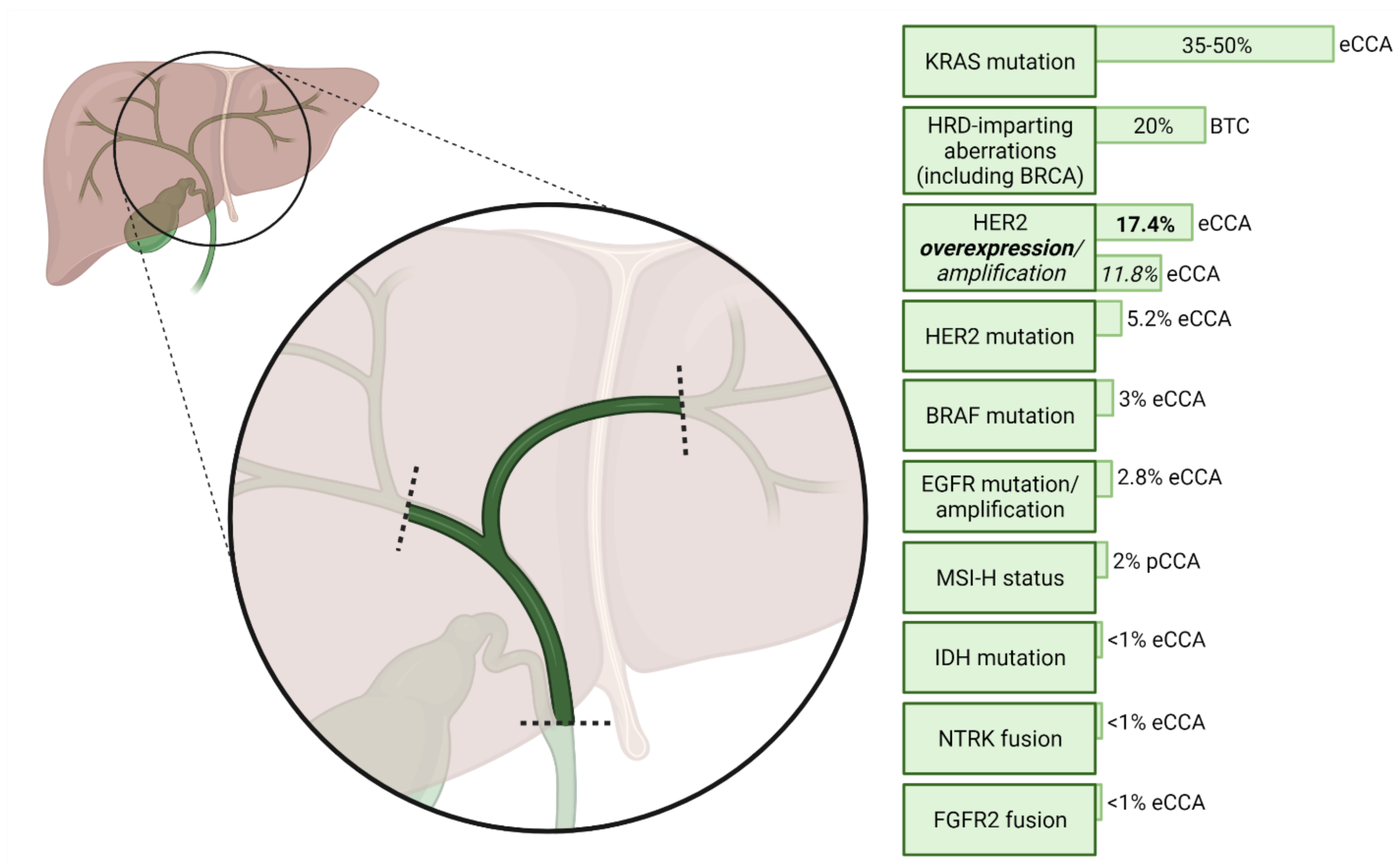
2. Molecular Characterisation of pCCA
3. Potentially Actionable Molecular Aberrations in Perihilar Cholangiocarcinoma
3.1. Isocitrate Dehydrogenase-1
3.2. Fibroblast Growth Factor Receptor
3.3. The Epidermal Growth Factor Receptor Family
3.4. Microsatellite Instability and Immune Checkpoint Inhibition
3.5. Anti-Angiogenic Therapies
3.6. The RAS–RAF–MEK–ERK Pathway
3.7. Other Targeted Therapies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.; Aishima, S.; Clavien, P.-A.; Fowler, K.; Goodman, Z.; Gores, G.; Gouw, A.; Kagen, A.; Klimstra, D.; Komuta, M.; et al. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology 2018, 68, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.; Gores, G.J. Pathogenesis, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groot Koerkamp, B.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.; Coelen, R.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J.; et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komaya, K.; Ebata, T.; Shirai, K.; Ohira, S.; Morofuji, N.; Akutagawa, A.; Yamaguchi, R.; Nagino, M.; Aoba, T.; Kaneoka, Y.; et al. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br. J. Surg. 2017, 104, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Hatzaras, I.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.P.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L.; et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013, 153, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Neoptolemos, J.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.-P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef]
- ICD-11 for Mortality and Morbidity Statistics. Available online: https://icd.who.int/browse11/l-m/en (accessed on 27 January 2022).
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2019, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Prueksapanich, P.; Piyachaturawat, P.; Aumpansub, P.; Ridtitid, W.; Chaiteerakij, R.; Rerknimitr, R. Liver Fluke-Associated Biliary Tract Cancer. Gut Liver 2018, 12, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.-J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2021. [Google Scholar] [CrossRef]
- Bismuth, H.; Nakache, R.; Diamond, T. Management Strategies in Resection for Hilar Cholangiocarcinoma. Ann. Surg. 1992, 215, 31–38. [Google Scholar] [CrossRef]
- Franssen, S.; de Jong, D.M.; van Driel, L.M.J.W.; Koerkamp, B.G. Challenges in Diagnosing Cholangiocarcinoma: Pulling Together Biochemical, Radiological, and Cytopathological Data. In Diagnosis and Management of Cholangiocarcinoma; Tabibian, J.H., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e296. [Google Scholar] [CrossRef] [Green Version]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Ni Huang, M.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [Green Version]
- Montal, R.; Sia, D.; Montironi, C.; Leow, W.Q.; Esteban-Fabró, R.; Pinyol, R.; Torres-Martin, M.; Bassaganyas, L.; Moeini, A.; Peix, J.; et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Lowery, M.; Ptashkin, R.N.; Jordan, E.J.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, O.; Ross, J.S.; Goshen-Lago, T.; Haddad, R.; Moore, A.; Sulkes, A.; Brenner, B.; Ben-Aharon, I. ERBB2 Pathway in Biliary Tract Carcinoma: Clinical Implications of a Targetable Pathway. Oncol. Res. Treat. 2020, 44, 20–27. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; Mcnamara, M.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2016, 36, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Goeppert, B.; Roessler, S.; Renner, M.; Singer, S.; Mehrabi, A.; Vogel, M.N.; Pathil, A.; Czink, E.; Köhler, B.; Springfeld, C.; et al. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in non-liver fluke associated cholangiocarcinoma. Br. J. Cancer 2018, 120, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Simbolo, M.; Fassan, M.; Ruzzenente, A.; Mafficini, A.; Wood, L.D.; Corbo, V.; Melisi, D.; Malleo, G.; Vicentini, C.; Malpeli, G.; et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 2014, 5, 2839–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churi, C.R.; Shroff, R.; Wang, Y.; Rashid, A.; Kang, H.; Weatherly, J.; Zuo, M.; Zinner, R.; Hong, D.; Meric-Bernstam, F.; et al. Mutation Profiling in Cholangiocarcinoma: Prognostic and Therapeutic Implications. PLoS ONE 2014, 9, e115383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, D.H.; Bekaii-Saab, T. Biliary tract cancer and genomic alterations in homologous recombinant deficiency: Exploiting synthetic lethality with PARP inhibitors. Chin. Clin. Oncol. 2020, 9, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Demols, A.; Perez-Casanova, L.; Rocq, L.; Charry, M.; De Nève, N.; Verrellen, A.; Ramadhan, A.; Van Campenhout, C.; De Clercq, S.; Maris, C.; et al. O-4 NTRK gene fusions in bilio-pancreatic cancers. Ann. Oncol. 2020, 31, 233. [Google Scholar] [CrossRef]
- Mondesir, J.; Willekens, C.; Touat, M.; de Botton, S. IDH1 and IDH2 mutations as novel therapeutic targets: Current perspectives. J. Blood Med. 2016, 7, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borger, D.R.; Goyal, L.; Yau, T.C.C.; Poon, R.T.; Ancukiewicz, M.; Deshpande, V.; Christiani, D.C.; Liebman, H.M.; Yang, H.; Kim, H.; et al. Circulating Oncometabolite 2-Hydroxyglutarate Is a Potential Surrogate Biomarker in Patients with Isocitrate Dehydrogenase-Mutant Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 1884–1890. [Google Scholar] [CrossRef] [Green Version]
- Chan-On, W.; Nairismägi, M.-L.; Ong, C.K.; Lim, W.K.; Dima, S.; Pairojkul, C.; Lim, K.H.; McPherson, J.R.; Cutcutache, I.; Heng, H.L.; et al. Exome sequencing identifies distinct mutational patterns in liver fluke–related and non-infection-related bile duct cancers. Nat. Genet. 2013, 45, 1474–1478. [Google Scholar] [CrossRef]
- Fujimoto, A.; Furuta, M.; Shiraishi, Y.; Gotoh, K.; Kawakami, Y.; Arihiro, K.; Nakamura, T.; Ueno, M.; Ariizumi, S.-I.; Nguyen, H.H.; et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat. Commun. 2015, 6, 6120. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Stein, E.M.; DE Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib inIDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Lowery, M.A.; Burris, H.A.; Janku, F.; Shroff, R.T.; Cleary, J.M.; Azad, N.S.; Goyal, L.; Maher, E.A.; Gore, L.; Hollebecque, A.; et al. Safety and activity of ivosidenib in patients with idh1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 711–720. [Google Scholar] [CrossRef]
- Adeva, J. Current development and future perspective of IDH1 inhibitors in cholangiocarcinoma. Liver Cancer Int. 2021, 3, 17–31. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.A.; et al. Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 (IDH1) mutation. J. Clin. Oncol. 2021, 39, 266. [Google Scholar] [CrossRef]
- Presta, M.; Chiodelli, P.; Giacomini, A.; Rusnati, M.; Ronca, R. Fibroblast growth factors (FGFs) in cancer: FGF traps as a new therapeutic approach. Pharmacol. Ther. 2017, 179, 171–187. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef] [Green Version]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 22, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients with FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.S.; Zagonel, V.; et al. ARQ 087, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced intrahepatic cholangiocarcinoma (iCCA) with FGFR2 genetic aberrations. J. Clin. Oncol. 2017, 35, 4017. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Cleary, J.M.; Voss, M.H.; Meric-Bernstam, F.; Hierro, C.; Heist, R.S.; Ishii, N.; Kirpicheva, Y.; Nicolas-Metral, V.; Pokorska-Bocci, A.; Vaslin, A.; et al. Safety and efficacy of the selective FGFR inhibitor debio 1347 in phase I study patients with FGFR genomically activated advanced biliary tract cancer (BTC). J. Clin. Oncol. 2018, 36, 447. [Google Scholar] [CrossRef]
- Bahleda, R.; Italiano, A.; Hierro, C.; Mita, A.; Cervantes, A.; Chan, N.; Awad, M.; Calvo, E.; Moreno, V.; Govindan, R.; et al. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4888–4897. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Strickler, J.H.; Govindan, R.; Chai, S.; Chan, N.; Quiroga-Garcia, V.; Bahleda, R.; Hierro, C.; Zhong, B.; Gonzalez, M.; et al. Safety and activity of the pan-fibroblast growth factor receptor (FGFR) inhibitor erdafitinib in phase 1 study patients (Pts) with molecularly selected advanced cholangiocarcinoma (CCA). J. Clin. Oncol. 2017, 35, 4074. [Google Scholar] [CrossRef]
- Goyal, L.; Bahleda, R.; Furuse, J.; Valle, J.W.; Moehler, M.H.; Oh, D.-Y.; Chang, H.-M.; Kelley, R.K.; Javle, M.M.; Borad, M.J.; et al. FOENIX-101: A phase II trial of TAS-120 in patients with intrahepatic cholangiocarcinoma harboring FGFR2 gene rearrangements. J. Clin. Oncol. 2019, 37, TPS468. [Google Scholar] [CrossRef]
- Borad, M.J.; Bridgewater, J.A.; Morizane, C.; Shroff, R.T.; Oh, D.-Y.; Moehler, M.H.; Furuse, J.; Benhadji, K.A.; He, H.; Valle, J.W. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). J. Clin. Oncol. 2020, 38, TPS600. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Busset, M.D.D.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Busset, M.D.D.; Braun, S.; El-Rayes, B.; Harris, W.; Damjanov, N.; Masi, G.; Rimassa, L.; Bhoori, S.; Niger, M.; Personeni, N.; et al. Efficacy of derazantinib (DZB) in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) expressing FGFR2-fusion or FGFR2 mutations/amplifications. Ann. Oncol. 2019, 30, v276–v277. [Google Scholar] [CrossRef]
- Javle, M.M.; Abou-Alfa, G.K.; Macarulla, T.; Personeni, N.; Adeva, J.; Bergamo, F.; Malka, D.; Vogel, A.; Knox, J.J.; Evans, T.R.J.; et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma patients with FGFR2 mutations or amplifications: Interim results from the phase 2 study FIDES-01. J. Clin. Oncol. 2022, 40, 427. [Google Scholar] [CrossRef]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39, 265. [Google Scholar] [CrossRef]
- Hoy, S.M. Pemigatinib: First Approval. Drugs 2020, 80, 923–929. [Google Scholar] [CrossRef]
- Kang, C. Infigratinib: First Approval. Drugs 2021, 81, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.; Borad, M.; Gallinson, D.; Murphy, A.; et al. FIGHT-202: A phase II study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA). Ann. Oncol. 2019, 30, v876. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef] [PubMed]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. CMLS 2008, 65, 1566–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2007, 98, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.-C.; Goekkurt, E.; Danenberg, P.V.; Lehmann, S.; Ehninger, G.; Aust, D.E.; Stoehlmacher-Williams, J. EGFR, FLT1 and Heparanase as Markers Identifying Patients at Risk of Short Survival in Cholangiocarcinoma. PLoS ONE 2013, 8, e64186. [Google Scholar] [CrossRef]
- Vivaldi, C.; Fornaro, L.; Ugolini, C.; Niccoli, C.; Musettini, G.; Pecora, I.; Insilla, A.C.; Salani, F.; Pasquini, G.; Catanese, S.; et al. HER2 Overexpression as a Poor Prognostic Determinant in Resected Biliary Tract Cancer. Oncology 2020, 25, 886–893. [Google Scholar] [CrossRef]
- Javle, M.; Churi, C.; Kang, H.; Shroff, R.T.; Janku, F.; Surapaneni, R.; Zuo, M.; Barrera, C.; Alshamsi, H.O.; Krishnan, S.; et al. HER2/neu-directed therapy for biliary tract cancer. J. Hematol. Oncol. 2015, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Leone, F.; Cavalloni, G.; Pignochino, Y.; Sarotto, I.; Ferraris, R.; Piacibello, W.; Venesio, T.; Capussotti, L.; Risio, M.; Aglietta, M. Somatic Mutations of Epidermal Growth Factor Receptor in Bile Duct and Gallbladder Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 1680–1685. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-T.; Chang, M.-C.; Huang, K.-W.; Tung, C.-C.; Hsu, C.; Wong, J.-M. Clinicopathological and prognostic significances of EGFR, KRAS and BRAF mutations in biliary tract carcinomas in Taiwan. J. Gastroenterol. Hepatol. 2014, 29, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Gwak, G.-Y.; Yoon, J.-H.; Shin, C.M.; Ahn, Y.J.; Chung, J.K.; Kim, Y.A.; Kim, T.-Y.; Lee, H.-S. Detection of response-predicting mutations in the kinase domain of the epidermal growth factor receptor gene in cholangiocarcinomas. J. Cancer Res. Clin. Oncol. 2005, 131, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Van Cutsem, E.; Rougier, P.; Ciardiello, F.; Heeger, S.; Schlichting, M.; Celik, I.; Köhne, C.-H. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 2012, 48, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Chang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188. [Google Scholar] [CrossRef]
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148. [Google Scholar] [CrossRef]
- Malka, D.; Cervera, P.; Foulon, S.; Trarbach, T.; de la Fouchardière, C.; Boucher, E.; Fartoux, L.; Faivre, S.; Blanc, J.-F.; Viret, F.; et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): A randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014, 15, 819–828. [Google Scholar] [CrossRef]
- Leone, F.; Marino, D.; Cereda, S.; Filippi, R.; Belli, C.; Spadi, R.; Nasti, G.; Montano, M.; Amatu, A.; Aprile, G.; et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-typeKRASadvanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2015, 122, 574–581. [Google Scholar] [CrossRef]
- Vogel, A.; Kasper, S.; Bitzer, M.; Block, A.; Sinn, M.; Schulze-Bergkamen, H.; Moehler, M.; Pfarr, N.; Endris, V.; Goeppert, B.; et al. PICCA study: Panitumumab in combination with cisplatin/gemcitabine chemotherapy in KRAS wild-type patients with biliary cancer—A randomised biomarker-driven clinical phase II AIO study. Eur. J. Cancer 2018, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.; Wei, L.; Zalupski, M.; O’Neil, B.; Calero, M.V.; Bekaii-Saab, T. HER2/neu May Not Be an Interesting Target in Biliary Cancers: Results of an Early Phase II Study with Lapatinib. Oncology 2012, 82, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.K.; Belani, C.; Singh, D.A.; Tanaka, M.; Lenz, H.-J.; Yen, Y.; Kindler, H.L.; Iqbal, S.; Longmate, J.; Mack, P.C.; et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother. Pharmacol. 2009, 64, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Mondaca, S.; Razavi, P.; Xu, C.; Offin, M.; Myers, M.; Scaltriti, M.; Hechtman, J.F.; Bradley, M.; O’Reilly, E.M.; Berger, M.F.; et al. Genomic Characterization of ERBB2-Driven Biliary Cancer and a Case of Response to Ado-Trastuzumab Emtansine. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39, 320. [Google Scholar] [CrossRef]
- Roa, I.; de Toro, G.; Schalper, K.; de Aretxabala, X.; Churi, C.; Javle, M. Overexpression of the HER2/Neu Gene: A New Therapeutic Possibility for Patients with Advanced Gallbladder Cancer. Gastrointest. Cancer Res. 2014, 7, 42. [Google Scholar]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef] [Green Version]
- Dudley, J.C.; Lin, M.-T.; Le, D.T.; Eshleman, J.R. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Suto, T.; Habano, W.; Sugai, T.; Uesugi, N.; Kanno, S.; Saito, K.; Nakamura, S.I. Infrequent Microsatellite Instability in Biliary Tract Cancer. J. Surg. Oncol. 2001, 76, 121–126. [Google Scholar] [CrossRef]
- Winkelmann, R.; Schneider, M.; Hartmann, S.; Schnitzbauer, A.A.; Zeuzem, S.; Peveling-Oberhag, J.; Hansmann, M.L.; Walter, D. Microsatellite Instability Occurs Rarely in Patients with Cholangiocarcinoma: A Retrospective Study from a German Tertiary Care Hospital. Int. J. Mol. Sci. 2018, 19, 1421. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.Y.; Dibbern, M.E.; Mahadevan, M.S.; Fan, J.; Kunk, P.R.; Stelow, E.B. Mismatch Repair Protein Deficiency/Microsatellite Instability Is Rare in Cholangiocarcinomas and Associated with Distinctive Morphologies. Am. J. Clin. Pathol. 2019, 153, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ono, M.; Ohmori, G.; Ameda, S.; Yamada, M.; Abe, T.; Fujii, S.; Fujita, M.; Maeda, M. Successful pembrolizumab treatment of microsatellite instability-high intrahepatic cholangiocarcinoma: A case report. Clin. Case Rep. 2021, 9, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Czink, E.; Kloor, M.; Goeppert, B.; Fröhling, S.; Uhrig, S.; Weber, T.F.; Meinel, J.; Sutter, C.; Weiss, K.H.; Schirmacher, P.; et al. Successful immune checkpoint blockade in a patient with advanced stage microsatellite-unstable biliary tract cancer. Cold Spring Harbor Mol. Case Stud. 2017, 3, a001974. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueno, M.; Hayami, S.; Kawai, M.; Miyamoto, A.; Suzaki, N.; Hirono, S.; Okada, K.-I.; Miyazawa, M.; Kitahata, Y.; et al. Effective Response of Intrahepatic Cholangiocarcinoma to Pembrolizumab: A Case Report. Anticancer Res. 2020, 40, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, A.; Sakuda, T.; Murakami, T.; Aihara, K.; Watanuki, Y.; Suzuki, Y.; Shibasaki, E.; Masuda, T.; Uehara, S.; Yasuoka, H.; et al. Microsatellite Instability-high Intrahepatic Cholangiocarcinoma with Portal Vein Tumor Thrombosis Successfully Treated with Pembrolizumab. Intern. Med. 2020, 59, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, H.S.; Lee, S.H.; Woo, S.M.; Kim, D.U.; Bang, S. Efficacy and Safety of Pembrolizumab for Gemcitabine/Cisplatin-Refractory Biliary Tract Cancer: A Multicenter Retrospective Study. J. Clin. Med. 2020, 9, 1769. [Google Scholar] [CrossRef]
- Kang, J.; Jeong, J.H.; Hwang, H.-S.; Lee, S.S.; Park, D.H.; Oh, D.W.; Song, T.J.; Kim, K.-H.; Hwang, S.; Hwang, D.W.; et al. Efficacy and Safety of Pembrolizumab in Patients with Refractory Advanced Biliary Tract Cancer: Tumor Proportion Score as a Potential Biomarker for Response. Cancer Res. Treat. 2020, 52, 594–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Thelen, A.; Scholz, A.; Benckert, C.; Schröder, M.; Weichert, W.; Wiedenmann, B.; Neuhaus, P.; Jonas, S. Microvessel density correlates with lymph node metastases and prognosis in hilar cholangiocarcinoma. J. Gastroenterol. 2008, 43, 959–966. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Yu, G.; Ding, H. Lymphangiogenic and Angiogentic Microvessel Density in Gallbladder Carcinoma. Hepato-Gastroenterology 2011, 58, 20–25. [Google Scholar] [PubMed]
- Thelen, A.; Scholz, A.; Weichert, W.; Wiedenmann, B.; Neuhaus, P.; Geßner, R.; Benckert, C.; Jonas, S. Tumor-Associated Angiogenesis and Lymphangiogenesis Correlate with Progression of Intrahepatic Cholangiocarcinoma. Am. J. Gastroenterol. 2010, 105, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Guedj, N.; Zhan, Q.; Perigny, M.; Rautou, P.-E.; Degos, F.; Belghiti, J.; Farges, O.; Bedossa, P.; Paradis, V. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J. Hepatol. 2009, 51, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Guida, T.; D’Attino, R.; Perrotta, E.; Otero, M.; Masala, A.; Cartenì, G. Sunitinib: Bridging present and future cancer treatment. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18 (Suppl. S6), vi31–vi34. [Google Scholar] [CrossRef]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M.; et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152–4163. [Google Scholar] [CrossRef] [PubMed]
- Abdelgalil, A.A.; Alkahtani, H.M.; Al-Jenoobi, F.I. Sorafenib. Profiles Drug Substan. Excip. Relat. Methodol. 2019, 44, 239–266. [Google Scholar] [CrossRef]
- Ettrich, T.J.; Seufferlein, T. Regorafenib. Recent results in cancer research. Fortschritte der Krebsforschung. Prog. Recher. Cancer 2018, 211, 45–56. [Google Scholar] [CrossRef]
- Yi, J.H.; Thongprasert, S.; Lee, J.; Doval, D.; Park, S.H.; Park, J.O.; Park, Y.S.; Kang, W.K.; Lim, H.Y. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: A multicentre, multinational study. Eur. J. Cancer 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Valle, J.W.; Vogel, A.; Denlinger, C.S.; He, A.R.; Bai, L.-Y.; Orlova, R.; Van Cutsem, E.; Adeva, J.; Chen, L.-T.; Obermannova, R.; et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: A randomised, double-blind, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 1468–1482. [Google Scholar] [CrossRef]
- Taylor, M.H.; Schmidt, E.V.; Dutcus, C.; Pinheiro, E.M.; Funahashi, Y.; Lubiniecki, G.; Rasco, D. The LEAP program: Lenvatinib plus pembrolizumab for the treatment of advanced solid tumors. Futur. Oncol. 2021, 17, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Bengala, C.; Bertolini, F.; Malavasi, N.; Boni, C.; Aitini, E.; Dealis, C.; Zironi, S.; Depenni, R.; Fontana, A.; Del Giovane, C.; et al. Sorafenib in patients with advanced biliary tract carcinoma: A phase II trial. Br. J. Cancer 2009, 102, 68–72. [Google Scholar] [CrossRef] [Green Version]
- El-Khoueiry, A.B.; Rankin, C.J.; Ben-Josef, E.; Lenz, H.-J.; Gold, P.J.; Hamilton, R.D.; Govindarajan, R.; Eng, C.; Blanke, C.D. SWOG 0514: A phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Investig. New Drugs 2011, 30, 1646–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2018, 125, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Demols, A.; Borbath, I.; Van den Eynde, M.; Houbiers, G.; Peeters, M.; Marechal, R.; Delaunoit, T.; Goemine, J.-C.; Laurent, S.; Holbrechts, S.; et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN, a randomized, double-blind, phase II trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1169–1177. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Sasaki, T.; Nagashima, F.; Mizuno, N.; Shimizu, S.; Ikezawa, H.; Hayata, N.; Nakajima, R.; Morizane, C. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: Primary analysis results. BMC Cancer 2020, 20, 1105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Meyerhardt, J.A.; Blaszkowsky, L.S.; Kambadakone, A.R.; Muzikansky, A.; Zheng, H.; Clark, J.W.; Abrams, T.A.; Chan, J.A.; Enzinger, P.C.; et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: A phase 2 study. Lancet Oncol. 2010, 11, 48–54. [Google Scholar] [CrossRef]
- Iyer, R.V.; Pokuri, V.K.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.M.; Grande, C.; Saab, T.B. A Multicenter Phase II Study of Gemcitabine, Capecitabine, and Bevacizumab for Locally Advanced or Metastatic Biliary Tract Cancer. Am. J. Clin. Oncol. 2018, 41, 649–655. [Google Scholar] [CrossRef]
- Lee, J.K.; Capanu, M.; O’Reilly, E.M.; Ma, J.; Chou, J.F.; Shia, J.; Katz, S.S.; Gansukh, B.; Reidylagunes, D.; Segal, N.H.; et al. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br. J. Cancer 2013, 109, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Moehler, M.; Maderer, A.; Schimanski, C.; Kanzler, S.; Denzer, U.; Kolligs, F.; Ebert, M.; Distelrath, A.; Geissler, M.; Trojan, J.; et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: A double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur. J. Cancer 2014, 50, 3125–3135. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Gebbia, V.; Pressiani, T.; Testa, A.; Personeni, N.; Arrivas Bajardi, E.; Foa, P.; Buonadonna, A.; Bencardino, K.; Barone, C.; et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: The VanGogh study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 26, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.H.; Fernebro, E.; Ploen, J.; Eberhard, J.; Lindebjerg, J.; Jakobsen, A. Randomized phase II crossover trial exploring the clinical benefit from targeting EGFR or VEGF with combination chemotherapy in patients with non-resectable biliary tract cancer. J. Clin. Oncol. 2015, 33, 4071. [Google Scholar] [CrossRef]
- Valle, J.W.; Wasan, H.; Lopes, A.; Backen, A.C.; Palmer, D.H.; Morris, K.; Duggan, M.; Cunningham, D.; Anthoney, D.A.; Corrie, P.; et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): A randomised phase 2 trial. Lancet Oncol. 2015, 16, 967–978. [Google Scholar] [CrossRef]
- Lubner, S.J.; Mahoney, M.R.; Kolesar, J.L.; LoConte, N.K.; Kim, G.P.; Pitot, H.C.; Philip, P.A.; Picus, J.; Yong, W.-P.; Horvath, L.; et al. Report of a Multicenter Phase II Trial Testing a Combination of Biweekly Bevacizumab and Daily Erlotinib in Patients with Unresectable Biliary Cancer: A Phase II Consortium Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3491–3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khoueiry, A.B.; Rankin, C.; Siegel, A.B.; Iqbal, S.; Gong, I.-Y.; Micetich, K.; Kayaleh, O.R.; Lenz, H.-J.; Blanke, C.D. S0941: A phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br. J. Cancer 2014, 110, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, X.; Long, J.; Zhao, S.; Mao, J.; Wang, D.; Bai, Y.; Bian, J.; Zhang, L.; Yang, X.; et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg. Nutr. 2020, 9, 414–424. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Zhang, S.S.; Nagasaka, M. Spotlight on Sotorasib (AMG 510) for KRASG12C Positive Non-Small Cell Lung Cancer. Lung Cancer 2021, 12, 115–122. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Spira, A.I.; Yaeger, R.; Buchschacher, G.L.; McRee, A.J.; Sabari, J.K.; Johnson, M.L.; Barve, M.A.; Hafez, N.; Velastegui, K.; et al. KRYSTAL-1: Updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation. J. Clin. Oncol. 2022, 40, 519. [Google Scholar] [CrossRef]
- Li, W.; Cui, Y.; Yin, F.; Peng, L.; Liu, X.; Shen, Y.; Guo, Y.; Wen, S.; Shi, J.; Lei, M.; et al. BRAF mutation in Chinese biliary tract cancer patients. J. Clin. Oncol. 2020, 38, e16678. [Google Scholar] [CrossRef]
- Tannapfel, A.; Sommerer, F.; Benicke, M.; Katalinic, A.; Uhlmann, D.; Witzigmann, H.; Hauss, J.; Wittekind, C. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 2003, 52, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Hyder, O.; Dodson, R.; Nayar, S.K.; Poling, J.; Beierl, K.; Eshleman, J.R.; Lin, M.-T.; Pawlik, T.M.; Anders, R.A. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum. Pathol. 2013, 44, 2768–2773. [Google Scholar] [CrossRef] [Green Version]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.L.; Raje, N.S.; Diamond, E.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.; Stenger, M. Dabrafenib in advanced melanoma with BRAF V600E mutation. J. Commun. Support. Oncol. 2014, 12, 48–49. [Google Scholar] [CrossRef] [Green Version]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.W.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-Institutional Phase II Study of Selumetinib in Patients with Metastatic Biliary Cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2357–2363. [Google Scholar] [CrossRef] [Green Version]
- Bridgewater, J.; Lopes, A.; Beare, S.; Duggan, M.; Lee, D.; Ricamara, M.; McEntee, D.; Sukumaran, A.; Wasan, H.; Valle, J.W. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: The ABC-04 study. BMC Cancer 2016, 16, 153. [Google Scholar] [CrossRef]
- Doherty, M.; Tam, V.C.; McNamara, M.G.; Hedley, D.W.; Dhani, N.C.; Chen, E.X.; Jang, R.W.-J.; Tang, P.A.; Sim, H.-W.; O’Kane, G.M.; et al. Selumetinib (Sel) and cisplatin/gemcitabine (CisGem) for advanced biliary tract cancer (BTC): A randomized trial. J. Clin. Oncol. 2018, 36, 4084. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Infante, J.R.; Janku, F.; Wong, D.J.L.; Sosman, J.A.; Keedy, V.; Patel, M.R.; Shapiro, G.I.; Mier, J.W.; Tolcher, A.W.; et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study. Cancer Discov. 2018, 8, 184–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, P.L.; Corso, C.D.; Robinson, N.D.; Scanlon, S.E.; Purshouse, K.R.; Bai, H.; Liu, Y.; Sundaram, R.K.; Hegan, D.C.; Fons, N.R.; et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci. Transl. Med. 2017, 99, eaal2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herberger, B.; Puhalla, H.; Lehnert, M.; Wrba, F.; Novak, S.; Brandstetter, A.; Gruenberger, B.; Gruenberger, T.; Pirker, R.; Filipits, M. Activated Mammalian Target of Rapamycin Is an Adverse Prognostic Factor in Patients with Biliary Tract Adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 4795–4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansel, D.E.; Rahman, A.; Hidalgo, M.; Thuluvath, P.J.; Lillemoe, K.D.; Schulick, R.; Ku, J.-L.; Park, J.-G.; Miyazaki, K.; Ashfaq, R.; et al. Identification of Novel Cellular Targets in Biliary Tract Cancers Using Global Gene Expression Technology. Am. J. Pathol. 2003, 163, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Riener, M.-O.; Bawohl, M.; Clavien, P.-A.; Jochum, W. RarePIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosom. Cancer 2008, 47, 363–367. [Google Scholar] [CrossRef]
- Buzzoni, R.; Pusceddu, S.; Bajetta, E.; de Braud, F.G.M.; Platania, M.; Iannacone, C.; Cantore, M.; Mambrini, A.; Bertolini, A.; Alabiso, O.; et al. Activity and safety of RAD001 (everolimus) in patients affected by biliary tract cancer progressing after prior chemotherapy: A phase II ITMO study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1597–1603. [Google Scholar] [CrossRef]
- Lau, D.K.; Tay, R.Y.; Yeung, Y.H.; Chionh, F.; Mooi, J.; Murone, C.; Skrinos, E.; Price, T.J.; Mariadason, J.M.; Tebbutt, N.C. Phase II study of everolimus (RAD001) monotherapy as first-line treatment in advanced biliary tract cancer with biomarker exploration: The RADiChol Study. Br. J. Cancer 2018, 118, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.S.; Cao, B.; Kim, J.; Al-Toubah, T.E.; Mehta, R.; Centeno, B.A.; Kim, R.D. Phase 2 study of copanlisib in combination with gemcitabine and cisplatin in advanced biliary tract cancers. Cancer 2020, 127, 1293–1300. [Google Scholar] [CrossRef]
- Vaishnavi, A.; Le, A.T.; Doebele, R.C. TRKing Down an Old Oncogene in a New Era of Targeted Therapy. Cancer Discov. 2014, 5, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.-L.; Deng, X.; Huang, F.; Tucker, M.; Crosby, K.; Rimkunas, V.; Wang, Y.; Deng, G.; Zhu, L.; Tan, Z.-P.; et al. Survey of Tyrosine Kinase Signaling Reveals ROS Kinase Fusions in Human Cholangiocarcinoma. PLoS ONE 2011, 6, e15640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; Dubois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
| Trial; Number of Patients Enrolled | iCCA | pCCA | dCCA | GBC | AVC |
|---|---|---|---|---|---|
| ABC-02 [9]; n = 410 | 241 (59% CCA) | 149 (36%) | 20 (5%) | ||
| BT22 [10]; n = 83 | 28 (34%) | 19 (23% eCCA) | 32 (39%) | 4 (5%) | |
| ABC-06 [12]; n = 162 | 72 (44%) | 45 (28% eCCA) | 34 (21%) | 11 (7%) | |
| NIFTY [13]; n = 174 | 74 (43%) | 47 (27% eCCA) | 53 (30%) | 0 | |
| ClarIDHy [21]; n = 185 | 169 (91%) | 4 (2%) | 2 (1%) | 0 | 0 |
| KEYNOTE-158 [22] (CCA patients only); n = 22 | 22 (100%; CCA subtype not reported) | 0 | 0 | ||
| ROAR [23]; n = 43 | 39 (91%) | 1 (2%) | 0 | 1 (2%) | 0 |
| MyPathway [24] (BTC patients only); n = 39 | 7 (18%) | 7 (18% eCCA) | 16 (41%) | 5 (13%) | |
| Trial; Year of Publication | KRAS Selection | Treatment Arm(s); Number of Patients | ORR | mPFS (95% CI) | mOS (95% CI) |
|---|---|---|---|---|---|
| Gruenberger et al., 2010 [80] | None | Gem/Ox + cetuximab (n = 30) | 63% (including 10% CR) | - | - |
| Malka et al. (BINGO trial), 2014 [81] | None | Gem/Ox (n = 74) | 23% (including 3% CR) | 5.5 (3.7–6.6) | 12.4 (8.6–16.0) |
| Gem/Ox + cetuximab (n = 76) | 24% (including 1% CR) | 6.1 (5.1–7.6) | 11.0 (9.1–13.7) | ||
| Leone et al. (Vecti-BIL trial), 2016 [82] | Wild-type only | Gem/Ox (n = 44) | 18% (including 2% CR) | 4.4 (2.6–6.2) | 10.2 (6.4–13.9) |
| Gem/Ox + panitumumab (n = 45) | 27% (including 2% CR) | 5.3 (3.3–7.2); p = 0.27 | 9.9 (5.4–14.3) | ||
| Vogel et al. (PICCA trial), 2018 [83] | Wild-type only | Cis/Gem (n = 28) | 39% (% CR unknown) | - | 20.1 |
| Cis/Gem + panitumumab (n = 62) | 45% (% CR unknown) | - | 12.8 |
| Trial; Year of Publication | Setting | Treatment Arm(s); Number of Patients | ORR | mPFS (95% CI) | mOS (95% CI) |
|---|---|---|---|---|---|
| VEGF inhibition as monotherapy | |||||
| Bengala et al., 2010 [114] | 1st- or 2nd-line | Sorafenib (n = 46) | 2% | 2.3 | 4.4 |
| Yi et al., 2012 [105] | 2nd-line | Sunitinib (n = 56) | 8.9% | 1.7 (1.0–2.4) | 4.8 (3.8–4.8) |
| El-Khoueiry et al. (SWOG 0514 trial), 2012 [115] | 1st-line | Sorafenib (n = 31) | 0% | 3.0 (2.0–4.0) | 9.0 (4.0–12.0) |
| Sun et al., 2019 [116] | 2nd-line | Regorafenib (n = 43) | 11% | 15.6 wk | 31.8 wk |
| Demols et al. (REACHIN trial), 2020 [117] | 2nd-line | Regorafenib (n = 33) | 0% | 3.0 (2.3–4.9) | 5.3 (2.7–10.5) |
| Placebo (n = 33) | 0% | 1.5 (1.2–2.0) | 5.1 (3.0–6.4) | ||
| Ueno et al., 2020 [118] | 2nd-line | Lenvatinib (n = 26) | 11.5% | 1.6 (1.4–3.2) | 7.35 (4.5–11.3) |
| Chemotherapy plus VEGF inhibition | |||||
| Zhu et al., 2010 [119] | 1st-line | Gem/Ox plus bevacizumab (n = 35) | 40% | 7 (5.3–10.3) | 12.7 (7.3–18.1) |
| Iyer et al., 2018 [120] | 1st-line | Gem/Cap plus bevacizumab (n = 50) | 24% | 8.1 (5.3–9.9) | 10.2 (7.5–13.7) |
| Lee et al., 2013 [121] | 1st-line | Cis/Gem plus sorafenib (n = 39) | 12% | 6.5 (3.5–8.3) | 14.4 (11.6–19.2) |
| Moehler et al., 2014 [122] | 1st-line | Gem plus sorafenib (n = 49) | 14% | 3.0 | 8.4 |
| Gem plus placebo (n = 48) | 10% | 4.9 | 11.2 | ||
| Santoro et al. (VanGogh trial), 2015 [123] | 1st-line | Vandetanib (n = 56) | 3.6% | 15 wk (10.3–22.1) | 32.6 wk (27.1–52) |
| Vandetanib plus Gem (n = 57) | 19.3% | 16.3 wk (13–27.6) | 40.6 wk (30.4–51.3) | ||
| Placebo plus Gem (n = 52) | 13.5% | 21.1 wk (10.1–32.1) | 43.9 wk (36.3–74.7) | ||
| Jensen et al., 2015 [124] | 1st-line | Panitumumab + Gem/Ox/Cap (n = 44) | 46% | 6.1 (5.8–8.1) | 9.5 (8.3–13.3) |
| Gem/Ox/Cap + bevacizumab (n = 44) | 18% | 8.2 (5.3–10.6) | 12.3 (8.8–13.3) | ||
| Valle et al. (ABC-03 trial), 2015 [125] | 1st-line | Cis/Gem plus cediranib (n = 62) | 44% (including 3% CR) | 8 (6.5–9.3) | 14.1 (10.2–16.4) |
| Cis/Gem plus placebo (n = 62) | 19% | 7.4 (5.7–8.5) | 11.9 (9.2–14.3) | ||
| Valle et al., 2021 [112] | 1st-line | Cis/Gem plus ramucirumab (n = 106) | 31.1% | 6.5 (80% CI 5.7–7.1) | 10.5 (80% CI 8.5–11.8) |
| Cis/Gem plus merestinib (n = 102) | 19.6% | 7.9 (80% CI 6.2–7.1) | 14.0 (80% CI 12.0–16.4) | ||
| Cis/Gem plus placebo (n = 101) | 32.7% (including 2% CR) | 6.6 (80% CI 5.6–6.8) | 13.0 (80% CI 11.4–15.3) | ||
| Targeted therapy combinations including VEGF inhibition | |||||
| Lubner et al., 2010 [126] | 1st-line | Bevacizumab plus erlotinib (n = 49) | 12% | 4.4 (3–7.8) | 9.9 (7.2–13.6) |
| El-Khouiery et al. (SWOG S0941 trial), 2014 [127] | 1st-line | Sorafenib plus erlotinib (n = 34) | 6% | 2 (2–3) | 6 (3–8) |
| Lin et al., 2020 [128] | 2nd-line | Lenvatinib plus pembrolizumab (n = 32) | 25% | 4.9 (4.7–5.2) | 11 (9.6–12.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gray, S.; Lamarca, A.; Edeline, J.; Klümpen, H.-J.; Hubner, R.A.; McNamara, M.G.; Valle, J.W. Targeted Therapies for Perihilar Cholangiocarcinoma. Cancers 2022, 14, 1789. https://doi.org/10.3390/cancers14071789
Gray S, Lamarca A, Edeline J, Klümpen H-J, Hubner RA, McNamara MG, Valle JW. Targeted Therapies for Perihilar Cholangiocarcinoma. Cancers. 2022; 14(7):1789. https://doi.org/10.3390/cancers14071789
Chicago/Turabian StyleGray, Simon, Angela Lamarca, Julien Edeline, Heinz-Josef Klümpen, Richard A. Hubner, Mairéad G. McNamara, and Juan W. Valle. 2022. "Targeted Therapies for Perihilar Cholangiocarcinoma" Cancers 14, no. 7: 1789. https://doi.org/10.3390/cancers14071789






