Overexpression of GATA5 Inhibits Prostate Cancer Progression by Regulating PLAGL2 via the FAK/PI3K/AKT Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Specimens and Cell Culture
2.2. Western Blotting
2.3. Immunoprecipitation (IP)
2.4. Quantitative Real-Time PCR
2.5. Cell Transfection Assay
2.6. Cell Proliferation Assay
2.7. Colony Formation Assay
2.8. Wound-Healing Assay
2.9. Transwell Invasion Assay
2.10. Cell Apoptosis and Cell Cycle
2.11. Immunohistochemistry (IHC)
2.12. Immunofluorescence
2.13. Xenograft Assay
2.14. Statistical Analysis
3. Results
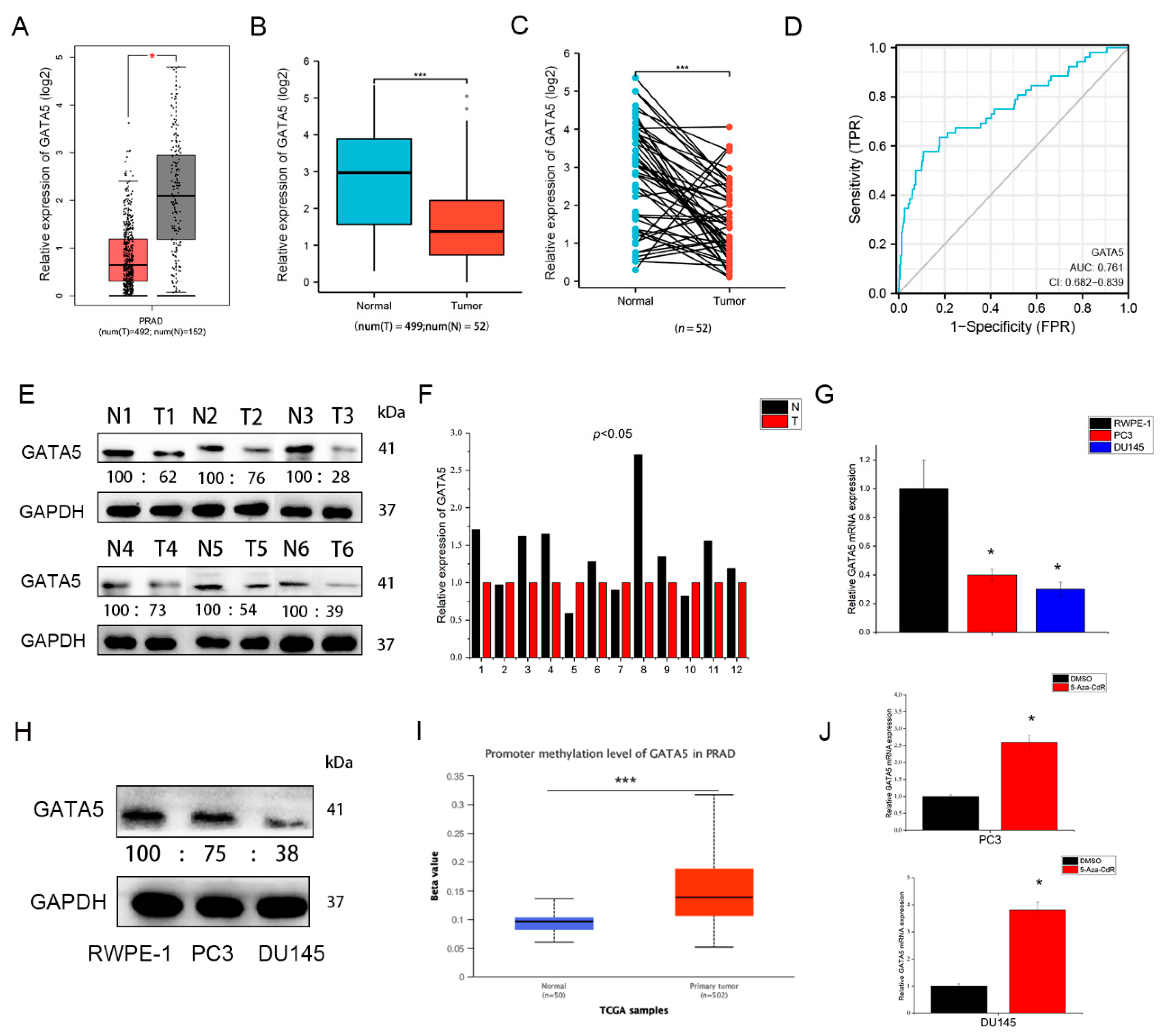
3.1. GATA5 Is Downregulated in PCa
3.2. Overexpression of GATA5 Inhibits PCa Cell Proliferation, Migration, and Invasion In Vitro
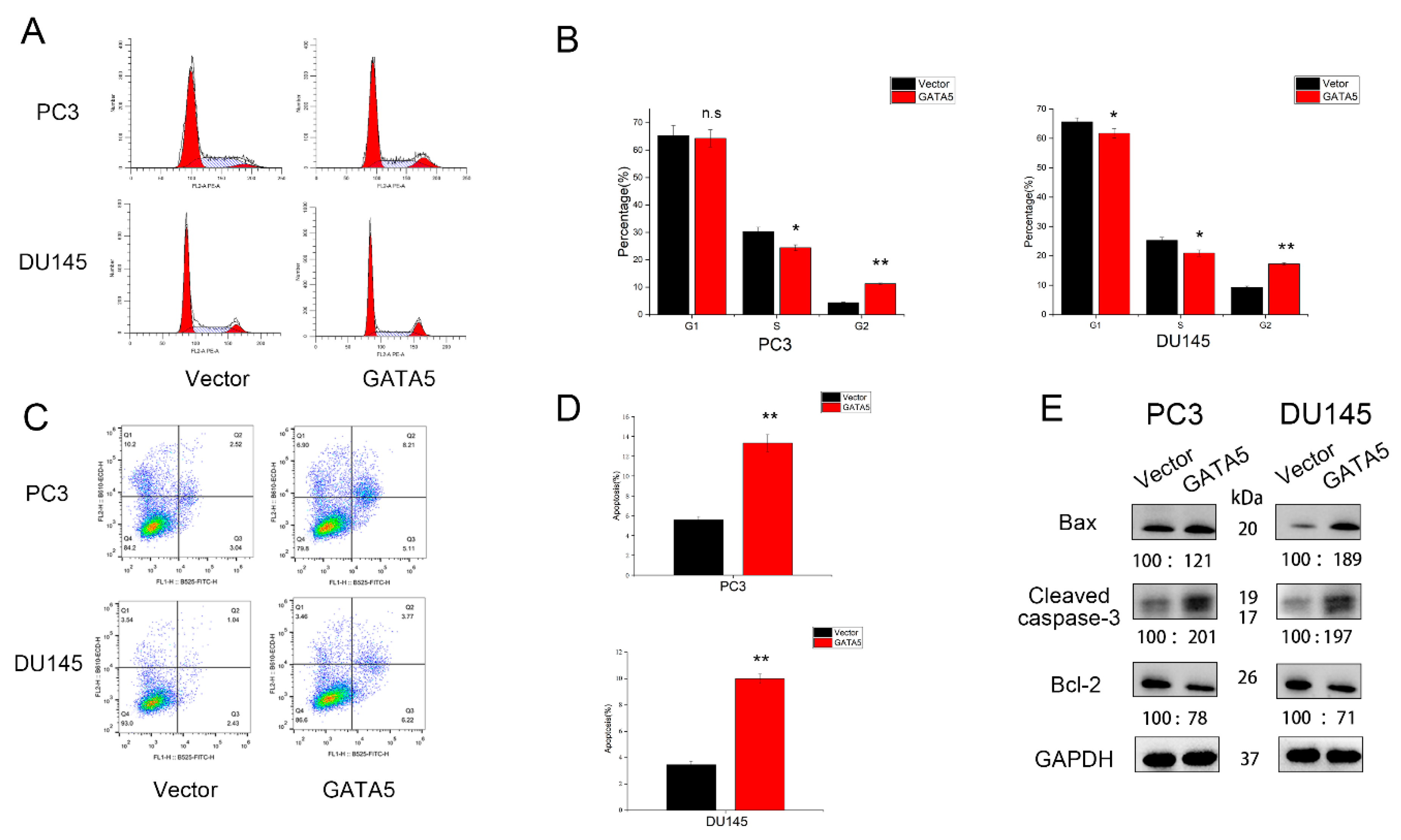
3.3. Effects of GATA5 on PCa Cell Cycle and Apoptosis
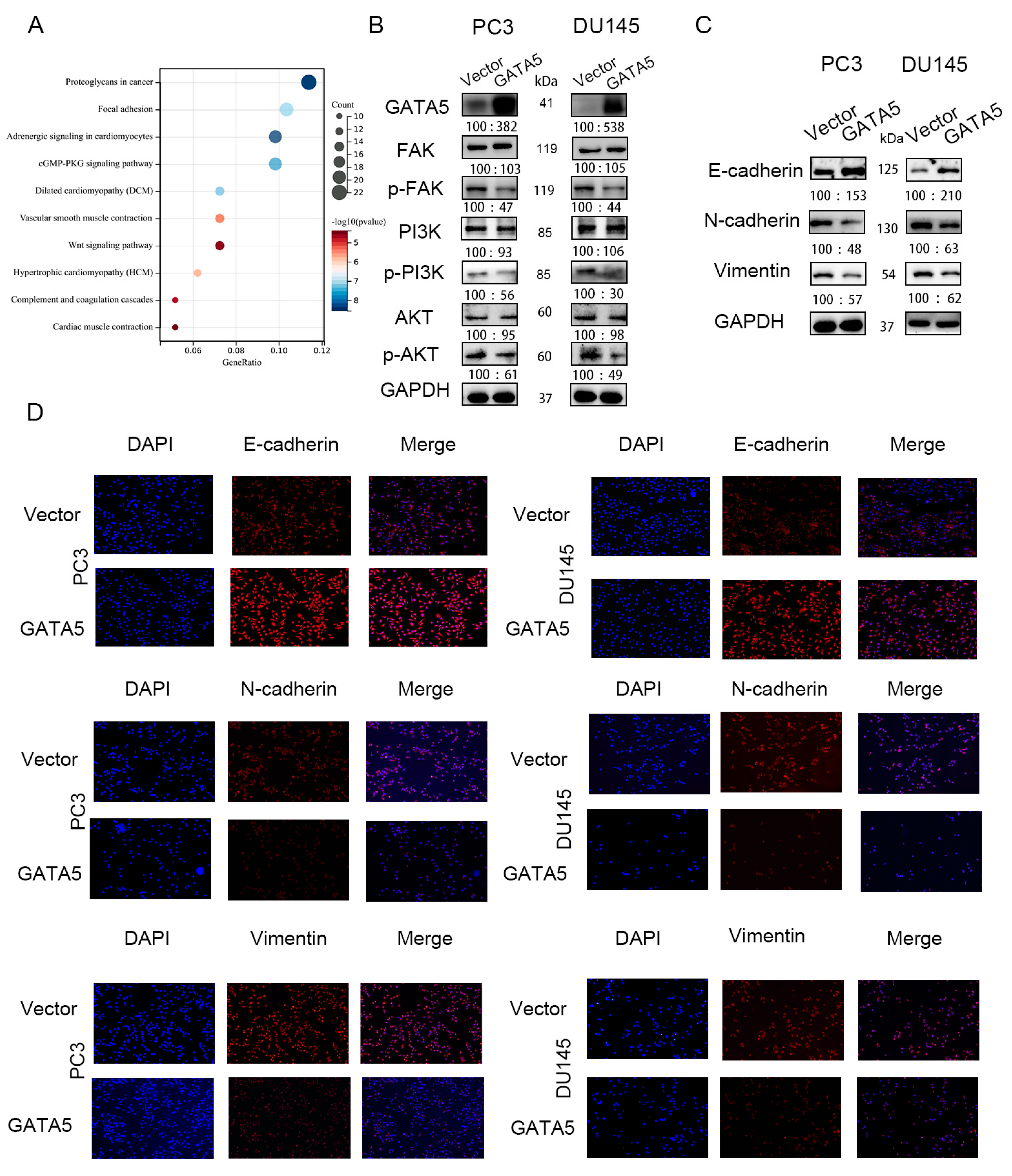
3.4. GATA5 Mediates EMT via the FAK/PI3K/AKT Pathway
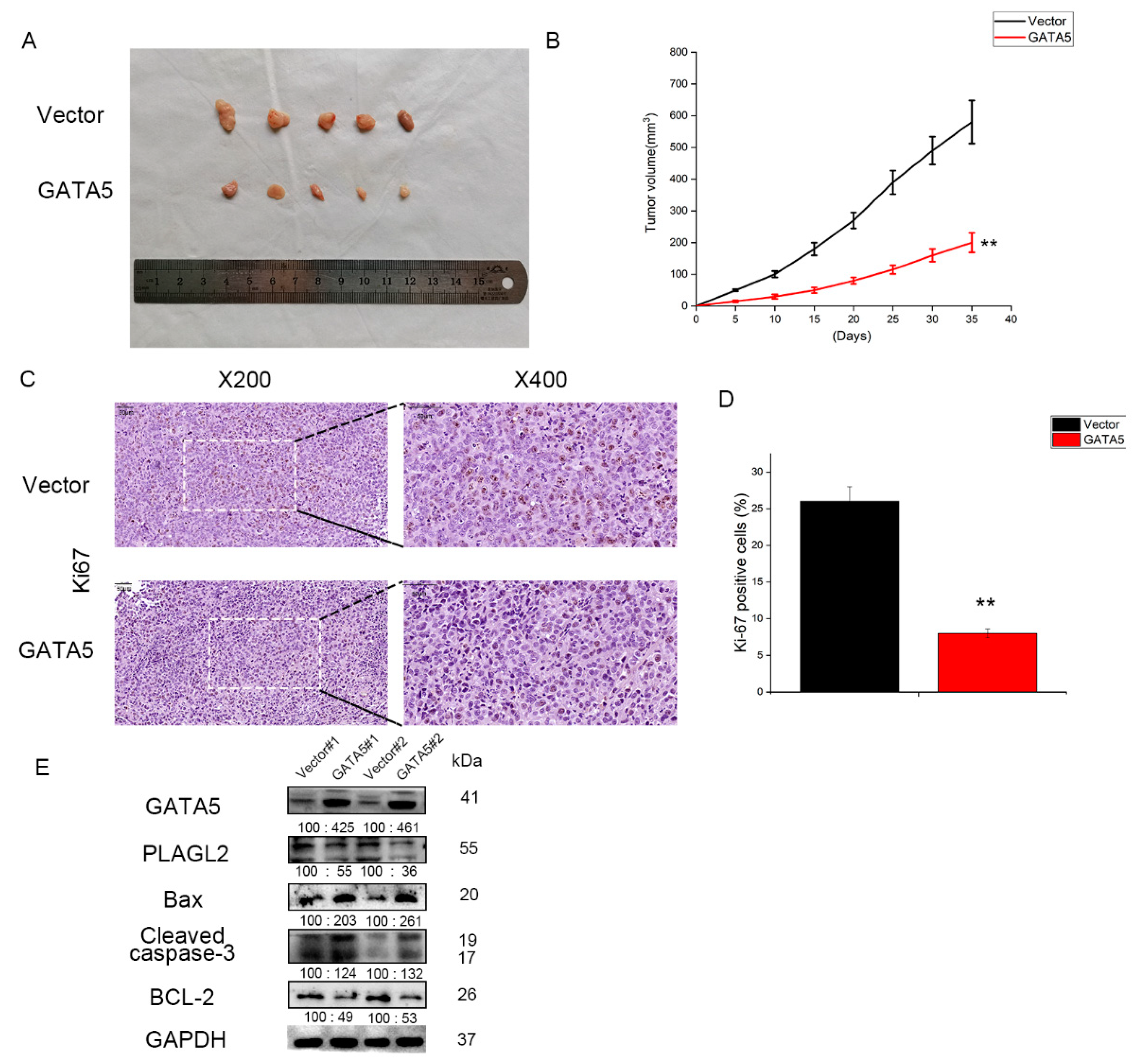
3.5. GATA5 Suppresses PCa Cell Growth In Vivo
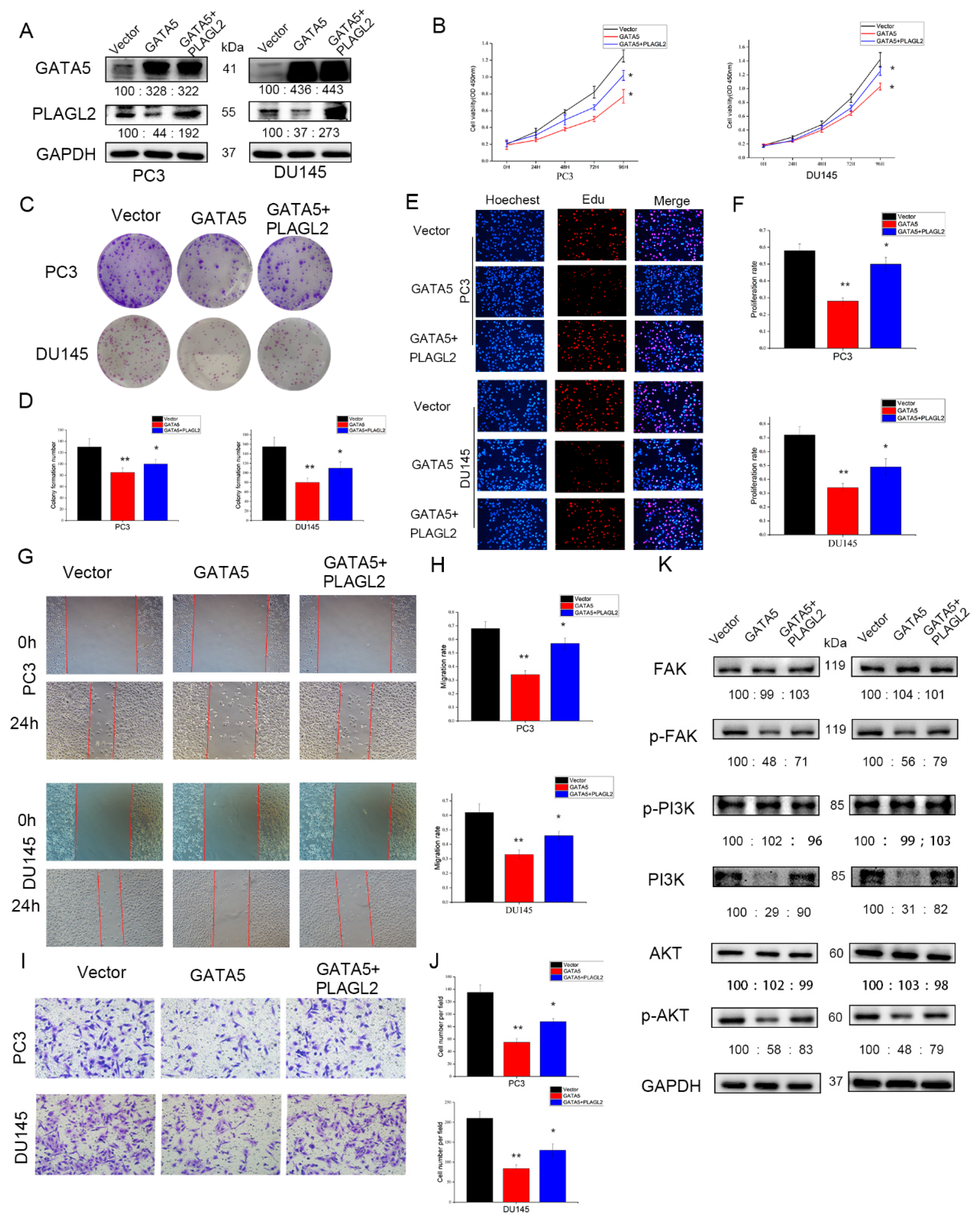
3.6. GATA5 Inhibits PCa Cell Growth by Regulating PLAGL2 In Vitro
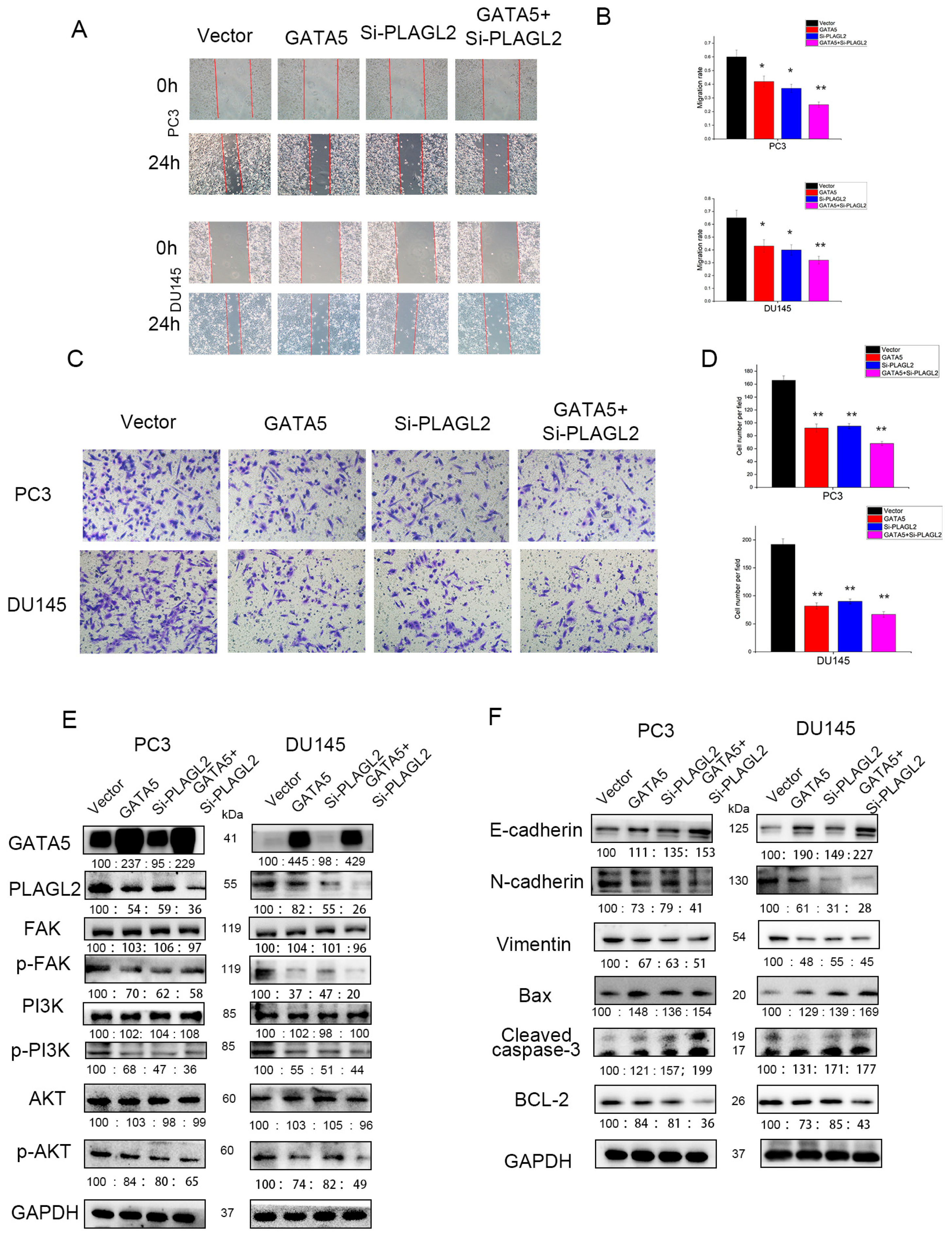
3.7. GATA5 Participates in PCa Progression through Regulating PLAGL2 via the FAK/PI3K/AKT Pathway
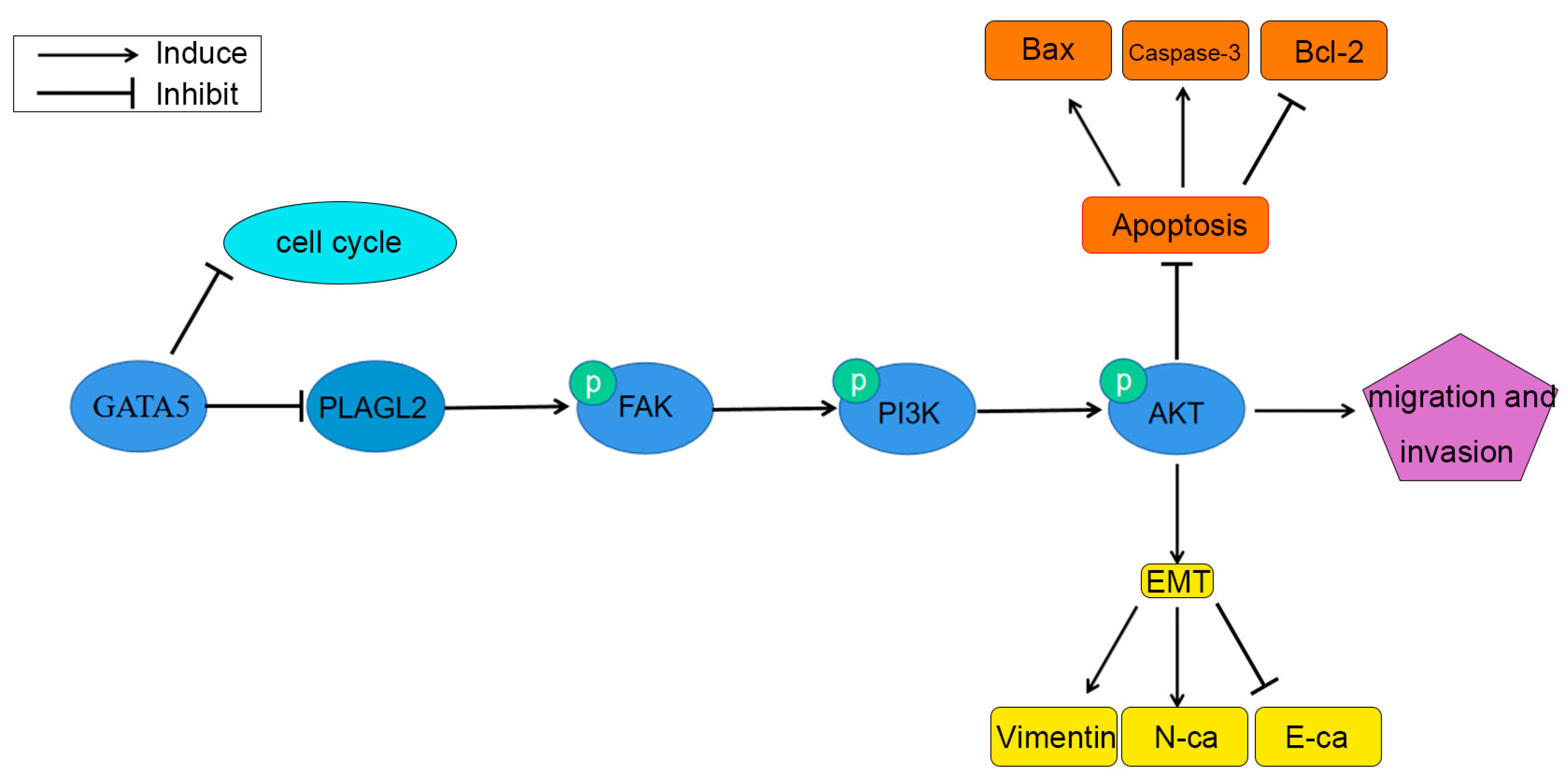
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, J.; Gu, W.; Qin, X.; Dai, B.; Lin, G.; Gan, H.; Freedland, S.J.; Zhu, Y.; Ye, D. Germline DNA repair gene mutation landscape in chinese prostate cancer patients. Eur. Urol. 2019, 76, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Simmons, L.H. Prevention of prostate cancer morbidity and mortality: Primary prevention and early detection. Med. Clin. N. Am. 2017, 101, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Gamat, M.; McNeel, D.G. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr.-Relat. Cancer 2017, 24, T297–T310. [Google Scholar] [CrossRef]
- Ku, S.Y.; Gleave, M.E.; Beltran, H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 2019, 16, 645–654. [Google Scholar] [CrossRef]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of advanced prostate cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef]
- Simon, M.C. Gotta have GATA. Nat. Genet. 1995, 11, 9–11. [Google Scholar] [CrossRef]
- Molkentin, J.D. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 2000, 275, 38949–38952. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T. GATA transcription factors: Basic principles and related human disorders. Tohoku J. Exp. Med. 2017, 242, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Surendran, P.; Feofanova, E.V.; Lahrouchi, N.; Ntalla, I.; Karthikeyan, S.; Cook, J.; Chen, L.; Mifsud, B.; Yao, C.; Kraja, A.T.; et al. Discovery of rare variants associated with blood pressure regulation through meta-analysis of 1.3 million individuals. Nat. Genet. 2020, 52, 1314–1332. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.P.; Yan, B. Potential roles of GATA binding protein 5 in cardiovascular diseases. Rev. Cardiovasc. Med. 2020, 21, 253–261. [Google Scholar] [PubMed]
- Song, Z.; Chen, L.; Pang, S.; Yan, B. Molecular genetic study on GATA5 gene promoter in acute myocardial infarction. PLoS ONE 2021, 16, e248203. [Google Scholar] [CrossRef] [PubMed]
- Sobota, R.S.; Kodaman, N.; Mera, R.; Piazuelo, M.B.; Bravo, L.E.; Pazos, A.; Zabaleta, J.; Delgado, A.G.; El-Rifai, W.; Morgan, D.R.; et al. Epigenetic and genetic variation in GATA5 is associated with gastric disease risk. Hum. Genet. 2016, 135, 895–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellebrekers, D.M.; Lentjes, M.H.; Van Den Bosch, S.M.; Melotte, V.; Wouters, K.A.; Daenen, K.L.; Smits, K.M.; Akiyama, Y.; Yuasa, Y.; Sanduleanu, S.; et al. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 3990–3997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Song, Y.F.; Lu, H.N.; Wang, D.P.; Zhang, X.S.; Huang, S.L.; Sun, B.L.; Huang, Z.G. Combined detection of plasma GATA5 and SFRP2 methylation is a valid noninvasive biomarker for colorectal cancer and adenomas. World J. Gastroenterol. 2015, 21, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Peters, I.; Dubrowinskaja, N.; Kogosov, M.; Abbas, M.; Hennenlotter, J.; von Klot, C.; Merseburger, A.S.; Stenzl, A.; Scherer, R.; Kuczyk, M.A.; et al. Decreased GATA5 mRNA expression associates with CpG island methylation and shortened recurrence-free survival in clear cell renal cell carcinoma. BMC Cancer 2014, 14, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, I.; Eggers, H.; Atschekzei, F.; Hennenlotter, J.; Waalkes, S.; Tränkenschuh, W.; Großhennig, A.; Merseburger, A.S.; Stenzl, A.; Kuczyk, M.A.; et al. GATA5 CpG island methylation in renal cell cancer: A potential biomarker for metastasis and disease progression. BJU Int. 2012, 110, E144–E152. [Google Scholar] [CrossRef]
- Mžik, M.; Chmelařová, M.; John, S.; Laco, J.; Slabý, O.; Kiss, I.; Bohovicová, L.; Palička, V.; Nekvindová, J. Aberrant methylation of tumour suppressor genes WT1, GATA5 and PAX5 in hepatocellular carcinoma. Clin. Chem. Lab. Med. 2016, 54, 1971–1980. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, M.; Zhang, R.; Wang, Q.; Li, W.; Dong, X.; Chen, Y.; Lu, Y.; Liu, K.; Lin, B.; et al. GATA5 inhibits hepatocellular carcinoma cells malignant behaviours by blocking expression of reprogramming genes. J. Cell. Mol. Med. 2019, 23, 2536–2548. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhao, N.; Zhou, Z.; Chen, J.; Han, S.; Zhang, X.; Bao, H.; Yuan, W.; Shu, X. PLAGL2 promotes the proliferation and migration of gastric cancer cells via USP37-mediated deubiquitination of Snail1. Theranostics 2021, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, Z.; Han, S.; Chen, J.; Liu, Z.; Zhang, X.; Yuan, W.; Ji, J.; Shu, X. PLAGL2 promotes epithelial-mesenchymal transition and mediates colorectal cancer metastasis via β-catenin-dependent regulation of ZEB1. Br. J. Cancer 2020, 122, 578–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Huo, J.; Xu, R.; Su, Q.; Tang, W.; Zhang, D.; Zhu, M.; Zhan, Y.; Dai, B.; Zhang, Y. Selenium sulfide disrupts the PLAGL2/C-MET/STAT3-induced resistance against mitochondrial apoptosis in hepatocellular carcinoma. Clin. Transl. Med. 2021, 11, e536. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, M.; Wang, Z.; Liu, X. Overexpression of pleomorphic adenoma Gene-Like 2 is a novel poor prognostic marker of prostate cancer. PLoS ONE 2017, 11, e158667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Canc. Res. 2019, 38, 250. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Liang, Y.; Jiang, L.; Wang, H.; Wang, S.; Dong, J. CX3CL1/fractalkine enhances prostate cancer spinal metastasis by activating the Src/FAK pathway. Int. J. Oncol. 2018, 53, 1544–1556. [Google Scholar] [CrossRef]
- Chen, F.; Wu, J.; Teng, J.; Li, W.; Zheng, J.; Bai, J. HCRP-1 regulates cell migration, invasion and angiogenesis via Src/FAK signaling in human prostate cancer. Int. J. Biol. Sci. 2020, 16, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Kishan, A.U.; Nickols, N.G. Treatment of the primary tumor in metastatic prostate cancer. World J. Urol. 2019, 37, 2597–2606. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, T.F.; Qiu, B.A.; Yang, Y.X.; Zhu, Y.J.; An, Y.; Zhao, W.C.; Wu, Y.T.; Ma, P.F.; Li, J.B.; et al. Methylation-Mediated silencing of GATA5 gene suppresses cholangiocarcinoma cell proliferation and metastasis. Transl. Oncol. 2018, 11, 585–592. [Google Scholar] [CrossRef]
- Loo, L.W.; Fong, A.Y.; Cheng, I.; Le Marchand, L. In silico functional pathway annotation of 86 established prostate cancer risk variants. PLoS ONE 2015, 10, e117873. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, H.; Zhang, L.; Liu, X.; Zhang, C.; Wang, Y.; He, Q.; Zhang, Y.; Li, Y.; Chen, Q.; et al. DJ-1 promotes colorectal cancer progression through activating PLAGL2/Wnt/BMP4 axis. Cell Death Dis. 2018, 9, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Li, D.; Du, Y.; Su, C.; Yang, C.; Lin, C.; Li, X.; Hu, G. Overexpressed PLAGL2 transcriptionally activates Wnt6 and promotes cancer development in colorectal cancer. Oncol. Rep. 2019, 41, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zheng, S.; Guo, H.; Dai, B.; Ni, J.; Shi, Y.; Bian, H.; Li, L.; Shen, Y.; Wu, M.; et al. PLAGL2-EGFR-HIF-1/2α signaling loop promotes HCC progression and Erlotinib insensitivity. Hepatology 2020, 73, 674–691. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Xu, Y.; Wan, S.P.; Yang, G. Expression of PLAGL2 in bladder urothelial carcinoma and its relationship to lymph node metastasis and survival. Sci. Rep. 2018, 8, 6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brullo, C.; Tasso, B. New Insights on Fak and Fak Inhibitors. Curr. Med. Chem. 2021, 28, 3318–3338. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [Green Version]
- Mittal, V. Epithelial mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal transition (EMT) and prostate cancer. Adv. Exp. Med. Biol. 2018, 1095, 101–110. [Google Scholar]
- Sroka, I.C.; Chopra, H.; Das, L.; Gard, J.M.; Nagle, R.B.; Cress, A.E. Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding a6 integrin. J. Cell. Biochem. 2016, 117, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Yao, J.F.; Deng, X.F.; Zheng, X.D.; Jia, M.; Wang, Y.Q.; Huang, Y.; Zhu, J.H. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin αvβ3 and activating FAK/PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 2018, 37, 23. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Liu, S.Q.; Qin, M.B.; Zhuge, C.F.; Qin, L.; Qin, N.; Lai, M.Y.; Huang, J.A. SphK1 modulates cell migration and EMT-related marker expression by regulating the expression of p-FAK in colorectal cancer cells. Int. J. Mol. Med. 2017, 39, 1277–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Dilution Rate | ||||||
|---|---|---|---|---|---|---|
| Antibody | Specificity | WB | IF | IHC | IP | Company |
| GATA5 | Rabbit | 1:500 | - | - | 1:200 | Proteintech |
| PLAGL2 | Rabbit | 1:500 | - | - | 1:100 | Proteintech |
| E-cadherin | Rabbit | 1:1000 | 1:100 | - | - | Proteintech |
| N-cadherin | Rabbit | 1:1000 | 1:100 | - | - | Proteintech |
| Vimentin | Rabbit | 1:1000 | 1:100 | - | - | Proteintech |
| FAK | Rabbit | 1:1000 | - | - | - | ABclonal |
| p-FAK | Rabbit | 1:1000 | - | - | - | ABclonal |
| PI3K | Rabbit | 1:1000 | - | - | - | ABclonal |
| p-PI3K | Rabbit | 1:1000 | - | - | - | ABclonal |
| AKT | Mouse | 1:1000 | - | - | - | ABclonal |
| p-AKT | Mouse | 1:1000 | - | - | - | ABclonal |
| Bax | Rabbit | 1:1000 | - | - | - | CST |
| Bcl-2 | Mouse | 1:1000 | - | - | - | CST |
| Cleaved caspase-3 | Rabbit | 1:1000 | - | - | - | CST |
| Ki67 | Rabbit | - | - | 1:200 | - | Abcam |
| GAPDH | Rabbit | 1:1000 | - | - | - | Servicebio |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, Z.; Zhai, G.; Yu, X.; Ke, S.; Shao, H.; Guo, J. Overexpression of GATA5 Inhibits Prostate Cancer Progression by Regulating PLAGL2 via the FAK/PI3K/AKT Pathway. Cancers 2022, 14, 2074. https://doi.org/10.3390/cancers14092074
Wang Q, Liu Z, Zhai G, Yu X, Ke S, Shao H, Guo J. Overexpression of GATA5 Inhibits Prostate Cancer Progression by Regulating PLAGL2 via the FAK/PI3K/AKT Pathway. Cancers. 2022; 14(9):2074. https://doi.org/10.3390/cancers14092074
Chicago/Turabian StyleWang, Qinghua, Zelin Liu, Guanzhong Zhai, Xi Yu, Shuai Ke, Haoren Shao, and Jia Guo. 2022. "Overexpression of GATA5 Inhibits Prostate Cancer Progression by Regulating PLAGL2 via the FAK/PI3K/AKT Pathway" Cancers 14, no. 9: 2074. https://doi.org/10.3390/cancers14092074
APA StyleWang, Q., Liu, Z., Zhai, G., Yu, X., Ke, S., Shao, H., & Guo, J. (2022). Overexpression of GATA5 Inhibits Prostate Cancer Progression by Regulating PLAGL2 via the FAK/PI3K/AKT Pathway. Cancers, 14(9), 2074. https://doi.org/10.3390/cancers14092074





