Immune-Based Therapy in Triple-Negative Breast Cancer: From Molecular Biology to Clinical Practice
Abstract
Simple Summary
Abstract
1. Introduction
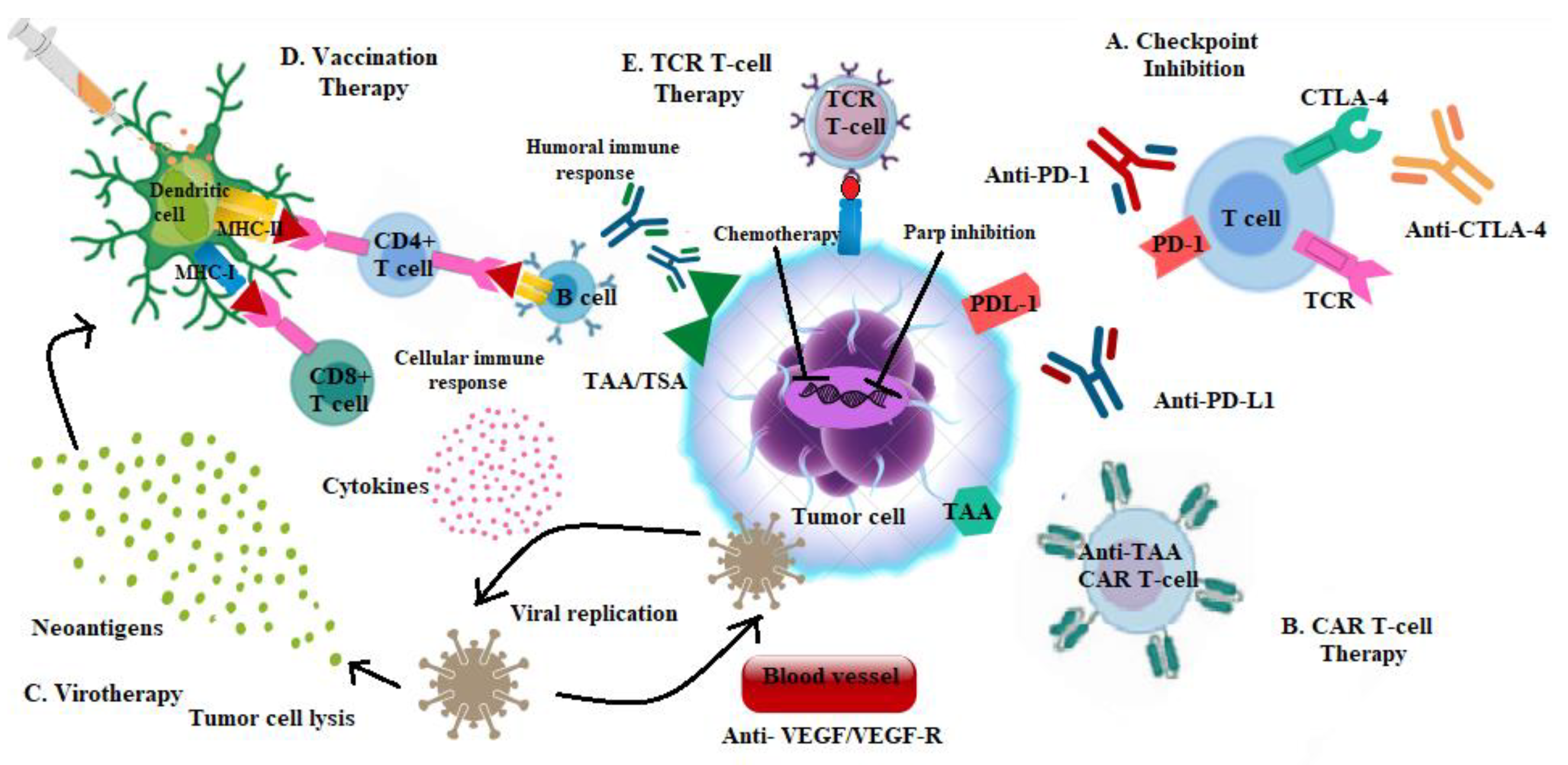
Rationale of Immune-Based Therapy in Breast Cancer
2. Breast Cancer “Immunogram”
Predictors of Response to Immune-Based Therapy
3. Anti PD-1 Antibodies in Metastatic TNBC: Available Results from Clinical Trials
3.1. Pembrolizumab
3.2. Nivolumab
4. Anti PD-L1 Antibodies in Metastatic TNBC: Available Results from Clinical Trials
4.1. Atezolizumab
4.2. Avelumab
4.3. Durvalumab
5. Anti PD-1 Antibodies in Early TNBC: Available Results from Clinical Trials
Pembrolizumab
6. Anti PD-L1 Antibodies in Early TNBC: Available Results from Clinical Trials
6.1. Atezolizumab
6.2. Durvalumab
7. Strategies to Overcome Immune Resistance
8. Combining Immunotherapy and Targeted Therapy
8.1. ICIs and PARP Inhibitors
8.2. ICIs and Small Molecules
8.3. ICIs and Novel Immune-Modulators
8.4. ICIs and Epigenetic Agents
9. Adoptive Cell Therapy (ACT)
10. Vaccines and Oncolytic Virotherapy
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henriques, B.; Mendes, F.; Martins, D. Immunotherapy in Breast Cancer: When, How, and What Challenges? Biomedicines 2021, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.; Livingstone, E.; Roesch, A.; Schilling, B.; Schadendorf, D. Immunotherapy in melanoma: Recent advances and future directions. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Davies, M. New modalities of cancer treatment for NSCLC: Focus on immunotherapy. Cancer Manag. Res. 2014, 6, 63–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, L.; Dong, Y.; Pan, Y.; Zhang, Y.; Liu, P.; Wang, J.; Chen, C.; Lu, J.; Yu, Y.; Deng, R. Identification and development of an independent immune-related genes prognostic model for breast cancer. BMC Cancer 2021, 21, 329. [Google Scholar] [CrossRef] [PubMed]
- Burugu, S.; Asleh-Aburaya, K.; Nielsen, T.O. Immune infiltrates in the breast cancer microenvironment: Detection, characterization and clinical implication. Breast Cancer 2017, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Bianchini, G.; Qi, Y.; Alvarez, R.H.; Iwamoto, T.; Coutant, C.; Ibrahim, N.K.; Valero, V.; Cristofanilli, M.; Green, M.C.; Radvanyi, L.; et al. Molecular Anatomy of Breast Cancer Stroma and Its Prognostic Value in Estrogen Receptor–Positive and –Negative Cancers. J. Clin. Oncol. 2010, 28, 4316–4323. [Google Scholar] [CrossRef]
- Karn, T.; Pusztai, L.; Holtrich, U.; Iwamoto, T.; Shiang, C.Y.; Schmidt, M.; Müller, V.; Solbach, C.; Gaetje, R.; Hanker, L.; et al. Homogeneous Datasets of Triple Negative Breast Cancers Enable the Identification of Novel Prognostic and Predictive Signatures. PLoS ONE 2011, 6, e28403. [Google Scholar] [CrossRef]
- Lee, H.J.; Song, I.H.; Park, I.A.; Heo, S.-H.; Kim, Y.-A.; Ahn, J.-H.; Gong, G. Differential expression of major histocompatibility complex class I in subtypes of breast cancer is associated with estrogen receptor and interferon signaling. Oncotarget 2016, 7, 30119–30132. [Google Scholar] [CrossRef]
- Chester, C.; Sanmamed, M.F.; Wang, J.; Melero, I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood 2018, 131, 49–57. [Google Scholar] [CrossRef]
- Harding, J.J.; Patnaik, A.; Moreno, V.; Stein, M.; Jankowska, A.M.; de Mendizabal, N.V.; Liu, Z.T.; Koneru, M.; Calvo, E. A phase Ia/Ib study of an anti-TIM-3 antibody (LY3321367) monotherapy or in combination with an anti-PD-L1 antibody (LY3300054): Interim safety, efficacy, and pharmacokinetic findings in advanced cancers. J. Clin. Oncol. 2019, 37 (Suppl. S8), 12. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Masuda, J.; Ozaki, Y.; Hara, F.; Kitano, S.; Takano, T. Pembrolizumab plus chemotherapy in triple-negative breast cancer. Lancet 2021, 398, 24. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Bernal, M.G.C.; Cervantes-Badillo, M.G.; Martínez-Herrera, J.F.; Lara-Torres, C.O.; Gerson-Cwilich, R.; Zentella-Dehesa, A.; Ibarra-Sánchez, M.D.J.; Esparza-López, J.; Montesinos, J.J.; Cortés-Morales, V.A.; et al. Biological Landscape of Triple Negative Breast Cancers Expressing CTLA-4. Front. Oncol. 2020, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- Kassardjian, A.; Shintaku, P.I.; Moatamed, N.A. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), in female breast carcinomas. PLoS ONE 2018, 13, e0195958. [Google Scholar] [CrossRef]
- Lan, G.; Li, J.; Wen, Q.; Lin, L.; Chen, L.; Chen, L.; Chen, X. Cytotoxic T lymphocyte associated antigen 4 expression predicts poor prognosis in luminal B HER2-negative breast cancer. Oncol. Lett. 2018, 15, 5093–5097. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.H.S.; McArthur, H. Breast Cancer Immunotherapy: From Biology to Current Clinical Applications. Eur. Med. J. 2020, 5, 113–124. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Schütz, F.; Stefanovic, S.; Mayer, L.; Von Au, A.; Domschke, C.; Sohn, C. PD-1/PD-L1 Pathway in Breast Cancer. Oncol. Res. Treat. 2017, 40, 294–297. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Sabatier, R.; Finetti, P.; Mamessier, E.; Adelaide, J.; Chaffanet, M.; Ali, H.R.; Viens, P.; Caldas, C.; Birnbaum, D.; Bertucci, F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2014, 6, 5449–5464. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Kim, J.W.; Kim, M.K.; Chung, B.W.; Ahn, S.K. Programmed cell death-ligand 1 expression in stromal immune cells is a marker of breast cancer outcome. J. Cancer 2020, 11, 7246–7252. [Google Scholar] [CrossRef] [PubMed]
- Fountzila, E.; Ignatiadis, M. Neoadjuvant immunotherapy in breast cancer: A paradigm shift? Ecancermedicalscience 2020, 14, 1147. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Lee, J.S.; Ruppin, E. Multiomics Prediction of Response Rates to Therapies to Inhibit Programmed Cell Death 1 and Programmed Cell Death 1 Ligand 1. JAMA Oncol. 2019, 5, 1614–1618. [Google Scholar] [CrossRef]
- Miglietta, F.; Griguolo, G.; Guarneri, V.; Dieci, M.V. Programmed Cell Death Ligand 1 in Breast Cancer: Technical Aspects, Prognostic Implications, and Predictive Value. Oncologist 2019, 24, e1055–e1069. [Google Scholar] [CrossRef]
- Szekely, B.; Bossuyt, V.; Li, X.; Wali, V.; Patwardhan, G.; Frederick, C.; Silber, A.; Park, T.; Harigopal, M.; Pelekanou, V.; et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 2018, 29, 2232–2239. [Google Scholar] [CrossRef]
- Cimino-Mathews, A.; Ye, X.; Meeker, A.; Argani, P.; Emens, L.A. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: A pilot study. Hum. Pathol. 2013, 44, 2055–2063. [Google Scholar] [CrossRef]
- Cimino-Mathews, A.; Thompson, E.; Taube, J.M.; Ye, X.; Lu, Y.; Meeker, A.; Xu, H.; Sharma, R.; Lecksell, K.; Cornish, T.; et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 2015, 47, 52–63. [Google Scholar] [CrossRef]
- Ogiya, R.; Niikura, N.; Kumaki, N.; Bianchini, G.; Kitano, S.; Iwamoto, T.; Hayashi, N.; Yokoyama, K.; Oshitanai, R.; Terao, M.; et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016, 107, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Loi, S.; Adams, S.; Schmid, P.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Winer, E.P.; Kockx, M.M.; et al. PD-L1 Immunohistochemistry Assay Comparison in Atezolizumab Plus nab-Paclitaxel–Treated Advanced Triple-Negative Breast Cancer. JNCI J. Natl. Cancer Inst. 2021, 113, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Noske, A.; Wagner, D.-C.; Schwamborn, K.; Foersch, S.; Steiger, K.; Kiechle, M.; Oettler, D.; Karapetyan, S.; Hapfelmeier, A.; Roth, W.; et al. Interassay and interobserver comparability study of four programmed death-ligand 1 (PD-L1) immunohistochemistry assays in triple-negative breast cancer. Breast 2021, 60, 238–244. [Google Scholar] [CrossRef]
- Ghebeh, H.; Mansour, F.A.; Colak, D.; Alfuraydi, A.A.; Al-Thubiti, A.A.; Monies, D.; Al-Alwan, M.; Al-Tweigeri, T.; Tulbah, A. Higher PD-L1 Immunohistochemical Detection Signal in Frozen Compared to Matched Paraffin-Embedded Formalin-Fixed Tissues. Antibodies 2021, 10, 24. [Google Scholar] [CrossRef]
- Dieci, M.V.; Tsvetkova, V.; Orvieto, E.; Piacentini, F.; Ficarra, G.; Griguolo, G.; Miglietta, F.; Giarratano, T.; Omarini, C.; Bonaguro, S.; et al. Immune characterization of breast cancer metastases: Prognostic implications. Breast Cancer Res. 2018, 20, 62. [Google Scholar] [CrossRef]
- Li, Y.; Vennapusa, B.; Chang, C.-W.; Tran, D.; Nakamura, R.; Sumiyoshi, T.; Hegde, P.; Molinero, L. Prevalence Study of PD-L1 SP142 Assay in Metastatic Triple-negative Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2020, 29, 258–264. [Google Scholar] [CrossRef]
- Rozenblit, M.; Huang, R.; Danziger, N.; Hegde, P.; Alexander, B.; Ramkissoon, S.; Blenman, K.; Ross, J.S.; Rimm, D.L.; Pusztai, L. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J. Immunother. Cancer 2020, 8, e001558. [Google Scholar] [CrossRef]
- Bianchini, G.; Smart, C.; Mansutti, M.; Lück, H.-J.; Zambelli, S.; Olier, C.; Anton, A.; Bisagni, G.; Merlini, L.; Murillo, S.M.; et al. Modulation by treatment of tumor infiltrating lymphocytes (TILs) and PDL1 expression in triple-negative breast cancer in the ETNA trial. J. Clin. Oncol. 2020, 38, 555. [Google Scholar] [CrossRef]
- Grandal, B.; Mangiardi-Veltin, M.; Laas, E.; Laé, M.; Meseure, D.; Bataillon, G.; El-Alam, E.; Darrigues, L.; Dumas, E.; Daoud, E.; et al. PD-L1 Expression after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancers Is Associated with Aggressive Residual Disease, Suggesting a Potential for Immunotherapy. Cancers 2021, 13, 746. [Google Scholar] [CrossRef]
- Parkes, E.E.; Walker, S.M.; Taggart, L.E.; McCabe, N.; Knight, L.A.; Wilkinson, R.; McCloskey, K.D.; Buckley, N.E.; Savage, K.I.; Salto-Tellez, M.; et al. Activation of STING-Dependent Innate Immune Signaling By S-Phase-Specific DNA Damage in Breast Cancer. JNCI J. Natl. Cancer Inst. 2016, 109, djw199. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; De Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.; Finotello, F.; Rieder, D.; Trajanoski, Z. Neoantigens Generated by Individual Mutations and Their Role in Cancer Immunity and Immunotherapy. Front. Immunol. 2017, 8, 1679. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Shi, L.Z.; Fu, T.; Guan, B.; Chen, J.; Blando, J.M.; Allison, J.; Xiong, L.; Subudhi, S.K.; Gao, J.; Sharma, P. Interdependent IL-7 and IFN-γ signalling in T-cell controls tumour eradication by combined α-CTLA-4+α-PD-1 therapy. Nat. Commun. 2016, 7, 12335. [Google Scholar] [CrossRef]
- Kitano, A.; Ono, M.; Yoshida, M.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017, 2, e000150. [Google Scholar] [CrossRef]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. npj Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef]
- Thomas, A.; Routh, E.; Pullikuth, A.; Jin, G.; Su, J.; Chou, J.W.; Hoadley, K.; Print, C.; Knowlton, N.; Black, M.A.; et al. Tumor mutational burden is a determinant of immune-mediated survival in breast cancer. OncoImmunology 2018, 7, e1490854. [Google Scholar] [CrossRef]
- Criscitiello, C.; Guerini-Rocco, E.; Viale, G.; Fumagalli, C.; Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Malapelle, U.; Fusco, N. Immunotherapy in Breast Cancer Patients: A Focus on the Use of the Currently Available Biomarkers in Oncology. Anti-Cancer Agents Med. Chem. 2021, 21, 6144112. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Jain, E.; Cohen, O.; Kim, D.; Buendia-Buendia, J.; Winer, E.; Lin, N.; Tolaney, S.; Wagle, N. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann. Oncol. 2020, 31, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Alva, A.S.; Mangat, P.K.; Garrett-Mayer, E.; Halabi, S.; Hansra, D.; Calfa, C.J.; Khalil, M.F.; Ahn, E.R.; Cannon, T.L.; Crilley, P.; et al. Pembrolizumab in Patients With Metastatic Breast Cancer With High Tumor Mutational Burden: Results From the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J. Clin. Oncol. 2021, 39, 2443–2451. [Google Scholar] [CrossRef]
- O’Meara, T.A.; Tolaney, S.M. Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget 2021, 12, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Sinn, B.V.; Karn, T.; Untch, M.; Sinn, H.-P.; Weber, K.E.; Hanusch, C.; Huober, J.B.; Staib, P.; Lorenz, R.; et al. Exome analysis of oncogenic pathways and tumor mutational burden (TMB) in triple-negative breast cancer (TNBC): Results of the translational biomarker program of the neoadjuvant double-blind placebo controlled GeparNuevo trial. J. Clin. Oncol. 2019, 37, 509. [Google Scholar] [CrossRef]
- Poulogiannis, G.; Frayling, I.M.; Arends, M.J. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology 2009, 56, 167–179. [Google Scholar] [CrossRef] [PubMed]
- VanderWalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef]
- Venetis, K.; Sajjadi, E.; Haricharan, S.; Fusco, N. Mismatch repair testing in breast cancer: The path to tumor-specific immuno-oncology biomarkers. Transl. Cancer Res. 2020, 9, 4060–4064. [Google Scholar] [CrossRef]
- Davies, H.; Morganella, S.; Purdie, C.A.; Jang, S.J.; Borgen, E.; Russnes, H.; Glodzik, D.; Zou, X.; Viari, A.; Richardson, A.L.; et al. Whole-Genome Sequencing Reveals Breast Cancers with Mismatch Repair Deficiency. Cancer Res. 2017, 77, 4755–4762. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Fremd, C.; Hlevnjak, M.; Zapatka, M.; Zoernig, I.; Halama, N.; Fejzibegovic, N.; Thewes, V.; Lichter, P.; Schirmacher, P.; Kloor, M.; et al. Mismatch Repair Deficiency Drives Durable Complete Remission by Targeting Programmed Death Receptor 1 in a Metastatic Luminal Breast Cancer Patient. Breast Care 2018, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M. Evaluation of BRCA1 and BRCA2 as Indicators of Response to Immune Checkpoint Inhibitors. JAMA Netw. Open 2021, 4, e217728. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.; Molinero, L.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Diéras, V.; Iwata, H.; Barrios, C.H.; Nechaeva, M.; Duc, A.N.; et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. J. Natl. Cancer Inst. 2021, 113, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Filleron, T.; Bieche, I.; Arnedos, M.; Campone, M.; Dalenc, F.; Coussy, F.; Sablin, M.-P.; Debled, M.; Lefeuvre-Plesse, C.; et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat. Med. 2021, 27, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab vs. investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The Interplay of Immunotherapy and Chemotherapy: Harnessing Potential Synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef]
- Shah, N.; Aiello, J.; Avigan, E.D.; Berdeja, J.G.; Borrello, I.M.; Chari, A.; Cohen, A.D.; Ganapathi, K.; Gray, L.; Green, D.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of multiple myeloma. J. Immunother. Cancer 2020, 8, e000734. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Kalinsky, K.; Kaklamani, V.G.; D’Adamo, D.R.; Aktan, G.; Tsai, M.L.; O’Regan, R.M.; Kaufman, P.A.; Wilks, S.T.; Andreopoulou, E.; et al. Eribulin Plus Pembrolizumab in Patients with Metastatic Triple-Negative Breast Cancer (ENHANCE 1): A Phase Ib/II Study. Clin. Cancer Res. 2021, 27, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy vs. placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; et al. Abstract GS1-02: Final results of KEYNOTE-355: Randomized, double-blind, phase 3 study of pembrolizumab + chemotherapy vs. placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. Cancer Res. 2022, 82, GS1-02. [Google Scholar] [CrossRef]
- Ozaki, Y.; Matsumoto, K.; Takahashi, M.; Mukohara, T.; Futamura, M.; Masuda, N.; Tsurutani, J.; Yoshimura, K.; Minami, H.; Takano, T. Phase II study of a combination therapy of nivolumab, bevacizumab and paclitaxel in patients with HER2-negative metastatic breast cancer as a first-line treatment (WJOG9917B, NEWBEAT trial). J. Clin. Oncol. 2018, 36, TPS1110. [Google Scholar] [CrossRef]
- Ozaki, Y.; Kitano, S.; Tsurutani, J.; Iwasa, T.; Takahashi, M.; Mukohara, T.; Masuda, N.; Futamura, M.; Minami, H.; Matsumoto, K.; et al. Abstract PS4-14: Immunological analysis of the combination therapy of nivolumab, paclitaxel and bevacizumab in patients with HER2-negative MBC in NEWBEAT trial (WJOG9917BTR). Cancer Res. 2021, 81, PS4-14. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; Van De Vijver, K.K.; De Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Mavratzas, A.; Seitz, J.; Smetanay, K.; Schneeweiss, A.; Jäger, D.; Fremd, C. Atezolizumab for use in PD-L1-positive unresectable, locally advanced or metastatic triple-negative breast cancer. Futur. Oncol. 2020, 16, 4439–4453. [Google Scholar] [CrossRef]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.-P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients with Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Adams, S.; Diamond, J.R.; Hamilton, E.; Pohlmann, P.R.; Tolaney, S.M.; Chang, C.-W.; Zhang, W.; Iizuka, K.; Foster, P.G.; Molinero, L.; et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer with 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol. 2019, 5, 334–342. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Diéras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 2021, 32, 983–993. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Juliá, E.P.; Amante, A.; Pampena, M.B.; Mordoh, J.; Levy, E.M. Avelumab, an IgG1 anti-PD-L1 Immune Checkpoint Inhibitor, Triggers NK Cell-Mediated Cytotoxicity and Cytokine Production against Triple Negative Breast Cancer Cells. Front. Immunol. 2018, 9, 2140. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Morrow, M.; Hammond, S.A.; Mulgrew, K.; Marcus, D.; Poon, E.; Watkins, A.; Mullins, S.; Chodorge, M.; Andrews, J.; et al. Identification and Characterization of MEDI4736, an Antagonistic Anti–PD-L1 Monoclonal Antibody. Cancer Immunol. Res. 2015, 3, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Al-Sayed, A.; Eiada, R.; Cabangon, L.; Ajarim, D.; Suleman, K.; Tulbah, A.; Al-Tweigeri, T. Weekly Paclitaxel given concurrently with Durvalumab has a favorable safety profile in triple-negative metastatic breast cancer. Sci. Rep. 2021, 11, 19154. [Google Scholar] [CrossRef]
- Schmid, P.; Im, S.A.; Armstrong, A.; Park, Y.H.; Chung, W.P.; Nowecki, Z.; Lord, S.; Wysocki, P.J.; Lu, Y.S.; Dry, H.; et al. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J. Clin. Oncol. 2021, 39 (Suppl. S15), 1023. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women with Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Schmid, P.; Salgado, R.; Park, Y.; Muñoz-Couselo, E.; Kim, S.; Sohn, J.; Im, S.-A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Abstract GS1-01: KEYNOTE-522 study of neoadjuvant pembrolizumab + chemotherapy vs. placebo + chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC: Event-free survival sensitivity and subgroup analyses. Cancer Res. 2022, 82, GS1-01. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy vs. placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Gianni, L.; Huang, C.-S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Abstract GS3-04: Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Cancer Res. 2020, 80, GS3-04. [Google Scholar] [CrossRef]
- Bianchini, G.; Huang, C.-S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Russo, S.; Ciruelos, E.; et al. LBA13 Tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Ann. Oncol. 2020, 31, S1145–S1146. [Google Scholar] [CrossRef]
- Bailly, C.; Thuru, X.; Quesnel, B. Combined cytotoxic chemotherapy and immunotherapy of cancer: Modern times. NAR Cancer 2020, 2, zcaa002. [Google Scholar] [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-U.; Grischke, E.-M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef]
- Karn, T.; Denkert, C.; Weber, K.; Holtrich, U.; Hanusch, C.; Sinn, B.; Higgs, B.; Jank, P.; Sinn, H.; Huober, J.; et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann. Oncol. 2020, 31, 1216–1222. [Google Scholar] [CrossRef]
- Loibl, S.; Schneeweiss, A.; Huober, J.B.; Braun, M.; Rey, J.; Blohmer, J.U.; Furlanetto, J.; Zahm, D.M.; Hanusch, C.; Thomalla, J.; et al. Durvalumab improves long-term outcome in TNBC: Results from the phase II randomized GeparNUEVO study investigating neodjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). J. Clin. Oncol. 2021, 39, 506. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Rieth, J.; Subramanian, S. Mechanisms of Intrinsic Tumor Resistance to Immunotherapy. Int. J. Mol. Sci. 2018, 19, 1340. [Google Scholar] [CrossRef]
- Yoshida, G.J. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 2015, 34, 111. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; Meylan, M.; De Reyniès, A.; Sautes-Fridman, C.; Fridman, W.H. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Front. Immunol. 2020, 11, 784. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.; Saddawi-Konefka, R.; Vermi, W.; Koebel, C.M.; Arthur, C.; White, J.M.; Uppaluri, R.; Andrews, D.; Ngiow, S.F.; Teng, M.; et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J. Exp. Med. 2012, 209, 1869–1882. [Google Scholar] [CrossRef]
- Segal, N.H.; Parsons, D.W.; Peggs, K.S.; Velculescu, V.; Kinzler, K.W.; Vogelstein, B.; Allison, J.P. Epitope Landscape in Breast and Colorectal Cancer. Cancer Res. 2008, 68, 889–892. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, K.; Xiao, Y.; Feng, B.; Mikule, K.; Ma, X.; Feng, N.; Vellano, C.P.; Federico, L.; Marszalek, J.R.; et al. Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models. Sci. Rep. 2019, 9, 1853. [Google Scholar] [CrossRef]
- Pantelidou, C.; Sonzogni, O.; De Oliveria Taveira, M.; Mehta, A.K.; Kothari, A.; Wang, D.; Visal, T.; Li, M.K.; Pinto, J.; Castrillon, J.A.; et al. PARP Inhibitor Efficacy Depends on CD8+ T-cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 722–737. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, W.; Ju, Z.; Wang, L.; Peng, Y.; Labrie, M.; Yap, T.A.; Mills, G.B.; Peng, G. PARPi Triggers the STING-Dependent Immune Response and Enhances the Therapeutic Efficacy of Immune Checkpoint Blockade Independent of BRCAness. Cancer Res. 2018, 79, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Postel-Vinay, S.; Im, S.-A.; Park, Y.H.; Delord, J.-P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G.; et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef]
- Vinayak, S.; Tolaney, S.M.; Schwartzberg, L.; Mita, M.; McCann, G.; Tan, A.R.; Wahner-Hendrickson, A.E.; Forero, A.; Anders, C.; Wulf, G.M.; et al. Open-label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Yau, C.; Wolf, D.M.; Han, H.S.; Du, L.; Wallace, A.M.; String-Reasor, E.; Boughey, J.C.; Chien, A.J.; Elias, A.D.; et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell 2021, 39, 989–998.e5. [Google Scholar] [CrossRef]
- Fares, M.C.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- Ellis, H.; Ma, C.X. PI3K Inhibitors in Breast Cancer Therapy. Curr. Oncol. Rep. 2019, 21, 110. [Google Scholar] [CrossRef]
- Kane, L.P. T Cell Ig and Mucin Domain Proteins and Immunity. J. Immunol. 2010, 184, 2743–2749. [Google Scholar] [CrossRef]
- Finlay, D.; Cantrell, D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration: PI3K and MTOR Pathways Control T-Cell Migration. Ann. N. Y. Acad. Sci. 2010, 1183, 149–157. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell–Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Loi, S.; Dushyanthen, S.; Beavis, P.A.; Salgado, R.; Denkert, C.; Savas, P.; Combs, S.; Rimm, D.L.; Giltnane, J.M.; Estrada, M.V.; et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin. Cancer Res. 2016, 22, 1499–1509. [Google Scholar] [CrossRef]
- Ebert, P.J.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D.A.; James, J.L.; Axelrod, M.L.; Balko, J.M. MEK inhibition activates STAT signaling to increase breast cancer immunogenicity via MHC-I expression. Cancer Drug Resist 2020, 3, 603–612. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, L.; Jiang, J.; Zhang, L.; Zhang, Z.; Pan, J.; Ni, C.; Chen, Z. Antiangiogenic therapy reverses the immunosuppressive breast cancer microenvironment. Biomark. Res. 2021, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Cauley, L.S.; Kim, I.-J.; Blackman, M.A.; Woodland, D.L.; Vignali, D.A.A. Lymphocyte Activation Gene-3 (CD223) Regulates the Size of the Expanding T Cell Population Following Antigen Activation In Vivo. J. Immunol. 2004, 172, 5450–5455. [Google Scholar] [CrossRef]
- Hong, D.S.; Schoffski, P.; Calvo, A.; Sarantopoulos, J.; De Olza, M.O.; Carvajal, R.D.; Prawira, A.; Kyi, C.; Esaki, T.; Akerley, W.L.; et al. Phase I/II study of LAG525 ± spartalizumab (PDR001) in patients (pts) with advanced malignancies. J. Clin. Oncol. 2018, 36, 3012. [Google Scholar] [CrossRef]
- Chan, A.S.H.; Jonas, A.B.; Qiu, X.; Ottoson, N.R.; Walsh, R.M.; Gorden, K.B.; Harrison, B.; Maimonis, P.J.; Leonardo, S.M.; Ertelt, K.E.; et al. Imprime PGG-Mediated Anti-Cancer Immune Activation Requires Immune Complex Formation. PLoS ONE 2016, 11, e0165909. [Google Scholar] [CrossRef]
- O’Day, S.; Borges, V.F.; Chmielowski, B.; Rao, R.D.; Abu-Khalaf, M.M.; Stopeck, A.; Nikolinakos, P.; Telli, M.L.; Xie, B.; Shaheen, M.F.; et al. An open label, multicenter phase II study combining imprime PGG (PGG) with pembrolizumab (P) in previously treated metastatic triple-negative breast cancer (mTNBC). J. Clin. Oncol. 2019, 37, 2550. [Google Scholar] [CrossRef]
- Tolaney, S.; Baldini, C.; Spira, A.; Cho, D.; Grignani, G.; Sawka, D.; Racca, F.; Bedard, P.; Rutten, A.; Liao10, E.J.; et al. Clinical activity of BEMPEG plus NIVO observed in metastatic TNBC: Preliminary results from the TNBC cohort of the Ph1/2 PIVOT-02 study. In Proceedings of the 5th International Cancer Immunotherapy Conference, Paris, France, 25–28 September 2019; p. 2. [Google Scholar]
- Terranova-Barberio, M.; Thomas, S.; Ali, N.; Pawlowska, N.; Park, J.; Krings, G.; Rosenblum, M.D.; Budillon, A.; Munster, P.N. HDAC inhibition potentiates immunotherapy in triple negative breast cancer. Oncotarget 2017, 8, 114156–114172. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Moroose, R.L.; Babu, S.; Baramidze, K.; Chan, D.; Leitner, S.P.; Nemsadze, G.; Ordentlich, P.; Quaranto, C.; Meyers, M.L.; et al. Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J. Clin. Oncol. 2020, 38, 1014. [Google Scholar] [CrossRef]
- Fuentes-Antrás, J.; Guevara-Hoyer, K.; Baliu-Piqué, M.; García-Sáenz, J.A.; Pérez-Segura, P.; Pandiella, A.; Ocaña, A. Adoptive Cell Therapy in Breast Cancer: A Current Perspective of Next-Generation Medicine. Front. Oncol. 2020, 10, 605633. [Google Scholar] [CrossRef] [PubMed]
- Albinger, N.; Hartmann, J.; Ullrich, E. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Ther. 2021, 28, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Schüßler-Lenz, M.; Bondanza, A.; Buchholz, C.J. Clinical development of CAR T cells—Challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017, 9, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Tchou, J.; Zhao, Y.; Levine, B.L.; Zhang, P.J.; Davis, M.M.; Melenhorst, J.J.; Kulikovskaya, I.; Brennan, A.L.; Liu, X.; Lacey, S.F.; et al. Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol. Res. 2017, 5, 1152–1161. [Google Scholar] [CrossRef]
- Wang, L.-C.S.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting Fibroblast Activation Protein in Tumor Stroma with Chimeric Antigen Receptor T Cells Can Inhibit Tumor Growth and Augment Host Immunity without Severe Toxicity. Cancer Immunol. Res. 2013, 2, 154–166. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, G.; Yan, X.; Xiao, X.; Ma, X.; Lin, S.; Wu, J.; Li, X.; Wang, W.; Liu, J.; et al. Prospects of Immunotherapy for Triple-Negative Breast Cancer. Front. Oncol. 2022, 11, 797092. [Google Scholar] [CrossRef]
- Ge, Y.; Xi, H.; Ju, S.; Zhang, X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett. 2013, 336, 253–259. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Fong, Y. Viroimmunotherapy for breast cancer: Promises, problems and future directions. Cancer Gene Ther. 2020, 28, 757–768. [Google Scholar] [CrossRef]
- Bernstein, V.; Ellard, S.L.; Dent, S.F.; Tu, D.; Mates, M.; Dhesy-Thind, S.K.; Panasci, L.; Gelmon, K.A.; Salim, M.; Song, X.; et al. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: Final analysis of Canadian Cancer Trials Group IND.213. Breast Cancer Res. Treat. 2018, 167, 485–493. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Meyers, D.E.; Thirukkumaran, C.M.; Liu, P.J.; Gratton, K.; Spurrell, J.; Shi, Q.; Thakur, S.; Morris, D.G. Oncolytic Reovirus and Immune Checkpoint Inhibition as a Novel Immunotherapeutic Strategy for Breast Cancer. Cancers 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed]
| NCT | Anti-PD-1/PD-L1 | Non IT Drugs | IT Drugs | Comparator Arms | Experimental Arms | Phase | Primary Endpoint | Status |
|---|---|---|---|---|---|---|---|---|
| NCT03424005 | Atezolizumab | Capecitabine Atezolizumab Ipatasertib SGN-LIV1A Bevacizumab Nab-Paclitaxel Sacituzumab Govitecan Gemcitabine Carboplatine | Tocilizumab Selicrelumab | Atezolizumab + Nab-Paclitaxel Capecitabine | Atezolizumab + Nab-Paclitaxel + Tocilizumab Atezolizumab + Sacituzumab Govitecan Atezolizumab + Ipatasertib Atezolizumab + SGN-LIV1A Atezolizumab + Selicrelumab + Bevacizumab Atezolizumab + CT | I/II | ORR Safety | Recruiting |
| NCT02849496 | Atezolizumab | Olaparib | Olaparib Olaparib + Atezolizumab | III | PFS | Recruiting | ||
| NCT03202316 | Atezolizumab | Cobimetinib Eribulin | Atezolizumab + Cobimetinib, + Eribulin Atezolizumab + Eribulin | II | ORR | Recruiting | ||
| NCT02425891 | Atezolizumab | Nab-paclitaxel | Placebo Plus Nab-Paclitaxel | Atezolizumab + Nab-Paclitaxel | III | PFS in ITT PFS in PD-L1 + OS in ITT OS in PD-L1+ | Completed | |
| NCT04177108 | Atezolizumab | Ipatasertib Paclitaxel | Cohort 1: PD-L1- Paclitaxel+ Placebo+ Placebo Cohort 2: PD-L1+ Paclitaxel + Atezolizumab + Placebo | Cohort 1: PD-L1-Paclitaxel + Atezolizumab + Ipatasertib Paclitaxel + Ipatasertib + Placebo Cohort 2: PD-L1+ Paclitaxel + Atezolizumab + Ipatasertib | III | PFS OS | Active, not recruiting | |
| NCT03961698 | Atezolizumab | Eganelisib Nab-Paclitaxel Bevacizumab | Cohort 1: PD-L1+ Eganelisib + Nab-Paclitaxel + Atezolizumab Cohort 2: PD-L1+ Eganelisib + Nab-Paclitaxel + Atezolizumab | II | CRR | Recruiting | ||
| NCT04408118 | Atezolizumab | Paclitaxel Bevacizumab | Atezolizumab + Paclitaxel + Bevacizumab | II | PFS | Recruiting | ||
| NCT02322814 | Atezolizumab | Cobimetinib Paclitaxel Nab-Paclitaxel | Cohort 1: Placebo + Paclitaxel | Cohort 1: Cobimetinib + Paclitaxel Cohort 2: Cobimetinib + Paclitaxel + Atezolizumab Cohort 3: Cobimetinib + Nab-Paclitaxel + Atezolizumab | II | Cohort 1: PFS Cohort 2, 3: OR, CR, PR | Completed | |
| NCT03829501 | Atezolizumab | KY1044 (Alomfilimab) | KY1044 KY1044 + Aezolizumab | I/II | Safety Tolerability ORR DLTs | Recruiting | ||
| NCT03101280 | Atezolizumab | Rucaparib | Rucaparib + Atezolizumab | I | Safety DLTs RP2D | Completed | ||
| NCT03915678 | Atezolizumab | BDB001 | Atezolizumab + BDB001+ RT | II | Activity measured in terms of CR, PR, SD | Recruiting | ||
| NCT04639245 | Atezolizumab | Cyclophosphamide Fludarabine | MAGE-A1-specific T Cell Receptor-transduced Autologous T-cells | FH-MagIC TCR-T cells + Atezolizumab After lymphodepletion with cyclophosphamide + fludarabine | I/II | Safety ORR | Recruiting | |
| NCT02708680 | Atezolizumab | Entinostat | Atezolizumab+ Placebo | Atezolizumab + Entinostat | II | DLT MTD PFS | Active, not recruiting | |
| NCT02819518 | Pembrolizumab | Nab-paclitaxel Paclitaxel Gemcitabine Carboplatin | Placebo + CT | Pembrolizumab + Nab-paclitaxel Pembrolizumab + Paclitaxel Pembrolizumab + Gemcitabine/Carboplatin | III | Safety PFS in ITT PFS in PD-L1 CPS ≥1 Tumors PFS in PD-L1 CPS ≥10 Tumors OS in ITT OS in PD-L1 CPS ≥1 Tumors OS in PD-L1 CPS ≥10 Tumors | Active, not recruiting | |
| NCT02971761 | Pembrolizumab | Enobosarm | Pembrolizumab + Enobosarm | II | Safety CBR DLTs | Active, not recruiting | ||
| NCT02657889 | Pembrolizumab | Niraparib | Pembrolizumab + Niraparib | I/II | ORR DLTs | Completed | ||
| NCT03106415 | Pembrolizumab | Binimetinib | Pembrolizumab + Binimetinib | I/II | ORR MTD | Active, not recruiting | ||
| NCT03797326 | Pembrolizumab | Lenvatinib | Pembrolizumab + Lenvatinib Lenvatinib | II | ORR Safety | Active, not recruiting | ||
| NCT03184558 | Pembrolizumab | Bemcetinib | Pembrolizumab + Bemcetinib | II | ORR | |||
| NCT03272334 | Pembrolizumab | HER2Bi armed activated T-cells | Pembrolizumab + HER2Bi armed activated T-cells | I/II | DLTs | Recruiting | ||
| NCT03012230 | Pembrolizumab | Ruxolitinib | Pembrolizumab + Ruxolitinib phosphate | I | MTD Safety | Recruiting | ||
| NCT04468061 | Pembrolizumab | Sacituzumab Govitecan | Pembrolizumab + Sacituzumab govitecan Sacituzumab govitecan | II | PFS | Recruiting | ||
| NCT04683679 | Pembrolizumab | Olaparib | Pembrolizumab + Olaparib + RT Pembrolizumab + RT | II | ORR | Recruiting | ||
| NCT02411656 | Pembrolizumab | Pembrolizumab | II | DCR | Recruiting | |||
| NCT02981303 | Pembrolizumab | Imprime PGG | Imprime PGG + Pembrolizumab | II | ORR | Completed | ||
| NCT03650894 | Nivolumab | Bicalutamide | Ipilimumab | Nivolumab + Ipilimumab, + Bicalutamide | II | CBR | Recruiting | |
| NCT03098550 | Nivolumab | Daratumumab | Nivolumab + Daratumumab Nivolumab | I/II | Safety | Completed | ||
| NCT04159818 | Nivolumab | Cisplatin Doxorubicin | Nivolumab Nivolumab + Cisplatin as induction therapy Nivolumab + Doxorubicin as induction therapy | II | PFS | Recruiting | ||
| NCT02393794 | Nivolumab | Romidepsin Cisplatin | Romidepsin + Cisplatin Romidepsin + Cisplatin + Nivolumab | I/II | RP2D ORR | Active not recruiting | ||
| NCT03414684 | Nivolumab | Carboplatin | Nivolumab + Carboplatin Carboplatin | II | PFS | Active not recruiting | ||
| NCT03316586 | Nivolumab | Cabozantinib | Nivolumab + Cabozantinib | II | ORR | Completed | ||
| NCT03829436 | Nivolumab | TPST-1120 | Nivolumab + TPST-1120 | I | DLTs MTD | Recruiting | ||
| NCT03241173 | Nivolumab | INCAGN2385 Ipilimumab | Nivolumab + INCAGN2385 INCAGN2385 + Ipilimumab Nivolumab + INCAGN2385 + Ipilimumab | I/II | Safety and Tolerability ORR | Completed | ||
| NCT03667716 | Nivolumab | COM 701 | COM 701 COM 701 + Nivolumab | I | Safety MTD | Recruiting | ||
| NCT03435640 | Nivolumab | Bempegaldesleukin NKTR-262 | NKTR-262 + Bempegaldesleukin NKTR-262 + Bempegaldesleukin + Nivolumab | I/II | Safety ORR | Active not recruiting | ||
| NCT02983045 | Nivolumab | Ipilimumab Bempegaldesleukin | Nivolumab + Bempegaldesleukin Nivolumab + Ipilimumab Bempegaldesleukin | I/II | ORR | Active not recruiting | ||
| NCT02499367 | Nivolumab | Cisplatin Doxorubicin Cyclophosphamid Cisplatin | RT Doxorubicin Doxorubicin Cyclophosphamid Cisplatin | Nivolumab + RT Nivolumab + Doxorubicin Nivolumab + Cyclophosphamid Nivolumab + Cispatin Nivolumab | II | PFS | Active not recruiting | |
| UMIN000030242 | Nivolumab | Bevacizumab Paclitaxel | Nivolumab + Paclitaxel + Bevacizumab | II | ORR | Active not recruiting | ||
| NCT03952325 | Nivolumab Pembrolizumab Atezolizumab | Tesetaxel | Tesetaxel + Nivoluamb Tesexatel + Pembrolizuamb Tesetaxel + Atezolizumab Tesetaxel | II | ORR PFS | Completed | ||
| NCT03971409 | Avelumab | Binimetinib Utomilumab Liposomal Doxorubicin Sacituzumab Govitecan | Binimetinib + Avelumab Anti-OX40 antibody PF-04518600 + Avelumab Utomilumab + Avelumab Avelumab + Binimetinib + Liposomal doxorubicin Avelumab + sacituzumab govitecan Avelumab + Liposomal doxorubicin | III | BORR | Recruiting | ||
| NCT04360941 | Avelumab | Palbociclib | Avelumab + Palbociclib | II | MTD ORR | Recruiting | ||
| NCT02802098 | Durvalumab | Bevacizumab | Bevacizumab + Durvalumab | I | Dynamic of peripheral blood mononuclear cells subpopulations PFS OS | Completed | ||
| NCT03616886 | Durvalumab | Paclitaxel Carboplatin | Oleclumab | Paclitaxel+ Carboplatin+ Durvalumab | Paclitaxel + Carboplatin + Durvalumab + Oleclumab | I/II | AES CBR | Recruiting |
| NCT03801369 | Durvalumab | Olaparib | Durvalumab + Olaparib | II | ORR | Recruiting | ||
| NCT03982173 | Durvalumab | Tremelimumab | Durvalumab + Tremelimumab | II | ORR | Active, not recruiting | ||
| NCT04837209 | Dostarlimab | Niraparib | Niraparib + Dostarlimab + Radiation therapy | II | ORR | Recruiting | ||
| NCT03742349 | Spartalizumab | Capmatinib | Canakinumab Lacnotuzumab NIR178 LAG525 | Spartalizumab + LAG525 + NIR178 Spartalizumab + LAG525 + Capmatinib Spartalizumab + LAG525 + MCS110 Spartalizumab + LAG525 + Canakinumab | I | Safety DLTs | Recruiting | |
| NCT03499899 | Spartalizuamb | Carboplatin | LAG525 | LAG525 + Spartalizumab LAG525 + Spartalizumab + Carboplatin LAG525 + Carboplatin | II | ORR | Completed | |
| NCT04673448 | Dostarlimab | Niraparib | Niraparib, Dostarlimab | I | ORR | Recruiting | ||
| NCT03579472 | Bintrafusp Alfa | Eribulin | Bintrafusp alfa, Eribulin mesylate | I | RP2D Safety | Recruiting | ||
| NCT04609215 | Carboplatin Gemcitabine | ALECSAT | ALECSAT + Carboplatin + Gemcitabine | I | Safety | Recruiting | ||
| NCT01516307 | Phosphate Buffer Saline Cyclophosphamide | Vaccine OPT-822/OPT-821 | Phosphate Buffer Saline + Cyclophosphamide | OPT-822/OPT-821 + Cyclophosphamide | II | PFS | Completed | |
| NCT02614833 | Paclitaxel | Eftilagimod alpha | Paclitaxel+ Placebo | Paclitaxel+ Eftilagimod alpha | I/II | Dose finding PFS | Completed | |
| NCT00179309 | Docetaxel | Panvac Vaccine | Docetaxel | PANVAC + Docetaxel | II | PFS | Completed | |
| NCT03066947 | Cyclophosphamide | SV-BR-1-GM Interferon-alpha-2b | Cyclophosphamide + SV-BR-1-GM + Interferon-alpha-2b | I/II | Safety | Completed | ||
| NCT04129996 | Camrelizumab | Nab-paclitaxel Famitinib | Camrelizumab + Nab-paclitaxel+ Famitinib | II | ORR | Active not recruiting | ||
| NCT04303741 | Camrelizumab | Apatinib Eribulin | Camrelizumab + Apatinib+ Eribulin | II | ORR | Active not recruiting | ||
| NCT03394287 | Camrelizumab | Apatinib | SHR-1210+ Apatinib daily dosing SHR-1210 + Apatinib intermittent dosing | II | ORR | Completed | ||
| NCT04405505 | TQB2450 | Nab-paclitaxel Anlotinib | Nab-paclitaxel | TQB2450 + Anlotinib | III | PFS | Not yet Recruiting | |
| NCT02936102 | FAZ 053 PDR001 | FAZ053 single agent FAZ053 + PDR001 | I | Safety and Tolerability | Active, not recruiting | |||
| NCT03872791 | KN046 | Nab-paclitaxel | KN046 KN046 + Nab-paclitaxel | I/II | ORR DOR | Active, not recruiting | ||
| NCT04085276 | Toripalimab | Nab-Paclitaxel | Nab-paclitaxel+ Placebo | Toripalimab + Nab-Paclitaxel | III | PFS | Recruiting | |
| NCT03893955 | Budigalimab (ABBV 181) | CBDCA Nab-Paclitaxel | ABBV 927 ABBV 368 | ABBV-927 + Nab-paclitaxel + ABBV-368 ABBV-927 + Carboplatin ABBV-927 + Carboplatin+ Budigalimab ABBV-927 + Carboplatin + ABBV-368 | I | ORR RP2D | Recruiting | |
| NCT03517488 | XmAb20717 | XmAb20717 | I | Safety and Tolerability | Recruiting | |||
| NCT03752398 | Ipilimumab XmAb20717 | XmAb20717 XmAb20717 + Ipilimumab | I | Safety and Tolerability | Recruiting | |||
| NCT03849469 | Pembrolizumab | XmAb22841 | XmAb22841 XmAb22841 + Pembrolizumab | I | Safety and Tolerability | Recruiting | ||
| NCT03538028 | INCAGN2385 | INCAGN02385 | I | Safety and Tolerability | Completed | |||
| NCT04254107 | Sasanlimab | SEA-TGT | SEA-TGT SEA-TGT+ Sasanlimab | I | Safety and Tolerability | Recruiting | ||
| NCT03665285 | NC318 | NC318 | I | Safety and Tolerability MTD | Recruiting |
| NCT | Anti PD-1/PD-L1 | Non-IT Drugs | IT Drugs | Compartor Arms | Experimental Arms | Phase | Primary Endpoints | Status |
|---|---|---|---|---|---|---|---|---|
| NCT04427293 | Pembrolizumab | Lenvatinib | Lenvatinib + Pembrolizumab | I | Effectiveness | Recruiting | ||
| NCT03639948 | Pembrolizumab | Carboplatin Docetaxel | Pembrolizumab + CT | II | pCR rate | Recruiting | ||
| NCT04373031 | Pembrolizumab | Epirubicin Cyclophosphamide Taxanes | IRX-2 | Pembrolizumab+ CT | Pembrolizumab + IRX-2 + CT | II | pCR rate | Recruiting |
| NCT05203445 | Pembrolizumab | Olaparib | Olaparib + Pembrolizumab | II | pathologically negative MRI-guided biopsy after 12 weeks of treatment | Recruiting | ||
| NCT02954874 | Pembrolizumab | No treatment | Pembrolizumab | III | iDFS Severity of Fatigue Physical function | Active not recruiting | ||
| NCT02957968 | Pembrolizumab | Decitabine Doxorubicin Cyclophosphamide Paclitaxel Carboplatin | Pembrolizumab + Decitabine Doxorubicin Cyclophosphamide Paclitaxel Carboplatin | II | Dynamic of TILs | Recruiting | ||
| NCT05177796 | Pembrolizumab | Panitumumab Paclitaxel Carboplatin Doxorubicin Cyclophosphamide | Panitumumab + Pembrolizumab + Paclitaxel + Carboplatin + Doxorubicin + Cyclophosphamide | II | pCR Rate | Active not yet recruiting | ||
| NCT03036488 | Pembrolizumab | Carboplatin Paclitaxel Doxorubicin/Epirubicin Cyclophosphamide | Placebo+ Carboplatin Paclitaxel Doxorubicin Epirubicin Cyclophosphamide | Pembrolizumab + CBDCA Paclitaxel Doxorubicin/Epirubicin Cyclophosphamide | III | pCR Rate EFS | Active, not recruiting | |
| NCT01986426 | Pembrolizumab | LTX-315 | LTX-315 + Pembrolizumab | I | DLT | Completed | ||
| NCT03197935 | Atezolizumab | Nab-paclitaxel Doxorubicin Cyclophosphamide | Placebo+ Nab-paclitaxel+ Doxorubicin+ Cyclophosphamide | Atezolizumab + Nab-paclitaxel Doxorubicin + Cyclophosphamide | III | pCR in ITT pCR in PD-L1+ | Completed | |
| NCT03498716 | Atezolizumab | Paclitaxel Dose-dense Doxorubicin/Epirubicin Cyclophosphamide | Placebo+ Paclitaxel+ Dose-dense Doxorubicin or Epirubicin+ Cyclophosphamide | Atezolizumab + Paclitaxel Dose-dense Doxorubicin/Epirubicin Cyclophosphamide | III | iDFS | Recruiting | |
| NCT03371017 | Atezolizumab | Gemcitabine Capecitabine Carboplatin | Placebo+ Gemcitabine+ Capecitabine+ Carboplatin | Atezolizumab + Gemcitabine + Capecitabine + Carboplatin | III | OS | Recruiting | |
| NCT03256344 | Atezolizumab | Talimogene laherparepvec | Talimogene + Laherparepvec + Atezolizumab | I | DLTs | Completed | ||
| NCT04102618 | Atezolizumab | Pelareorep | Atezolizumab + Pelareorep | I | CelTIL Score | Recruiting | ||
| NCT03356860 | Durvalumab | Paclitaxel Epirubicin Cyclophosphamide | Paclitaxel+ Epirubicin+ Cyclophosphamide | Durvalumab + Paclitaxel Epirubicin + Cyclophosphamide | I/II | pCR Rate Safety | Recruiting | |
| NCT05209529 | Durvalumab | Olaparib | Durvalumab + Olaparib Olaparib monotherapy | II | pCR Rate | Recruiting | ||
| NCT02489448 | Durvalumab | Nab-paclitaxel Doxorubicin Cyclophosphamide | Durvalumab + Nab-paclitaxel Doxorubicin + Cyclophosphamide | I/II | pCR Rate | Active not recruiting | ||
| NCT03740893 | Durvalumab | AZD6738 Olaparib | Standard CT | AZD6738 monotherapy Olaparib monotherapy Durvalumab monotherapy | II | Safety Immunomodulating action | Recruiting | |
| NCT03594396 | Durvalumab | Olaparib | Durvalumab + Olaparib | I/II | Changes in tumor biology | Active not recruiting | ||
| NCT02685059 | Durvalumab | Nab-Paclitaxel Epirubicin Cyclophosphamide | Placebo+ Nab-Paclitaxel Epirubicin Cyclophosphamide | Durvalumab + Nab-Paclitaxel Epirubicin + Cyclophosphamide | II | pCR Rate | Completed | |
| NCT01042379 | Pembrolizumab Cemiplimab Dostarlimab Durvalumab | AMG 386 Trastuzumab AMG 479 (Ganitumab) Metformin MK-2206 AMG 386 T-DM1 Pertuzumab and Trastuzumab Ganetespib ABT-888 Neratinib PLX3397 Talazoparib Irinotecan Patritumab SGN-LIV1A Olaparib SD-101 Tucatinib REGN3767 Trilaciclib Paclitaxel Encequidar Carboplatin Oral Paclitaxel | Standard Treatments depending on HR/HER2-status | Experimental agents added to standard neoadjuvant treatment | II | pCR rate | Recruiting | |
| NCT04613674 | Camrelizumab | Standard CT | Placebo+ Standard CT | Camrelizumab + Standard CT | III | pCR Rate | Recruiting | |
| NCT04301739 | HLX 10 | Nab-Paclitaxel Carboplatin Doxorubicin Cyclophosphamide | Placebo+ Nab-Paclitaxel Carboplatin Doxorubicin Cyclophosphamide | HLX 10 + Nab-Paclitaxel + Carboplatin Doxorubicin + Cyclophosphamide | III | pCR Rate | Not yet recruiting | |
| NCT03815890 | Nivolumab | Ipilimumab | Nivolumab Nivolumab + Ipilimumab | II | Immune activation after pre-operative Nivolumab | Recruiting | ||
| NCT03487666 | Nivolumab | Capecitabine | Capecitabine | Nivolumab Nivolumab + Capecitabine | II | Immune activation measured by changes in the peripheral immunoscore (PIS) at week 6 | Active not recruiting | |
| NCT03818685 | Nivolumab | Capecitabine | Ipilimumab | Capecitabine | Nivolumab + Ipilimumab | II | iDFS | Recruiting |
| NCT04185311 | Nivolumab | Ipilimumab+ Talimogene laherparepvec | Talimogene laherparepvec + Nivolumab + Ipilimumab | I | Safety | Active not recruiting | ||
| NCT02938442 | Doxorubicin Cyclophosphamide Paclitaxel | P10s-PADRE with MONTANIDE™ ISA 51 VG | Doxorubicin + Cyclophosphamide Paclitaxel | P10s-PADRE with MONTANIDE™ ISA 51 VG + Doxorubicin + Cyclophosphamide + Paclitaxel | I/II | Safety pCR Rate | Recruiting | |
| NCT02779855 | Paclitaxel | Talimogene laherparepvec | Talimogene laherparepvec + Paclitaxel | I/II | MTD RP2D pCR Rate | Active not recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlino, F.; Diana, A.; Piccolo, A.; Ventriglia, A.; Bruno, V.; De Santo, I.; Letizia, O.; De Vita, F.; Daniele, B.; Ciardiello, F.; et al. Immune-Based Therapy in Triple-Negative Breast Cancer: From Molecular Biology to Clinical Practice. Cancers 2022, 14, 2102. https://doi.org/10.3390/cancers14092102
Carlino F, Diana A, Piccolo A, Ventriglia A, Bruno V, De Santo I, Letizia O, De Vita F, Daniele B, Ciardiello F, et al. Immune-Based Therapy in Triple-Negative Breast Cancer: From Molecular Biology to Clinical Practice. Cancers. 2022; 14(9):2102. https://doi.org/10.3390/cancers14092102
Chicago/Turabian StyleCarlino, Francesca, Anna Diana, Antonio Piccolo, Anna Ventriglia, Vincenzo Bruno, Irene De Santo, Ortensio Letizia, Ferdinando De Vita, Bruno Daniele, Fortunato Ciardiello, and et al. 2022. "Immune-Based Therapy in Triple-Negative Breast Cancer: From Molecular Biology to Clinical Practice" Cancers 14, no. 9: 2102. https://doi.org/10.3390/cancers14092102
APA StyleCarlino, F., Diana, A., Piccolo, A., Ventriglia, A., Bruno, V., De Santo, I., Letizia, O., De Vita, F., Daniele, B., Ciardiello, F., & Orditura, M. (2022). Immune-Based Therapy in Triple-Negative Breast Cancer: From Molecular Biology to Clinical Practice. Cancers, 14(9), 2102. https://doi.org/10.3390/cancers14092102







