Tissue Microarray Analyses Suggest Axl as a Predictive Biomarker in HPV-Negative Head and Neck Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Material
2.2. Tissue Microarray Construction
2.3. Immunohistochemistry (IHC)
2.4. Analysis of Patient Survival
2.5. Further Data Analyses
3. Results
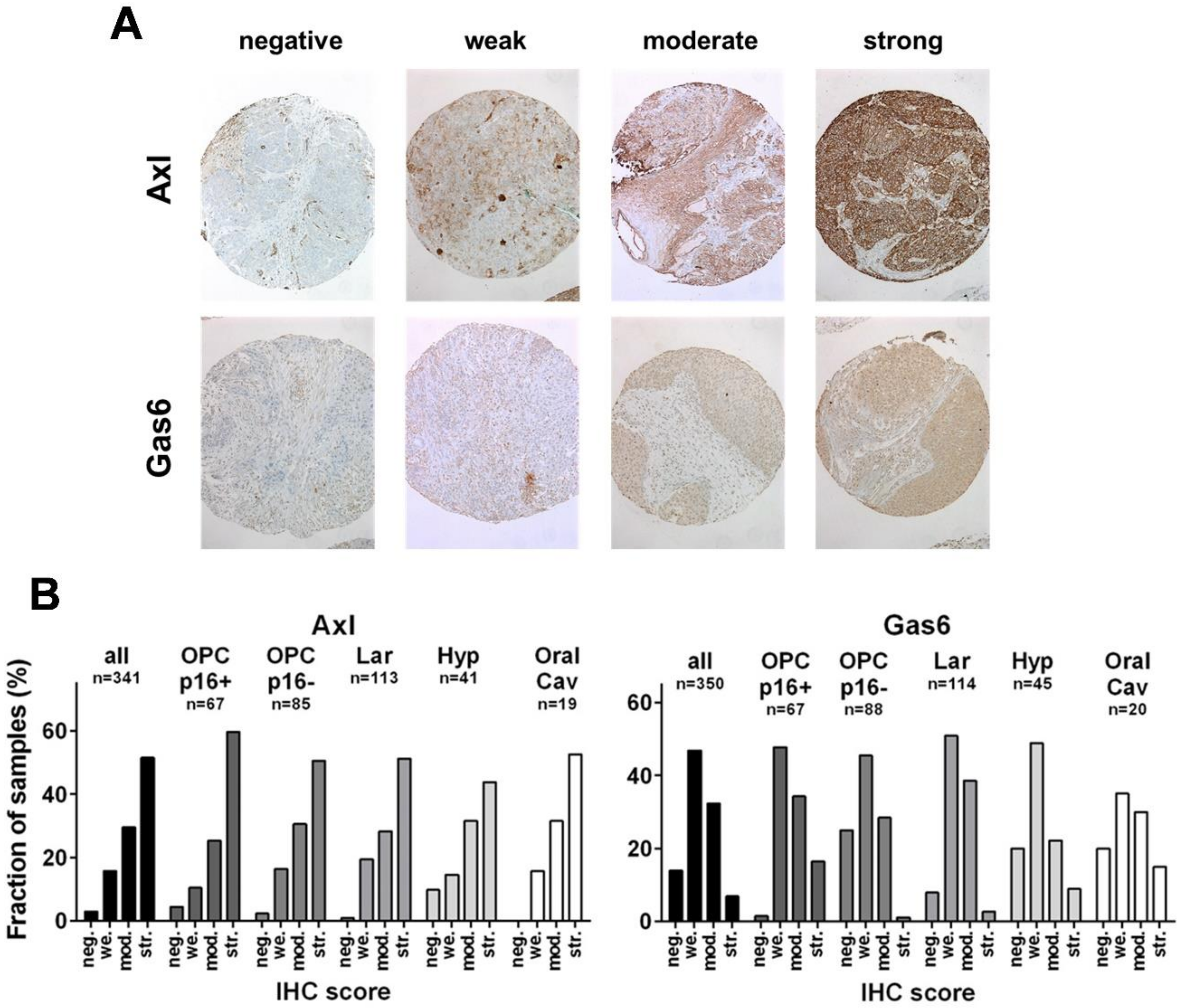
3.1. Expression of Axl and Gas6 in HNSCC Subsites
3.2. Impact of Axl Expression on Patient Survival
3.3. Impact of Gas6 Expression on Patient Survival
3.4. Multivariable Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, P.; Eriksen, J.G.; Hamilton-Dutoit, S.; Tramm, T.; Alsner, J.; Overgaard, J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2009, 27, 1992–1998. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.R.; Ludmir, E.B.; Augustyn, A.; Zaorsky, N.G.; Lehrer, E.J.; Ryali, R.; Trifiletti, D.M.; Adeberg, S.; Amini, A.; Verma, V. De-intensification of therapy in human papillomavirus associated oropharyngeal cancer: A systematic review of prospective trials. Oral. Oncol. 2020, 103, 104608. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Lemke, G. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- Linger, R.M.; Keating, A.K.; Earp, H.S.; Graham, D.K. TAM receptor tyrosine kinases: Biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 2008, 100, 35–83. [Google Scholar] [CrossRef] [Green Version]
- Scaltriti, M.; Elkabets, M.; Baselga, J. Molecular pathways: AXL, a membrane receptor mediator of resistance to therapy. Clin. Cancer Res. 2016, 22, 1313–1317. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ye, X.; Tan, C.; Hongo, J.A.; Zha, J.; Liu, J.; Kallop, D.; Ludlam, M.J.; Pei, L. Axl as a potential therapeutic target in cancer: Role of Axl in tumor growth, metastasis and angiogenesis. Oncogene 2009, 28, 3442–3455. [Google Scholar] [CrossRef] [Green Version]
- Byers, L.A.; Diao, L.; Wang, J.; Saintigny, P.; Girard, L.; Peyton, M.; Shen, L.; Fan, Y.; Giri, U.; Tumula, P.K.; et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 2013, 19, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linger, R.M.; Cohen, R.A.; Cummings, C.T.; Sather, S.; Migdall-Wilson, J.; Middleton, D.H.; Lu, X.; Baron, A.E.; Franklin, W.A.; Merrick, D.T.; et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene 2013, 32, 3420–3431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Lee, J.C.; Lin, L.; Olivas, V.; Au, V.; LaFramboise, T.; Abdel-Rahman, M.; Wang, X.; Levine, A.D.; Rho, J.K.; et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012, 44, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, M.K.; Beauchamp-Perez, F.D.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene 2014, 33, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Holland, S.J.; Pan, A.; Franci, C.; Hu, Y.; Chang, B.; Li, W.; Duan, M.; Torneros, A.; Yu, J.; Heckrodt, T.J.; et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010, 70, 1544–1554. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.S.; Miller, M.A.; Gertler, F.B.; Lauffenburger, D.A. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci. Signal. 2013, 6, ra66. [Google Scholar] [CrossRef] [Green Version]
- Dunne, P.D.; McArt, D.G.; Blayney, J.K.; Kalimutho, M.; Greer, S.; Wang, T.; Srivastava, S.; Ong, C.W.; Arthur, K.; Loughrey, M.; et al. AXL is a key regulator of inherent and chemotherapy-induced invasion and predicts a poor clinical outcome in early-stage colon cancer. Clin. Cancer Res. 2014, 20, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Paccez, J.D.; Vasques, G.J.; Correa, R.G.; Vasconcellos, J.F.; Duncan, K.; Gu, X.; Bhasin, M.; Libermann, T.A.; Zerbini, L.F. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene 2013, 32, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Rankin, E.B.; Fuh, K.C.; Taylor, T.E.; Krieg, A.J.; Musser, M.; Yuan, J.; Wei, K.; Kuo, C.J.; Longacre, T.A.; Giaccia, A.J. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010, 70, 7570–7579. [Google Scholar] [CrossRef] [Green Version]
- Brand, T.M.; Iida, M.; Stein, A.P.; Corrigan, K.L.; Braverman, C.M.; Coan, J.P.; Pearson, H.E.; Bahrar, H.; Fowler, T.L.; Bednarz, B.P.; et al. AXL is a logical molecular target in head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 2601–2612. [Google Scholar] [CrossRef] [Green Version]
- Giles, K.M.; Kalinowski, F.C.; Candy, P.A.; Epis, M.R.; Zhang, P.M.; Redfern, A.D.; Stuart, L.M.; Goodall, G.J.; Leedman, P.J. Axl mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol. Cancer Ther. 2013, 12, 2541–2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badarni, M.; Prasad, M.; Balaban, N.; Zorea, J.; Yegodayev, K.M.; Joshua, B.Z.; Dinur, A.B.; Grenman, R.; Rotblat, B.; Cohen, L.; et al. Repression of AXL expression by AP-1/JNK blockage overcomes resistance to PI3Ka therapy. JCI Insight 2019, 4, e125341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaji, K.; Vijayaraghavan, S.; Diao, L.; Tong, P.; Fan, Y.; Carey, J.P.; Bui, T.N.; Warner, S.; Heymach, J.V.; Hunt, K.K.; et al. AXL Inhibition Suppresses the DNA Damage Response and Sensitizes Cells to PARP Inhibition in Multiple Cancers. Mol. Cancer Res. 2017, 15, 45–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Massenhausen, A.; Bragelmann, J.; Billig, H.; Thewes, B.; Queisser, A.; Vogel, W.; Kristiansen, G.; Schrock, A.; Bootz, F.; Brossart, P.; et al. Implication of the receptor tyrosine kinase AXL in head and neck cancer progression. Int. J. Mol. Sci. 2016, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Elkabets, M.; Pazarentzos, E.; Juric, D.; Sheng, Q.; Pelossof, R.A.; Brook, S.; Benzaken, A.O.; Rodon, J.; Morse, N.; Yan, J.J.; et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 2015, 27, 533–546. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, N.K.; Iida, M.; Nickel, K.P.; Longhurst, C.A.; Fischbach, S.R.; Rodems, T.S.; Kranjac, C.A.; Bo, A.Y.; Luo, Q.; Gallagher, M.M.; et al. AXL mediates cetuximab and radiation resistance through tyrosine 821 and the c-ABL kinase pathway in head and neck cancer. Clin. Cancer Res. 2020, 26, 4349–4359. [Google Scholar] [CrossRef]
- Ruicci, K.M.; Meens, J.; Plantinga, P.; Stecho, W.; Pinto, N.; Yoo, J.; Fung, K.; MacNeil, D.; Mymryk, J.S.; Barrett, J.W.; et al. TAM family receptors in conjunction with MAPK signalling are involved in acquired resistance to PI3Kalpha inhibition in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 217. [Google Scholar] [CrossRef]
- Skinner, H.D.; Giri, U.; Yang, L.P.; Kumar, M.; Liu, Y.; Story, M.D.; Pickering, C.R.; Byers, L.A.; Williams, M.D.; Wang, J.; et al. Integrative analysis identifies a novel AXL-PI3 Kinase-PD-L1 signaling axis associated with radiation resistance in head and neck cancer. Clin. Cancer Res. 2017, 23, 2713–2722. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, N.K.; Cummings, C.T.; Iida, M.; Hulse, J.; Pearson, H.E.; Vasileiadi, E.; Parker, R.E.; Orbuch, R.A.; Ondracek, O.J.; Welke, N.B.; et al. MERTK mediates intrinsic and adaptive resistance to AXL-targeting agents. Mol. Cancer Ther. 2018, 17, 2297–2308. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Zhou, L.; Wang, H.; Zhang, Q.; Xu, Y. Axl is a potential cancer prognostic marker for the migration and invasion of nasopharyngeal carcinoma. Adv. Clin. Exp. Med. 2016, 25, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubendorf, L.; Kononen, J.; Koivisto, P.; Schraml, P.; Moch, H.; Gasser, T.C.; Willi, N.; Mihatsch, M.J.; Sauter, G.; Kallioniemi, O.P. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999, 59, 803–806. [Google Scholar] [PubMed]
- Dancau, A.M.; Simon, R.; Mirlacher, M.; Sauter, G. Tissue microarrays. Methods Mol. Biol. 2010, 576, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.; Kosinski, M. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0.4.3. 2018. Available online: https://CRAN.R-project.org/package=survminer (accessed on 15 March 2022).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 15 March 2022).
- Wickham, H. Reshaping data with the reshape package. J. Stat. Softw. 2007, 21, 1–20. Available online: http://www.jstatsoft.org/v21/i12/ (accessed on 15 March 2022). [CrossRef]
- Simon, R.; Mirlacher, M.; Sauter, G. Immunohistochemical analysis of tissue microarrays. Methods Mol. Biol. 2010, 664, 113–126. [Google Scholar] [CrossRef]
- Steurer, S.; Schneider, J.; Buscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Hoflmayer, D.; Weidemann, S.; Fraune, C.; et al. Immunohistochemically detectable thyroglobulin expression in extrathyroidal cancer is 100% specific for thyroidal tumor origin. Ann. Diagn Pathol. 2021, 54, 151793. [Google Scholar] [CrossRef]
- Nauta, I.H.; Heideman, D.A.M.; Brink, A.; van der Steen, B.; Bloemena, E.; Koljenovic, S.; Baatenburg de Jong, R.J.; Leemans, C.R.; Brakenhoff, R.H. The unveiled reality of human papillomavirus as risk factor for Oral. Cavity squamous cell carcinoma. Int. J. Cancer 2021, 149, 420–430. [Google Scholar] [CrossRef]
- Simoens, C.; Gorbaslieva, I.; Gheit, T.; Holzinger, D.; Lucas, E.; Ridder, R.; Rehm, S.; Vermeulen, P.; Lammens, M.; Vanderveken, O.M.; et al. HPV DNA genotyping, HPV E6* I mRNA detection, and p16(INK4a)/Ki-67 staining in Belgian head and neck cancer patient specimens, collected within the HPV-AHEAD study. Cancer Epidemiol. 2021, 72, 101925. [Google Scholar] [CrossRef]
- Taberna, M.; Resteghini, C.; Swanson, B.; Pickard, R.K.; Jiang, B.; Xiao, W.; Mena, M.; Kreinbrink, P.; Chio, E.; Gillison, M.L. Low etiologic fraction for human papillomavirus in larynx squamous cell carcinoma. Oral. Oncol. 2016, 61, 55–61. [Google Scholar] [CrossRef]
- Tagliabue, M.; Mena, M.; Maffini, F.; Gheit, T.; Quiros Blasco, B.; Holzinger, D.; Tous, S.; Scelsi, D.; Riva, D.; Grosso, E.; et al. Role of human papillomavirus infection in head and neck cancer in Italy: The HPV-AHEAD study. Cancers 2020, 12, 3567. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, C.; Wuerdemann, N.; Gattenlohner, S.; Brobeil, A.; Wierzbicka, M.; Wagner, S.; Klussmann, J.P. The role of high-risk human papillomavirus infections in laryngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2017, 274, 3837–3842. [Google Scholar] [CrossRef] [PubMed]
- Castellsague, X.; Alemany, L.; Quer, M.; Halec, G.; Quiros, B.; Tous, S.; Clavero, O.; Alos, L.; Biegner, T.; Szafarowski, T.; et al. HPV involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef] [PubMed]
- Wium, M.; Ajayi-Smith, A.F.; Paccez, J.D.; Zerbini, L.F. The role of the receptor tyrosine kinase Axl in carcinogenesis and development of therapeutic resistance: An overview of molecular mechanisms and future applications. Cancers 2021, 13, 1521. [Google Scholar] [CrossRef]
- Antony, J.; Huang, R.Y. AXL-Driven EMT state as a targetable conduit in cancer. Cancer Res. 2017, 77, 3725–3732. [Google Scholar] [CrossRef] [Green Version]
- Prigge, E.S.; Arbyn, M.; von Knebel Doeberitz, M.; Reuschenbach, M. Diagnostic accuracy of p16(INK4a) immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int. J. Cancer 2017, 140, 1186–1198. [Google Scholar] [CrossRef] [Green Version]



| Patient Characteristics | ||
|---|---|---|
| Interpretable staining, number (%) | ||
| Axl and/or Gas6 | 362 (100) | |
| Axl | 341 (94.2) | |
| Gas6 | 350 (96.7) | |
| Age, median (range) | 60 (32–85) | |
| Sex, number (%) | ||
| male | 281 (77.6) | |
| female | 81 (22.4) | |
| Location, number (%) | ||
| Oropharynx | 170 (47) | |
| p16+ (% of OPSCC) | 68 (40) | |
| p16− (% of OPSCC) | 90 (52.9) | |
| p16 n.a. (% of OPSCC) | 12 (7.1) | |
| Larynx | 120 (33.1) | |
| Hypopharynx | 46 (12.7) | |
| Oral cavity | 21 (5.8) | |
| Nasopharynx | 5 (1.4) | |
| T classification, number (%) | ||
| T1 | 83 (22.9) | |
| T2 | 106 (29.3) | |
| T3 | 88 (24.3) | |
| T4 | 84 (23.2) | |
| n.a. | 1 (0.3) | |
| N classification, number (%) | ||
| N0 | 159 (43.9) | |
| N1 | 53 (14.6) | |
| N2 | 131 (36.2) | |
| N3 | 19 (5.3) | |
| Therapy, number (%) | ||
| surgery | 111 (30.7) | |
| surgery + (chemo)radiation | 181 (50) | |
| chemoradiation | 50 (13.8) | |
| radiotherapy | 9 (2.5) | |
| other | 5 (1.4) | |
| n.a. | 6 (1.7) | |
| Variables | Overall Survival | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| HPV-, surgery only | |||
| Axl | 10.035 | 2.13–47.26 | ** 0.0036 |
| T-stage | 1.661 | 1.011–2.728 | * 0.0454 |
| N-stage | 1.339 | 0.762–2.355 | 0.3105 |
| age | 2.088 | 1.161–3.755 | * 0.0139 |
| sex (m,f) | 0.380 | 0.0848–1.704 | 0.2063 |
| HPV-, RT in any form | |||
| Gas6 | 0.340 | 0.184–0.628 | *** 0.0006 |
| T-stage | 1.269 | 0.962–1.676 | 0.0923 |
| N-stage | 1.598 | 1.190–2.145 | ** 0.0018 |
| age | 1.512 | 1.131–2.022 | ** 0.0053 |
| sex (m,f) | 0.880 | 0.467-1.656 | 0.6913 |
| Variables | Recurrence free survival | ||
| HR | 95% CI | p-value | |
| HPV-, surgery only | |||
| Axl | 3.061 | 1.338–7.002 | ** 0.0080 |
| T-stage | 1.722 | 1.206–2.458 | ** 0.0028 |
| N-stage | 1.212 | 0.7982–1.840 | 0.3670 |
| age | 1.003 | 0.6694–1.502 | 0.9895 |
| sex (m,f) | 1.034 | 0.4228–2.530 | 0.9410 |
| HPV-, RT in any form | |||
| Gas6 | 0.459 | 0.276–0.763 | ** 0.0027 |
| T-stage | 1.198 | 0.946–1.516 | 0.1337 |
| N-stage | 1.438 | 1.229–1.840 | ** 0.0040 |
| age | 1.233 | 0.965–1.576 | 0.0947 |
| sex (m,f) | 1.136 | 0.668–1.931 | 0.6380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busch, C.-J.; Hagel, C.; Becker, B.; Oetting, A.; Möckelmann, N.; Droste, C.; Möller-Koop, C.; Witt, M.; Blaurock, M.; Loges, S.; et al. Tissue Microarray Analyses Suggest Axl as a Predictive Biomarker in HPV-Negative Head and Neck Cancer. Cancers 2022, 14, 1829. https://doi.org/10.3390/cancers14071829
Busch C-J, Hagel C, Becker B, Oetting A, Möckelmann N, Droste C, Möller-Koop C, Witt M, Blaurock M, Loges S, et al. Tissue Microarray Analyses Suggest Axl as a Predictive Biomarker in HPV-Negative Head and Neck Cancer. Cancers. 2022; 14(7):1829. https://doi.org/10.3390/cancers14071829
Chicago/Turabian StyleBusch, Chia-Jung, Christian Hagel, Benjamin Becker, Agnes Oetting, Nikolaus Möckelmann, Conrad Droste, Christina Möller-Koop, Melanie Witt, Markus Blaurock, Sonja Loges, and et al. 2022. "Tissue Microarray Analyses Suggest Axl as a Predictive Biomarker in HPV-Negative Head and Neck Cancer" Cancers 14, no. 7: 1829. https://doi.org/10.3390/cancers14071829
APA StyleBusch, C.-J., Hagel, C., Becker, B., Oetting, A., Möckelmann, N., Droste, C., Möller-Koop, C., Witt, M., Blaurock, M., Loges, S., Rothkamm, K., Betz, C., Münscher, A., Clauditz, T. S., & Rieckmann, T. (2022). Tissue Microarray Analyses Suggest Axl as a Predictive Biomarker in HPV-Negative Head and Neck Cancer. Cancers, 14(7), 1829. https://doi.org/10.3390/cancers14071829








