Neoadjuvant Therapy for Primary Resectable Retroperitoneal Sarcomas—Looking Forward
Abstract
Simple Summary
Abstract
1. Background
2. Evidence Surrounding Neoadjuvant Radiotherapy
2.1. Historical Context
2.2. Histology-Specific Evidence
2.3. STRASS-1
2.4. Short-Course RT
2.5. Non-Photon RT
3. Evidence Surrounding Neoadjuvant Systemic Therapy
3.1. Historical Context
3.2. Cytotoxic Chemotherapy
3.3. Histology-Specific Evidence
3.4. STRASS2
3.5. Targeted Therapy
3.6. Immunotherapy
3.7. Active Trials
4. Multimodality Neoadjuvant Therapy
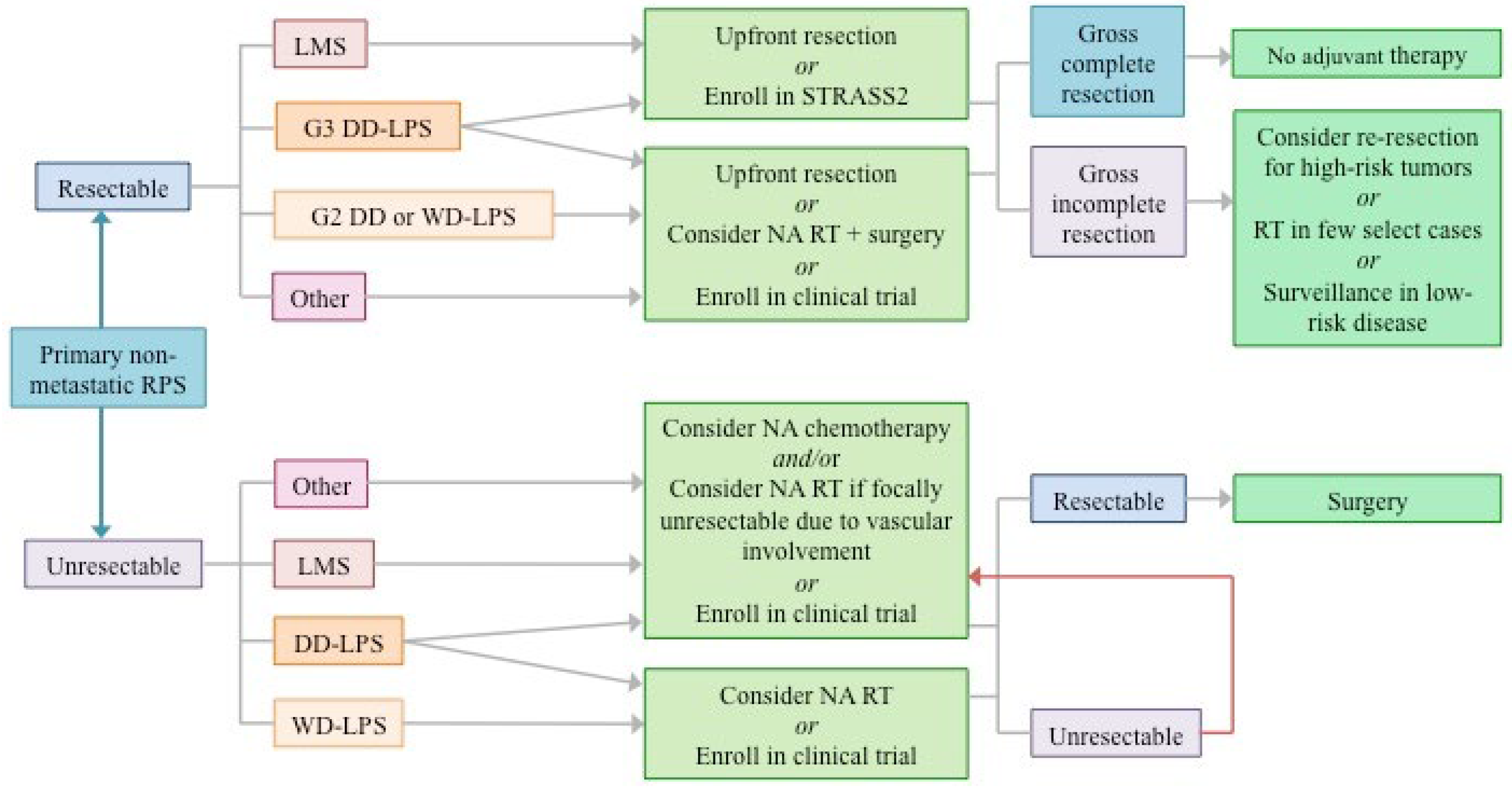
Intersection of STRASS1 and STRASS2
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nathan, H.; Raut, C.P.; Thornton, K.; Herman, J.M.; Ahuja, N.; Schulick, R.D.; Choti, M.A.; Pawlik, T.M. Predictors of Survival after Resection of Retroperitoneal Sarcoma: A Population-Based Analysis and Critical Appraisal of the AJCC Staging System. Ann. Surg. 2009, 250, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Rivoire, M.; Castaing, M.; Stoeckle, E.; Le Cesne, A.; Blay, J.Y.; Laplanche, A. Primary Retroperitoneal Sarcomas: A Multivariate Analysis of Surgical Factors Associated with Local Control. J. Clin. Oncol. 2009, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Albertsmeier, M.; Rauch, A.; Roeder, F.; Hasenhütl, S.; Pratschke, S.; Kirschneck, M.; Gronchi, A.; Jebsen, N.L.; Cassier, P.A.; Sargos, P.; et al. External Beam Radiation Therapy for Resectable Soft Tissue Sarcoma: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2018, 25, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, M.; Wang, J.; Jo, V.Y.; Baldini, E.H.; Bertagnolli, M.M.; Raut, C.P. Surgical Management of Primary Retroperitoneal Sarcomas: Rationale for Selective Organ Resection. Ann. Surg. Oncol. 2018, 25, 98–106. [Google Scholar] [CrossRef]
- Abdelfatah, E.; Guzzetta, A.A.; Nagarajan, N.; Wolfgang, C.L.; Pawlik, T.M.; Choti, M.A.; Schulick, R.; Montgomery, E.A.; Meyer, C.; Thornton, K.; et al. Long-term outcomes in treatment of retroperitoneal sarcomas: A 15 year single-institution evaluation of prognostic features: Retroperitoneal Sarcomas. J. Surg. Oncol. 2016, 114, 56–64. [Google Scholar] [CrossRef]
- Stahl, J.M.; Corso, C.D.; Park, H.S.; An, Y.; Rutter, C.E.; Han, D.; Roberts, K.B. The effect of microscopic margin status on survival in adult retroperitoneal soft tissue sarcomas. Eur. J. Surg. Oncol. EJSO 2017, 43, 168–174. [Google Scholar] [CrossRef]
- Berger, N.G.; Silva, J.P.; Mogal, H.; Clarke, C.N.; Bedi, M.; Charlson, J.; Christians, K.K.; Tsai, S.; Gamblin, T.C. Overall survival after resection of retroperitoneal sarcoma at academic cancer centers versus community cancer centers: An analysis of the National Cancer Data Base. Surgery 2018, 163, 318–323. [Google Scholar] [CrossRef]
- Tseng, W.H.; Martinez, S.R.; Do, L.; Tamurian, R.M.; Borys, D.; Canter, R.J. Lack of Survival Benefit Following Adjuvant Radiation in Patients with Retroperitoneal Sarcoma: A SEER Analysis. J. Surg. Res. 2011, 168, e173–e180. [Google Scholar] [CrossRef]
- Klooster, B.; Rajeev, R.; Chrabaszcz, S.; Charlson, J.; Miura, J.; Bedi, M.; Gamblin, T.C.; Johnston, F.; Turaga, K.K. Is long-term survival possible after margin-positive resection of retroperitoneal sarcoma (RPS)? Survival after R2 Resection in Sarcomas. J. Surg. Oncol. 2016, 113, 823–827. [Google Scholar] [CrossRef]
- Baldini, E.H.; Wang, D.; Haas, R.L.M.; Catton, C.N.; Indelicato, D.J.; Kirsch, D.G.; Roberge, D.; Salerno, K.; Deville, C.; Guadagnolo, B.A.; et al. Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int. J. Radiat. Oncol. 2015, 92, 602–612. [Google Scholar] [CrossRef]
- Yang, J.C.; Chang, A.E.; Baker, A.R.; Sindelar, W.F.; Danforth, D.N.; Topalian, S.L.; DeLaney, T.; Glatstein, E.; Steinberg, S.M.; Merino, M.J.; et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998, 16, 197–203. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Surgery with or without Radiation Therapy in Treating Patients with Primary Soft Tissue Sarcoma of the Retroperitoneum or Pelvis. 2004; NCT00091351. Available online: https://clinicaltrials.gov/ct2/show/NCT00091351 (accessed on 15 January 2022).
- Smith, M.J.F.; Ridgway, P.F.; Catton, C.N.; Cannell, A.J.; O’Sullivan, B.; Mikula, L.A.; Jones, J.J.; Swallow, C.J. Combined management of retroperitoneal sarcoma with dose intensification radiotherapy and resection: Long-term results of a prospective trial. Radiother. Oncol. 2014, 110, 165–171. [Google Scholar] [CrossRef]
- Sargos, P.; Dejean, C.; de Figueiredo, B.H.; Brouste, V.; Nguyen Bui, B.; Italiano, A.; Stoeckle, E.; Kantor, G. High-dose pre-operative helical tomotherapy (54 Gy) for retroperitoneal liposarcoma. Radiat. Oncol. 2012, 7, 214. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Pisters, P.W.T.; Mikula, L.; Feig, B.W.; Hunt, K.K.; Cormier, J.N.; Ballo, M.T.; Catton, C.N.; Jones, J.J.; O’Sullivan, B.; et al. Long-Term Results of Two Prospective Trials of Preoperative External Beam Radiotherapy for Localized Intermediate- or High-Grade Retroperitoneal Soft Tissue Sarcoma. Ann. Surg. Oncol. 2006, 13, 508–517. [Google Scholar] [CrossRef]
- Roeder, F.; Ulrich, A.; Habl, G.; Uhl, M.; Saleh-Ebrahimi, L.; Huber, P.E.; Schulz-Ertner, D.; Nikoghosyan, A.V.; Alldinger, I.; Krempien, R.; et al. Clinical Phase I/II trial to Investigate Preoperative Dose-Escalated Intensity-Modulated Radiation Therapy (IMRT) and Intraoperative Radiation Therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: Interim analysis. BMC Cancer 2014, 14, 617. [Google Scholar] [CrossRef]
- Cheng, H.; Miura, J.T.; Lalehzari, M.; Rajeev, R.; Donahue, A.E.; Bedi, M.; Gamblin, T.C.; Turaga, K.K.; Johnston, F.M. Neoadjuvant radiotherapy for retroperitoneal sarcoma: A systematic review: NART for RPS: A Systematic Review. J. Surg. Oncol. 2016, 113, 628–634. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in Patterns of Recurrence after Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients from the Multi-institutional Collaborative RPS Working Group. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef]
- Abraham, J.A.; Weaver, M.J.; Hornick, J.L.; Zurakowski, D.; Ready, J.E. Outcomes and Prognostic Factors for a Consecutive Case Series of 115 Patients with Somatic Leiomyosarcoma. J. Bone Jt. Surg. 2012, 94, 736–744. [Google Scholar] [CrossRef]
- Haas, R.L.; Floot, B.G.J.; Scholten, A.N.; van der Graaf, W.T.A.; van Houdt, W.; Schrage, Y.; van de Ven, M.; Bovée, J.V.M.G.; van Coevorden, F.; Vens, C. Cellular Radiosensitivity of Soft Tissue Sarcoma. Radiat. Res. 2021, 196, 23–30. [Google Scholar] [CrossRef]
- Allignet, B.; Meurgey, A.; Bouhamama, A.; Karanian, M.; Meeus, P.; Vaz, G.; Gouin, F.; Moncharmont, C.; Prapant, S.; Waissi, W.; et al. Impact of histological subtype on radiological and pathological response after neoadjuvant radiotherapy in soft tissue sarcoma. Eur. J. Surg. Oncol. 2021, 47, 2995–3003. [Google Scholar] [CrossRef]
- Pitson, G.; Robinson, P.; Wilke, D.; Kandel, R.A.; White, L.; Griffin, A.M.; Bell, R.S.; Catton, C.N.; Wunder, J.S.; O’Sullivan, B. Radiation response: An additional unique signature of myxoid liposarcoma. Int. J. Radiat. Oncol. 2004, 60, 522–526. [Google Scholar] [CrossRef]
- Tang, Z.; Zeng, Q.; Li, Y.; Zhang, X.; Ma, J.; Suto, M.J.; Xu, B.; Yi, N. Development of a radiosensitivity gene signature for patients with soft tissue sarcoma. Oncotarget 2017, 8, 27428–27439. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. REtroperitoneal SArcoma Registry: An International Prospective Initiative (RESAR). 2019; NCT03838718. Available online: https://clinicaltrials.gov/ct2/show/NCT03838718 (accessed on 22 January 2022).
- Callegaro, D.; Gronchi, A.; Taiwo, A.; Marreaud, S.; Litière, S.; Marreaud, S. Preoperative radiotherapy in patients with primary retroperitoneal sarcoma (RPS): Trial (STRASS) vs. off-trial (STREXIT) results. Connect. Tissue Oncol. Soc. Meet. Final Program 2020, 17, 3464763. [Google Scholar]
- Parsai, S.; Lawrenz, J.; Kilpatrick, S.; Rubin, B.; Hymes, C.; Gray, M.; Mesko, N.; Shah, C.; Nystrom, L.; Scott, J.G. Early Outcomes of Preoperative 5-Fraction Radiation Therapy for Soft Tissue Sarcoma Followed by Immediate Surgical Resection. Adv. Radiat. Oncol. 2020, 5, 1274–1279. [Google Scholar] [CrossRef]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Ladra, M.M.; Edgington, S.K.; Mahajan, A.; Grosshans, D.; Szymonifka, J.; Khan, F.; Moteabbed, M.; Friedmann, A.M.; MacDonald, S.M.; Tarbell, N.J.; et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother. Oncol. 2014, 113, 77–83. [Google Scholar] [CrossRef]
- DeLaney, T.F.; Chen, Y.-L.; Baldini, E.H.; Wang, D.; Adams, J.; Hickey, S.B.; Yeap, B.Y.; Hahn, S.M.; De Amorim Bernstein, K.; Nielsen, G.P.; et al. Phase 1 trial of preoperative image guided intensity modulated proton radiation therapy with simultaneously integrated boost to the high risk margin for retroperitoneal sarcomas. Adv. Radiat. Oncol. 2017, 2, 85–93. [Google Scholar] [CrossRef]
- Allen, A.M.; Pawlicki, T.; Dong, L.; Fourkal, E.; Buyyounouski, M.; Cengel, K.; Plastaras, J.; Bucci, M.K.; Yock, T.I.; Bonilla, L.; et al. An evidence based review of proton beam therapy: The report of ASTRO’s emerging technology committee. Radiother. Oncol. 2012, 103, 8–11. [Google Scholar] [CrossRef]
- Doyen, J.; Aloi, D.; Groulier, A.; Vidal, M.; Lesueur, P.; Calugaru, V.; Bondiau, P.Y. Role of proton therapy in reirradiation and in the treatment of sarcomas. Cancer/Radiothérapie 2021, 25, 550–553. [Google Scholar] [CrossRef]
- Schneider, R.A.; Vitolo, V.; Albertini, F.; Koch, T.; Ares, C.; Lomax, A.; Goitein, G.; Hug, E.B. Small bowel toxicity after high dose spot scanning-based proton beam therapy for paraspinal/retroperitoneal neoplasms. Strahlenther. Onkol. 2013, 189, 1020–1025. [Google Scholar] [CrossRef]
- Swanson, E.L.; Indelicato, D.J.; Louis, D.; Flampouri, S.; Li, Z.; Morris, C.G.; Paryani, N.; Slopsema, R. Comparison of Three-Dimensional (3D) Conformal Proton Radiotherapy (RT), 3D Conformal Photon RT, and Intensity-Modulated RT for Retroperitoneal and Intra-Abdominal Sarcomas. Int. J. Radiat. Oncol. 2012, 83, 1549–1557. [Google Scholar] [CrossRef]
- De Kruijff, R.M. FLASH radiotherapy: Ultra-high dose rates to spare healthy tissue. Int. J. Radiat. Biol. 2020, 96, 419–423. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Karagounis, I.V.; Cramer, G.M.; Kim, M.M.; Skoufos, G.; Goia, D.; Hagan, S.; Verginadis, I.I.; Shoniyozov, K.; Chiango, J.; et al. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021, 81, 4808–4821. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int. J. Radiat. Oncol. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Levy, K.; Natarajan, S.; Wang, J.; Chow, S.; Eggold, J.T.; Loo, P.E.; Manjappa, R.; Melemenidis, S.; Lartey, F.M.; Schüler, E.; et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci. Rep. 2020, 10, 21600. [Google Scholar] [CrossRef]
- Brodowicz, T.; Schwameis, E.; Widder, J.; Amann, G.; Wiltschke, C.; Dominkus, M.; Windhager, R.; Ritschl, P.; Pötter, R.; Kotz, R.; et al. Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 2000, 4, 151–160. [Google Scholar] [CrossRef]
- Petrioli, R.; Coratti, A.; Correale, P.; D’Aniello, C.; Grimaldi, L.; Tanzini, G.; Civitelli, S.; Marsili, S.; Messinese, S.; Marzocca, G.; et al. Adjuvant Epirubicin with or without Ifosfamide for Adult Soft-Tissue Sarcoma. Am. J. Clin. Oncol. 2002, 25, 468–473. [Google Scholar] [CrossRef]
- Gortzak, E.; Azzarelli, A.; Buesa, J.; Bramwell, V.H.C.; van Coevorden, F.; van Geel, A.N.; Ezzat, A.; Santoro, A.; Oosterhuis, J.W.; van Glabbeke, M.; et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur. J. Cancer 2001, 37, 1096–1103. [Google Scholar] [CrossRef]
- Frustaci, S.; Gherlinzoni, F.; De Paoli, A.; Bonetti, M.; Azzarelli, A.; Comandone, A.; Olmi, P.; Buonadonna, A.; Pignatti, G.; Barbieri, E.; et al. Adjuvant Chemotherapy for Adult Soft Tissue Sarcomas of the Extremities and Girdles: Results of the Italian Randomized Cooperative Trial. J. Clin. Oncol. 2001, 19, 1238–1247. [Google Scholar] [CrossRef]
- Austrian Cooperative Soft Tissue Sarcoma Study Group; Fakhrai, N.; Ebm, C.; Kostler, W.J.; Jantsch, M.; Abdolvahab, F.; Dominkus, M.; Pokrajac, B.; Kauer-Dorner, D.; Zielinski, C.C.; et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: Long-term follow-up of a prospective randomized feasibility trial. Wien. Klin. Wochenschr. 2010, 122, 614–619. [Google Scholar]
- Eilber, F.C.; Rosen, G.; Eckardt, J.; Forscher, C.; Nelson, S.D.; Selch, M.; Dorey, F.; Eilber, F.R. Treatment-Induced Pathologic Necrosis: A Predictor of Local Recurrence and Survival in Patients Receiving Neoadjuvant Therapy for High-Grade Extremity Soft Tissue Sarcomas. J. Clin. Oncol. 2001, 19, 3203–3209. [Google Scholar] [CrossRef]
- Donahue, T.R.; Kattan, M.W.; Nelson, S.D.; Tap, W.D.; Eilber, F.R.; Eilber, F.C. Evaluation of neoadjuvant therapy and histopathologic response in primary, high-grade retroperitoneal sarcomas using the sarcoma nomogram. Cancer 2010, 116, 3883–3891. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpal, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Swallow, C.J.; Strauss, D.C.; Bonvalot, S.; Rutkowski, P.; Desai, A.; Gladdy, R.A.; Gonzalez, R.; Gyorki, D.E.; Fairweather, M.; van Houdt, W.J.; et al. Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: An Updated Consensus Approach from the Transatlantic Australasian RPS Working Group. Ann. Surg. Oncol. 2021, 28, 7873–7888. [Google Scholar] [CrossRef]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef]
- Sarcoma Meta-analysis Collaboration (SMAC). Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst. Rev. 2000, 4, CD001419. [Google Scholar] [CrossRef]
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: Meta-analysis of individual data. Lancet 1997, 350, 1647–1654. [Google Scholar] [CrossRef]
- Istl, A.C.; Ruck, J.M.; Morris, C.D.; Levin, A.S.; Meyer, C.F.; Johnston, F.M. Call for improved design and reporting in soft tissue sarcoma studies: A systematic review and meta-analysis of chemotherapy and survival outcomes in resectable STS. J. Surg. Oncol. 2019, 119, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Miura, J.T.; Charlson, J.; Gamblin, T.C.; Eastwood, D.; Banerjee, A.; Johnston, F.M.; Turaga, K.K. Impact of chemotherapy on survival in surgically resected retroperitoneal sarcoma. Eur. J. Surg. Oncol. EJSO 2015, 41, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Bremjit, P.J.; Jones, R.L.; Chai, X.; Kane, G.; Rodler, E.T.; Loggers, E.T.; Pollack, S.M.; Pillarisetty, V.G.; Mann, G.N. A Contemporary Large Single-Institution Evaluation of Resected Retroperitoneal Sarcoma. Ann. Surg. Oncol. 2014, 21, 2150–2158. [Google Scholar] [CrossRef]
- Dangoor, A.; Seddon, B.; Gerrand, C.; Grimer, R.; Whelan, J.; Judson, I. UK guidelines for the management of soft tissue sarcomas. Clin. Sarcoma Res. 2016, 6, 20. [Google Scholar] [CrossRef]
- Kawai, A.; Yonemori, K.; Takahashi, S.; Araki, N.; Ueda, T. Systemic Therapy for Soft Tissue Sarcoma: Proposals for the Optimal Use of Pazopanib, Trabectedin, and Eribulin. Adv. Ther. 2017, 34, 1556–1571. [Google Scholar] [CrossRef]
- Palmerini, E.; Staals, E.L.; Alberghini, M.; Zanella, L.; Ferrari, C.; Benassi, M.S.; Picci, P.; Mercuri, M.; Bacci, G.; Ferrari, S. Synovial sarcoma: Retrospective analysis of 250 patients treated at a single institution. Cancer 2009, 115, 2988–2998. [Google Scholar] [CrossRef]
- Al-Hussaini, H.; Hogg, D.; Blackstein, M.E.; O’Sullivan, B.; Catton, C.N.; Chung, P.W.; Griffin, A.M.; Hodgson, D.; Hopyan, S.; Kandel, R.; et al. Clinical Features, Treatment, and Outcome in 102 Adult and Pediatric Patients with Localized High-Grade Synovial Sarcoma. Sarcoma 2011, 2011, 231789. [Google Scholar] [CrossRef]
- Ferrari, A.; Gronchi, A.; Casanova, M.; Meazza, C.; Gandola, L.; Collini, P.; Lozza, L.; Bertulli, R.; Olmi, P.; Casali, P.G. Synovial sarcoma: A retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 2004, 101, 627–634. [Google Scholar] [CrossRef]
- Wu, Y.; Bi, W.; Han, G.; Jia, J.; Xu, M. Influence of neoadjuvant chemotherapy on prognosis of patients with synovial sarcoma. World J. Surg. Oncol. 2017, 15, 101. [Google Scholar] [CrossRef]
- Minami, Y.; Matsumoto, S.; Ae, K.; Tanizawa, T.; Hayakawa, K.; Funauchi, Y.; Saito, M.; Tsuda, Y. The Role of Neoadjuvant Chemotherapy in Resectable Primary Synovial Sarcoma. Anticancer Res. 2020, 40, 1029–1034. [Google Scholar] [CrossRef]
- Canter, R.J.; Qin, L.-X.; Maki, R.G.; Brennan, M.F.; Ladanyi, M.; Singer, S. A Synovial Sarcoma-Specific Preoperative Nomogram Supports a Survival Benefit to Ifosfamide-Based Chemotherapy and Improves Risk Stratification for Patients. Clin. Cancer Res. 2008, 14, 8191–8197. [Google Scholar] [CrossRef]
- D’Ambrosio, L.; Touati, N.; Blay, J.; Grignani, G.; Flippot, R.; Czarnecka, A.M.; Piperno-Neumann, S.; Martin-Broto, J.; Sanfilippo, R.; Katz, D.; et al. Doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as a first-line treatment for advanced leiomyosarcoma: A propensity score matching analysis from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer 2020, 126, 2637–2647. [Google Scholar]
- Pautier, P.; Floquet, A.; Penel, N.; Piperno-Neumann, S.; Isambert, N.; Rey, A.; Bompas, E.; Cioffi, A.; Delcambre, C.; Cupissol, D.; et al. Randomized Multicenter and Stratified Phase II Study of Gemcitabine Alone Versus Gemcitabine and Docetaxel in Patients with Metastatic or Relapsed Leiomyosarcomas: A Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist 2012, 17, 1213–1220. [Google Scholar]
- Seddon, B.; Scurr, M.; Jones, R.L.; Wood, Z.; Propert-Lewis, C.; Fisher, C.; Flanagan, A.; Sunkersing, J.; A’Hern, R.; Whelan, J.; et al. A phase II trial to assess the activity of gemcitabine and docetaxel as first line chemotherapy treatment in patients with unresectable leiomyosarcoma. Clin. Sarcoma Res. 2015, 5, 13. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Gronchi, A.; Palmerini, E.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Brunello, A.; Blay, J.Y.; Tendero, O.; Beveridge, R.D.; et al. Neoadjuvant chemotherapy in high risk soft tissue sarcoma: Final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), Polish (PSG) sarcoma groups. J. Clin. Oncol. 2020, 38, 2178–2186. [Google Scholar] [CrossRef]
- Tseng, W.W.; Barretta, F.; Conti, L.; Grignani, G.; Tolomeo, F.; Albertsmeier, M.; Angele, M.K.; Rutkowski, P.; Skoczylas, J.; De Paoli, A.; et al. Defining the role of neoadjuvant systemic therapy in high-risk retroperitoneal sarcoma: A multi-institutional study from the Transatlantic Australasian Retroperitoneal Sarcoma Working Group. Cancer 2021, 127, 729–738. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Surgery with Our without Neoadjuvant Chemotherapy in High Risk RetroPeritoneal Sarcoma (STRASS2). 2022; NCT04031677. Available online: https://clinicaltrials.gov/ct2/show/NCT04031677?term=STRASS2&draw=2&rank=1 (accessed on 3 March 2022).
- Gronchi, A.; Miceli, R.; Allard, M.A.; Callegaro, D.; Le Péchoux, C.; Fiore, M.; Honoré, C.; Sanfilippo, R.; Coppola, S.; Stacchiotti, S.; et al. Personalizing the Approach to Retroperitoneal Soft Tissue Sarcoma: Histology-specific Patterns of Failure and Postrelapse Outcome after Primary Extended Resection. Ann. Surg. Oncol. 2015, 22, 1447–1454. [Google Scholar] [CrossRef]
- Sleijfer, S.; Ray-Coquard, I.; Papai, Z.; Le Cesne, A.; Scurr, M.; Schöffski, P.; Collin, F.; Pandite, L.; Marreaud, S.; De Brauwer, A.; et al. Pazopanib, a Multikinase Angiogenesis Inhibitor, in Patients with Relapsed or Refractory Advanced Soft Tissue Sarcoma: A Phase II Study from the European Organisation for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group (EORTC Study 62043). J. Clin. Oncol. 2009, 27, 3126–3132. [Google Scholar]
- Van der Graaf, W.T.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Chen, L.; Oke, T.; Siegel, N.; Cojocaru, G.; Tam, A.J.; Blosser, R.L.; Swailes, J.; Ligon, J.A.; Lebid, A.; Morris, C.; et al. The Immunosuppressive Niche of Soft-Tissue Sarcomas is Sustained by Tumor-Associated Macrophages and Characterized by Intratumoral Tertiary Lymphoid Structures. Clin. Cancer Res. 2020, 26, 4018–4030. [Google Scholar] [CrossRef]
- Delespaul, L.; Lesluyes, T.; Pérot, G.; Brulard, C.; Lartigue, L.; Baud, J.; Lagarde, P.; Le Guellec, S.; Neuville, A.; Terrier, P.; et al. Recurrent TRIO Fusion in Nontranslocation–Related Sarcomas. Clin. Cancer Res. 2017, 23, 857–867. [Google Scholar] [CrossRef]
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive Genomic Analysis of Rhabdomyosarcoma Reveals a Landscape of Alterations Affecting a Common Genetic Axis in Fusion-Positive and Fusion-Negative Tumors. Cancer Discov. 2014, 4, 216–231. [Google Scholar] [CrossRef]
- Keung, E.Z.; Burgess, M.; Salazar, R.; Parra, E.R.; Rodrigues-Canales, J.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Attia, S.; Riedel, R.F.; et al. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin. Cancer Res. 2020, 26, 1258–1266. [Google Scholar] [CrossRef]
- Boxberg, M.; Steiger, K.; Lenze, U.; Rechl, H.; von Eisenhart-Rothe, R.; Wörtler, K.; Weichert, W.; Langer, R.; Specht, K. PD-L1 and PD-1 and characterization of tumor-infiltrating lymphocytes in high grade sarcomas of soft tissue–prognostic implications and rationale for immunotherapy. OncoImmunology 2018, 7, e1389366. [Google Scholar] [CrossRef]
- Tseng, W.W.; Malu, S.; Zhang, M.; Chen, J.; Sim, G.C.; Wei, W.; Ingram, D.; Somaiah, N.; Lev, D.C.; Pollock, R.E.; et al. Analysis of the Intratumoral Adaptive Immune Response in Well Differentiated and Dedifferentiated Retroperitoneal Liposarcoma. Sarcoma 2015, 2015, 547460. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Nguyen, P.; Engle, E.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Green, B.; Soni, A.; Cuda, J.D.; Stein, J.E.; et al. Multidimensional, quantitative assessment of PD-1/PD-L1 expression in patients with Merkel cell carcinoma and association with response to pembrolizumab. J. Immunother. Cancer 2018, 6, 99. [Google Scholar] [CrossRef]
- Tan, K.W.; Chacko, A.-M.; Chew, V. PD-1 expression and its significance in tumour microenvironment of hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2019, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Yeong, J. Prognostic value of CD8+ PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J. Immunother. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Neoadjuvant Chemotherapy and Retifanlimab in Patients with Selected Retroperitoneal Sarcomas (TORNADO). 2021; NCT04968106. Available online: https://clinicaltrials.gov/ct2/show/NCT04968106?term=TORNADO&draw=2&rank=2 (accessed on 15 January 2022).
- Keung, E.Z.; Lazar, A.J.; Torres, K.E.; Wang, W.-L.; Cormier, J.N.; Ashleigh Guadagnolo, B.; Bishop, A.J.; Lin, H.; Hunt, K.K.; Bird, J.; et al. Phase II study of neoadjuvant checkpoint blockade in patients with surgically resectable undifferentiated pleomorphic sarcoma and dedifferentiated liposarcoma. BMC Cancer 2018, 18, 913. [Google Scholar] [CrossRef]
- Gronchi, A.; Hindi, N.; Cruz, J.; Blay, J.-Y.; Lopez-Pousa, A.; Italiano, A.; Alvarez, R.; Gutierrez, A.; Rincón, I.; Sangalli, C.; et al. Trabectedin and RAdiotherapy in Soft Tissue Sarcoma (TRASTS): Results of a Phase I Study in Myxoid Liposarcoma from Spanish (GEIS), Italian (ISG), French (FSG) Sarcoma Groups. EClinicalMedicine 2019, 9, 35–43. [Google Scholar] [CrossRef]
- Gronchi, A.; Hindi, N.; Blay, J.; Redondo, A.; Sanfilippo, R.; Morosi, C.; Cruz Jurado, J.; Luna Fra, P.; Martinez-Trufero, J.; Valverde Morales, C.M.; et al. Trabectedin and radiotherapy in soft tissue sarcoma (TRASTS) study: An international, prospective, phase II trial in localized myxoid liposarcoma—A collaborative Spanish (GEIS), Italian (ISG) and French (FSG) group study. J. Clin. Oncol. 2020, 38, 11514. [Google Scholar] [CrossRef]
- Guo, S.; Lopez-Marquez, H.; Fan, K.C.; Choy, E.; Cote, G.; Harmon, D.; Nielsen, G.P.; Yang, C.; Zhang, C.; Mankin, H.; et al. Synergistic Effects of Targeted PI3K Signaling Inhibition and Chemotherapy in Liposarcoma. PLoS ONE 2014, 9, e93996. [Google Scholar] [CrossRef][Green Version]
- Ray-Coquard, I.; Blay, J.-Y.; Italiano, A.; Le Cesne, A.; Penel, N.; Zhi, J.; Heil, F.; Rueger, R.; Graves, B.; Ding, M.; et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: An exploratory proof-of-mechanism study. Lancet Oncol. 2012, 13, 1133–1140. [Google Scholar] [CrossRef]
- Wagner, A.J.; Banerji, U.; Mahipal, A.; Somaiah, N.; Hirsch, H.; Fancourt, C.; Johnson-Levonas, A.O.; Lam, R.; Meister, A.K.; Russo, G.; et al. Phase I Trial of the Human Double Minute 2 Inhibitor MK-8242 in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2017, 35, 1304–1311. [Google Scholar] [CrossRef]
- Das, S. MDM2 Inhibition in a Subset of Sarcoma Cell Lines Increases Susceptibility to Radiation Therapy by Inducing Senescence in the Polyploid Cells. Adv. Radiat. Oncol. 2020, 5, 250–259. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. SU2C-SARC032: A Randomized Trial of Pembrolizumab & Radiotherapy Versus Radiotherapy in High-Risk Soft Tissue Sarcoma of the Extremity. 2017; NCT03092323. Available online: https://clinicaltrials.gov/ct2/show/NCT03092323?term=SARC032&draw=2&rank=1 (accessed on 15 January 2022).
- ClinicalTrials.gov. Neoadjuvant Durvalumab and Tremelimumab Plus Radiation for High Risk Soft-Tissue Sarcoma (NEXIS). 2017; NCT03116529. Available online: https://clinicaltrials.gov/ct2/show/NCT03116529?term=NEXIS&draw=2&rank=2 (accessed on 21 January 2022).
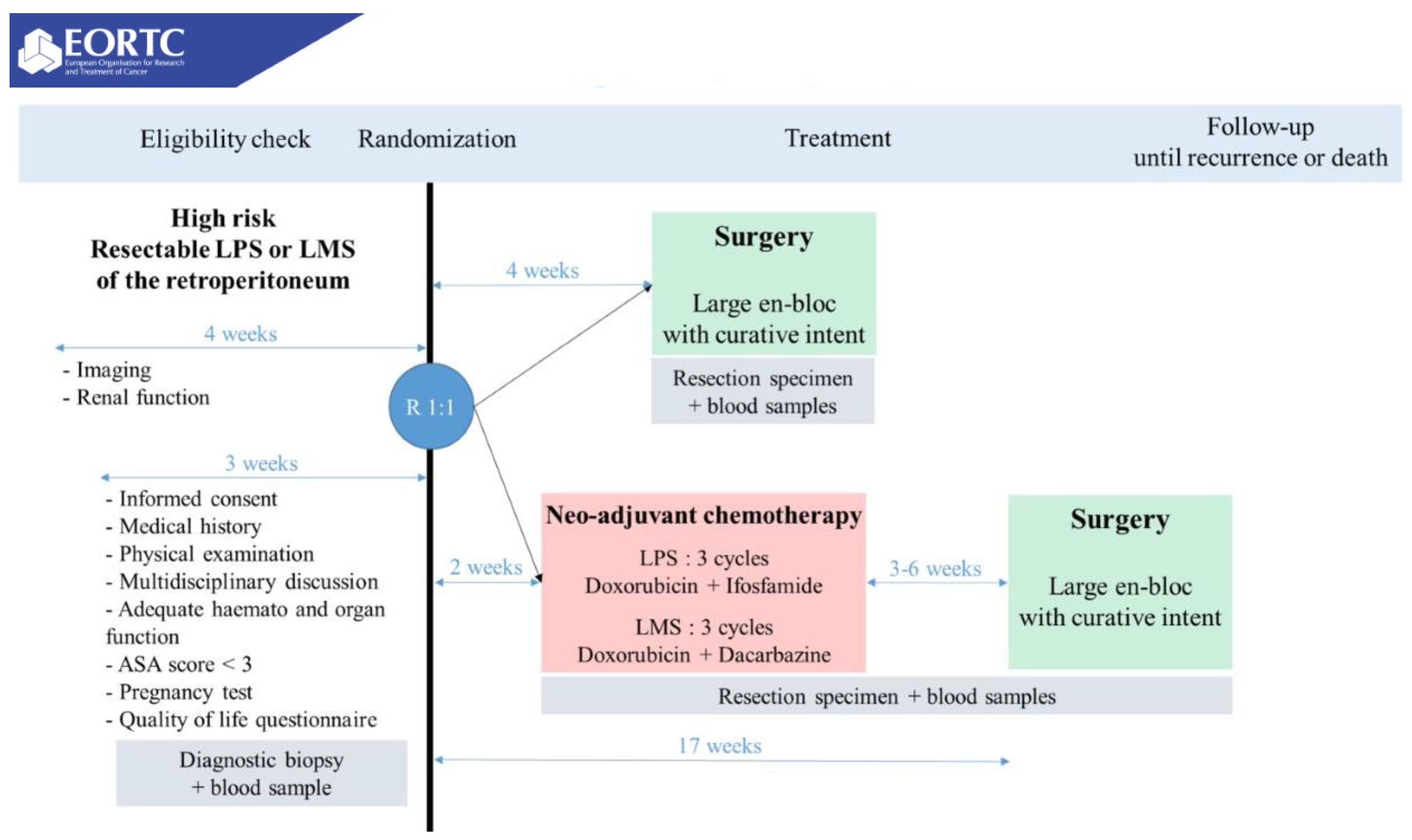
| Study | Design | N | Population | Intervention and Comparator | Outcomes | Findings |
|---|---|---|---|---|---|---|
| Radiotherapy | ||||||
| STRASS18 Bonvalot 2020 | Phase III RCT (1:1) | 266 | Resectable primary RPS | I: Neoadjuvant 3DCRT or IMRT (50.4 Gy in 28 fx of 1.8 Gy) + surgery C: surgery alone (en-bloc curative intent resection) | Primary: AFRS Secondary: tumor response, DMFS, ARFI, OS, safety, QoL |
|
| Systemic therapy | ||||||
| STRASS2 NCT04031677 | Phase III RCT (1:1) | 250 * | Resectable high-risk primary retroperitoneal LMS and LPS | I: 3 cycles neoadjuvant chemotherapy (LPS: ADM + ifosfamide, LMS: ADM+DTIC) C: surgery alone (en-bloc curative intent resection) | Primary: DFS Secondary: OS, LRFS, RFS, DMFS | Study in progress |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istl, A.C.; Gronchi, A. Neoadjuvant Therapy for Primary Resectable Retroperitoneal Sarcomas—Looking Forward. Cancers 2022, 14, 1831. https://doi.org/10.3390/cancers14071831
Istl AC, Gronchi A. Neoadjuvant Therapy for Primary Resectable Retroperitoneal Sarcomas—Looking Forward. Cancers. 2022; 14(7):1831. https://doi.org/10.3390/cancers14071831
Chicago/Turabian StyleIstl, Alexandra C., and Alessandro Gronchi. 2022. "Neoadjuvant Therapy for Primary Resectable Retroperitoneal Sarcomas—Looking Forward" Cancers 14, no. 7: 1831. https://doi.org/10.3390/cancers14071831
APA StyleIstl, A. C., & Gronchi, A. (2022). Neoadjuvant Therapy for Primary Resectable Retroperitoneal Sarcomas—Looking Forward. Cancers, 14(7), 1831. https://doi.org/10.3390/cancers14071831






