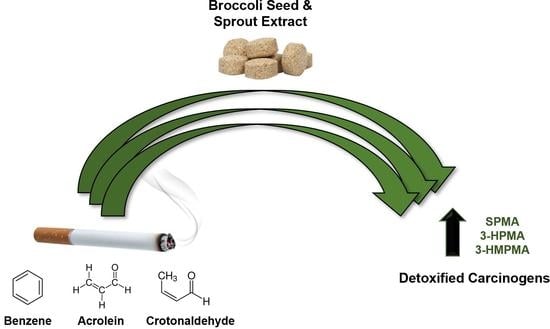
Randomized Crossover Trial Evaluating Detoxification of Tobacco Carcinogens by Broccoli Seed and Sprout Extract in Current Smokers
Abstract
Simple Summary
Abstract
1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Study Agent and Treatment Plan
2.3. Biomarker Analysis
2.3.1. Carcinogen Metabolites
2.3.2. SFN Metabolites
2.3.3. Buccal Cell mRNA
2.3.4. GSTM1 and GSTT1 Genotypes
2.4. Statistical Analysis
3. Results
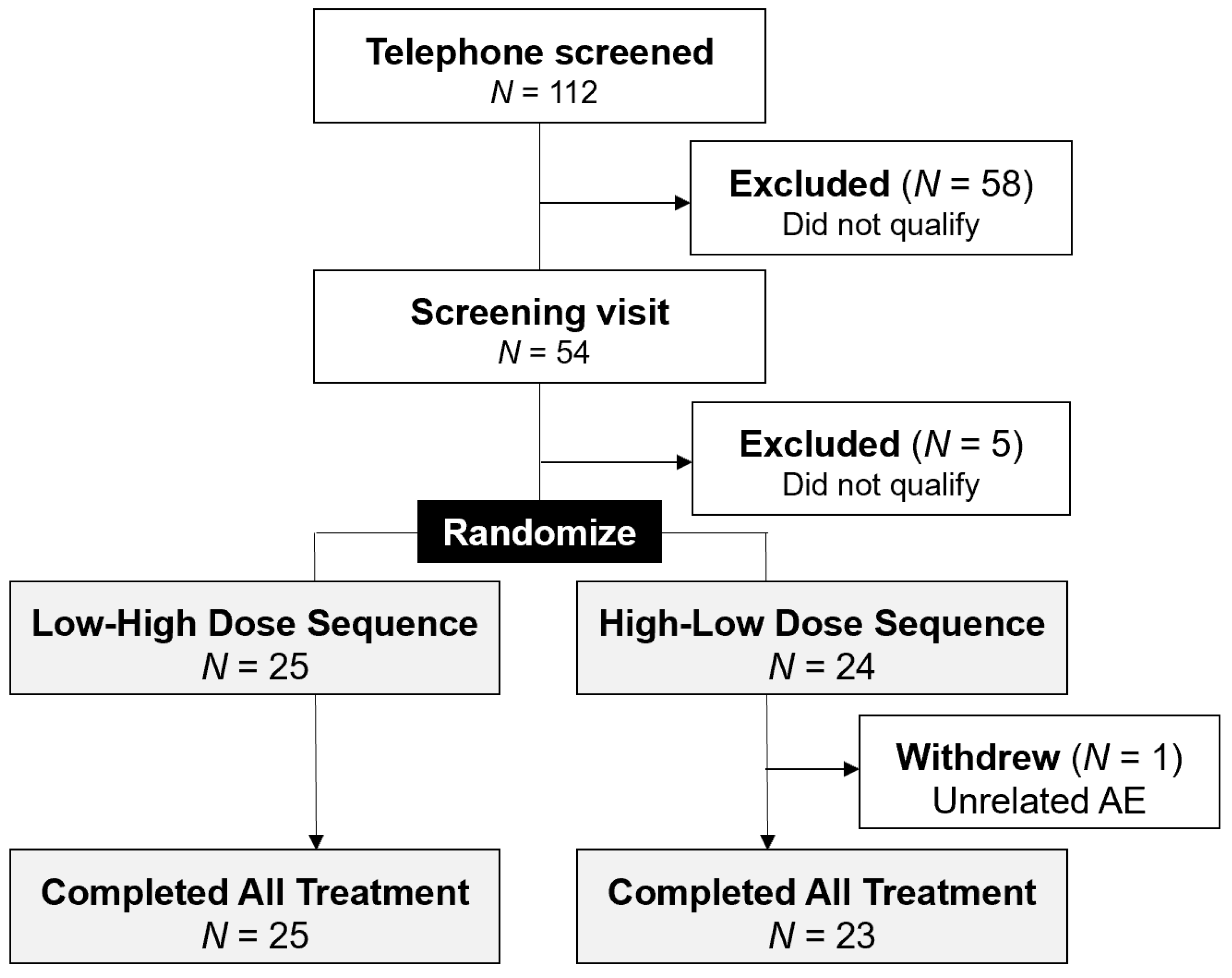
3.1. Study Population
3.2. Study Endpoints
3.2.1. Bioavailability
3.2.2. Carcinogen Detoxification
3.2.3. Buccal Cell Gene Expression
3.2.4. GSTT1 and GSTM1 Genotypes
3.2.5. Linear Regression Analysis for SPMA Detoxification
3.2.6. Safety and Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Leon, X.; Quer, M.; Diez, S.; Orus, C.; Lopez-Pousa, A.; Burgues, J. Second neoplasm in patients with head and neck cancer. Head Neck 1999, 21, 204–210. [Google Scholar] [CrossRef]
- Lee, D.H.; Roh, J.L.; Baek, S.; Jung, J.H.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Second cancer incidence, risk factor, and specific mortality in head and neck squamous cell carcinoma. Otolaryngol. Head Neck Surg. 2013, 149, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Day, G.L.; Blot, W.J.; Shore, R.E.; McLaughlin, J.K.; Austin, D.F.; Greenberg, R.S.; Liff, J.M.; Preston-Martin, S.; Sarkar, S.; Schoenberg, J.B.; et al. Second cancers following oral and pharyngeal cancers: Role of tobacco and alcohol. J. Natl. Cancer Inst. 1994, 86, 131–137. [Google Scholar] [CrossRef]
- Bosshart, S.L.; Morand, G.B.; Broglie, M.A. Frequency and localization of second primary tumors in patients with oropharyngeal carcinoma-the influence of the human papilloma virus. Cancers 2021, 13, 1755. [Google Scholar] [CrossRef]
- Lippman, S.M.; Hong, W.K. Second malignant tumors in head and neck squamous cell carcinoma: The overshadowing threat for patients with early-stage disease. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 691–694. [Google Scholar] [CrossRef]
- Massa, S.T.; Osazuwa-Peters, N.; Christopher, K.M.; Arnold, L.D.; Schootman, M.; Walker, R.J.; Varvares, M.A. Competing causes of death in the head and neck cancer population. Oral. Oncol. 2017, 65, 8–15. [Google Scholar] [CrossRef]
- Khuri, F.R.; Kim, E.S.; Lee, J.J.; Winn, R.J.; Benner, S.E.; Lippman, S.M.; Fu, K.K.; Cooper, J.S.; Vokes, E.E.; Chamberlain, R.M.; et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol. Biomark. Prev. 2001, 10, 823–829. [Google Scholar]
- Kashigar, A.; Habbous, S.; Eng, L.; Irish, B.; Bissada, E.; Irish, J.; Brown, D.; Gilbert, R.; Gullane, P.; Xu, W.; et al. Social environment, secondary smoking exposure, and smoking cessation among head and neck cancer patients. Cancer 2013, 119, 2701–2709. [Google Scholar] [CrossRef]
- Duffy, S.A.; Ronis, D.L.; Valenstein, M.; Lambert, M.T.; Fowler, K.E.; Gregory, L.; Bishop, C.; Myers, L.L.; Blow, F.C.; Terrell, J.E. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2203–2208. [Google Scholar] [CrossRef]
- Conlon, M.S.C.; Santi, S.A.; Meigs, M.L.; Davidson, S.M.; Saunders, D. Cigarette-smoking characteristics and interest in cessation in patients with head-and-neck cancer. Curr. Oncol. 2020, 27, e478–e485. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.H.; Mahoney, M.C.; Cummings, K.M.; Loree, T.R.; Sullivan, M.; King, B.A.; Warren, G.; Hyland, A. Evaluation of an intervention to enhance the delivery of smoking cessation services to patients with cancer. J. Cancer Educ. 2011, 26, 577–582. [Google Scholar] [CrossRef] [PubMed]
- McCarter, K.; Martinez, U.; Britton, B.; Baker, A.; Bonevski, B.; Carter, G.; Beck, A.; Wratten, C.; Guillaumier, A.; Halpin, S.A.; et al. Smoking cessation care among patients with head and neck cancer: A systematic review. BMJ Open 2016, 6, e012296. [Google Scholar] [CrossRef] [PubMed]
- Burris, J.L.; Borger, T.N.; Shelton, B.J.; Darville, A.K.; Studts, J.L.; Valentino, J.; Blair, C.; Davis, D.B.; Scales, J. Tobacco Use and Tobacco Treatment Referral Response of Patients With Cancer: Implementation Outcomes at a National Cancer Institute-Designated Cancer Center. JCO Oncol. Pract. 2022, 18, e261–e270. [Google Scholar] [CrossRef]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Diet and oral, pharyngeal, and esophageal cancer. Nutr. Cancer 2002, 44, 104–126. [Google Scholar] [CrossRef]
- Pavia, M.; Pileggi, C.; Nobile, C.G.; Angelillo, I.F. Association between fruit and vegetable consumption and oral cancer: A meta-analysis of observational studies. Am. J. Clin. Nutr. 2006, 83, 1126–1134. [Google Scholar] [CrossRef]
- Day, G.L.; Shore, R.E.; Blot, W.J.; McLaughlin, J.K.; Austin, D.F.; Greenberg, R.S.; Liff, J.M.; Preston-Martin, S.; Sarkar, S.; Schoenberg, J.B.; et al. Dietary factors and second primary cancers: A follow-up of oral and pharyngeal cancer patients. Nutr. Cancer 1994, 21, 223–232. [Google Scholar] [CrossRef]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef]
- Fowke, J.H. Head and neck cancer: A case for inhibition by isothiocyanates and indoles from cruciferous vegetables. Eur. J. Cancer Prev. 2007, 16, 348–356. [Google Scholar] [CrossRef]
- Burge-Bottenbley, A.; Shklar, G. Retardation of experimental oral cancer development by retinyl acetate. Nutr. Cancer 1983, 5, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Garewal, H.S.; Meyskens, F.L., Jr. Retinoids as potential chemopreventive agents in squamous cell carcinoma of the head and neck. Prev. Med. 1989, 18, 740–748. [Google Scholar] [CrossRef][Green Version]
- Hong, W.K.; Endicott, J.; Itri, L.M.; Doos, W.; Batsakis, J.G.; Bell, R.; Fofonoff, S.; Byers, R.; Atkinson, E.N.; Vaughan, C.; et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N. Engl. J. Med. 1986, 315, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.K.; Lippman, S.M.; Itri, L.M.; Karp, D.D.; Lee, J.S.; Byers, R.M.; Schantz, S.P.; Kramer, A.M.; Lotan, R.; Peters, L.J.; et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 1990, 323, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Khuri, F.R.; Lee, J.J.; Lippman, S.M.; Kim, E.S.; Cooper, J.S.; Benner, S.E.; Winn, R.; Pajak, T.F.; Williams, B.; Shenouda, G.; et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J. Natl. Cancer Inst. 2006, 98, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, H.J.; Santamaria, A.B.; Talalay, P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc. Natl. Acad. Sci. USA 1992, 89, 2394–2398. [Google Scholar] [CrossRef]
- Kensler, T.W. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ. Health Perspect. 1997, 105 (Suppl. 4), 965–970. [Google Scholar]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef]
- Hong, F.; Freeman, M.L.; Liebler, D.C. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 2005, 18, 1917–1926. [Google Scholar] [CrossRef]
- Kensler, T.W.; Egner, P.A.; Agyeman, A.S.; Visvanathan, K.; Groopman, J.D.; Chen, J.G.; Chen, T.Y.; Fahey, J.W.; Talalay, P. Keap1-nrf2 signaling: A target for cancer prevention by sulforaphane. Top. Curr. Chem. 2013, 329, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Bauman, J.E.; Zang, Y.; Sen, M.; Li, C.; Wang, L.; Egner, P.A.; Fahey, J.W.; Normolle, D.P.; Grandis, J.R.; Kensler, T.W.; et al. Prevention of carcinogen-induced oral cancer by sulforaphane. Cancer Prev. Res. 2016, 9, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Chen, J.G.; Egner, P.A.; Fahey, J.W.; Jacobson, L.P.; Stephenson, K.K.; Ye, L.; Coady, J.L.; Wang, J.B.; Wu, Y.; et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Chen, J.G.; Wang, J.B.; Wu, Y.; Sun, Y.; Lu, J.H.; Zhu, J.; Zhang, Y.H.; Chen, Y.S.; Friesen, M.D.; et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: Results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev. Res. 2011, 4, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Ng, D.; Carmella, S.G.; Chen, M.; Jacobson, L.P.; Munoz, A.; Egner, P.A.; Chen, J.G.; Qian, G.S.; Chen, T.Y.; et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis 2012, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Chen, J.G.; Zarth, A.T.; Ng, D.K.; Wang, J.B.; Kensler, K.H.; Jacobson, L.P.; Munoz, A.; Johnson, J.L.; Groopman, J.D.; et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: Results of a randomized clinical trial in China. Cancer Prev. Res. 2014, 7, 813–823. [Google Scholar] [CrossRef]
- Garland, L.L.; Guillen-Rodriguez, J.; Hsu, C.H.; Yozwiak, M.; Zhang, H.H.; Alberts, D.S.; Davis, L.E.; Szabo, E.; Merenstein, C.; Lel, J.; et al. Effect of intermittent versus continuous low-dose aspirin on nasal epithelium gene expression in current smokers: A randomized, double-blinded rial. Cancer Prev. Res. 2019, 12, 809–820. [Google Scholar] [CrossRef]
- Yuan, J.M.; Murphy, S.E.; Stepanov, I.; Wang, R.; Carmella, S.G.; Nelson, H.H.; Hatsukami, D.; Hecht, S.S. 2-Phenethyl Isothiocyanate, Glutathione S-transferase M1 and T1 Polymorphisms, and Detoxification of Volatile Organic Carcinogens and Toxicants in Tobacco Smoke. Cancer Prev. Res. 2016, 9, 598–606. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Panjwani, A.A.; Liu, H.; Cornblatt, G.; Cornblatt, B.S.; Ownby, S.L.; Fuchs, E.; Holtzclaw, W.D.; et al. Bioavailability of Sulforaphane Following Ingestion of Glucoraphanin-Rich Broccoli Sprout and Seed Extracts with Active Myrosinase: A Pilot Study of the Effects of Proton Pump Inhibitor Administration. Nutrients 2019, 11, 1489. [Google Scholar] [CrossRef]
- Geiger, J.L.; Cedars, E.D.; Zang, Y.; Normolle, D.P.; Li, H.; Grandis, J.R.; Centuori, S.; Johnson, D.E.; Bauman, J.E. Clinical trials optimizing investigator and self-collection of buccal cells for RNA yield. Laryngoscope Investig. Otolaryngol. 2021, 6, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Carmella, S.G.; Chen, M.; Han, S.; Briggs, A.; Jensen, J.; Hatsukami, D.K.; Hecht, S.S. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem. Res. Toxicol. 2009, 22, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, W.; Shi, L.; Hou, H.; Hu, Q. Simultaneous determination of five mercapturic acid derived from volatile organic compounds in human urine by LC-MS/MS and its application to relationship study. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2014, 967, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, K.T.; Nelson, H.H.; Wiencke, J.K.; Smith, C.M.; Levin, S. The glutathione S-transferase theta and mu deletion polymorphisms in asbestosis. Am. J. Ind. Med. 1997, 31, 274–279. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Mannucci, A.; Kimpton, C.P.; Gill, P. A rapid and quantitative DNA sex test: Fluorescence-based PCR analysis of X-Y homologous gene amelogenin. Biotechniques 1993, 15, 636–638, 640–641. [Google Scholar]
- Ducey, D.A.; Christ, C.M. Arizona Department of Health Services Biennial Report 2017–2018, Bureau of Tobacco and Chronic Disease; Arizona Department of Health Services: Phoenix, AZ, USA, 2018. Available online: https://www.azdhs.gov/documents/prevention/tobacco-chronic-disease/tobacco-free-az/reports/2018-biennial-evaluation-report.pdf (accessed on 16 March 2022).
- Wang, Z.; Wu, V.H.; Allevato, M.M.; Gilardi, M.; He, Y.; Luis Callejas-Valera, J.; Vitale-Cross, L.; Martin, D.; Amornphimoltham, P.; McDermott, J.; et al. Syngeneic animal models of tobacco-associated oral cancer reveal the activity of in situ anti-CTLA-4. Nat. Commun. 2019, 10, 5546. [Google Scholar] [CrossRef]
- Chen, J.G.; Johnson, J.; Egner, P.; Ng, D.; Zhu, J.; Wang, J.B.; Xue, X.F.; Sun, Y.; Zhang, Y.H.; Lu, L.L.; et al. Dose-dependent detoxication of the airborne pollutant benzene in a randomized trial of broccoli sprout beverage in Qidong, China. Am. J. Clin. Nutr. 2019, 110, 675–684. [Google Scholar] [CrossRef]
- Farnham, M.W.; Stephenson, K.K.; Fahey, J.W. Glucoraphanin level in broccoli seed is largely determined by genotype. Hortscience 2005, 40, 50–53. [Google Scholar] [CrossRef]
- Farnham, M.W.; Wilson, P.E.; Stephenson, K.K.; Fahey, J.W. Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breed. 2004, 123, 60–65. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Santin-Marquez, R.; Alarcon-Aguilar, A.; Lopez-Diazguerrero, N.E.; Chondrogianni, N.; Konigsberg, M. Sulforaphane—Role in aging and neurodegeneration. Geroscience 2019, 41, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Dong, N.; Su, X.; Duan, M.; Wei, Y.; Wei, J.; Liu, G.; Peng, Q.; Zhao, Y. Sulforaphane induces S-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells. Sci. Rep. 2021, 11, 2504. [Google Scholar] [CrossRef]
- Yang, G.; Xu, Y.; Chen, X.; Hu, G. IFITM1 plays an essential role in the antiproliferative action of interferon-gamma. Oncogene 2007, 26, 594–603. [Google Scholar] [CrossRef]
- Alteber, Z.; Sharbi-Yunger, A.; Pevsner-Fischer, M.; Blat, D.; Roitman, L.; Tzehoval, E.; Elinav, E.; Eisenbach, L. The anti-inflammatory IFITM genes ameliorate colitis and partially protect from tumorigenesis by changing immunity and microbiota. Immunol. Cell Biol. 2018, 96, 284–297. [Google Scholar] [CrossRef]
- Katiyar, T.; Yadav, V.; Maurya, S.S.; Ruwali, M.; Singh, M.; Hasan, F.; Pandey, R.; Mehrotra, D.; Singh, S.; Mishra, S.; et al. Interaction of glutathione-s-transferase genotypes with environmental risk factors in determining susceptibility to head and neck cancer and treatment response and survival outcome. Environ. Mol. Mutagen. 2020, 61, 574–584. [Google Scholar] [CrossRef]

| Characteristic | N = 49 a |
|---|---|
| Age, years: Median (range) | 57 (37, 73) |
| Sex: N (%) | |
| Male | 23 (47) |
| Female | 26 (53) |
| Race: N (%) | |
| Black or African American | 1 (2) |
| White | 44 (90) |
| More than one | 3 (6) |
| Unknown or not reported | 1 (2) |
| Ethnicity: N (%) | |
| Hispanic or Latino | 4 (8) |
| Not Hispanic or Latino | 44 (90) |
| Unknown or not reported | 1 (2) |
| Karnofsky performance status: N (%) | |
| 90% | 6 (12) |
| 100% | 43 (88) |
| Tobacco use: Median (range) | |
| Pack-years | 36 (24, 60) |
| Cigarettes per day | 20 (10, 30) |
| GST genotype: N (%) | |
| GSTM1 null | 23 (47) |
| GSTT1 null | 8 (16) |
| Carcinogen (Metabolite) a | BSSE Dose Level | Pre (95% CI) pmol/mgCr | Post (95% CI) pmol/mgCr | Post/Pre (95% CI) | p-Value d |
|---|---|---|---|---|---|
| Benzene (SPMA) | Low | 7.8 (6.3, 9.6) b | 9.0 (7.2, 11.3) | 1.2 (1.0, 1.3) c | 0.05 |
| High | 7.6 (6.2, 9.4) | 9.1 (7.3, 11.3) | 1.2 (1.0, 1.4) | 0.04 | |
| Acrolein (3-HPMA) | Low | 9934.5 (8008.0, 12,324.4) | 11,032.1 (9161.1, 13,285.2) | 1.1 (1.0, 1.3) | 0.11 |
| High | 9750.0 (7903.7, 12,027.6) | 12,450.1 (10,658.8, 14,542.5) | 1.3 (1.1, 1.5) | <0.01 | |
| Crotonaldehyde (3-HMPMA) | Low | 10,962.6 (9026.8, 13,313.6) | 11,482.0 (9677.5, 13,622.9) | 1.1 (0.9, 1.2) | 0.56 |
| High | 10,808.6 (8947.3, 13,057.0) | 12,795.1 (10,922.9, 14,988.1) | 1.2 (1.02, 1.37) | 0.02 |
| Toxicity a | Low Dose (N = 49) | High Dose (N = 49) | Both Arms (N = 49) b | p Value c |
|---|---|---|---|---|
| Abdominal Pain | 1 (2%) | 10 (20%) | 10 (20%) | <0.01 |
| Bloating | 0 (0%) | 1 (2%) | 1 (2%) | NA d |
| Diarrhea | 3 (6%) | 10 (20%) | 11 (22%) | 0.02 |
| Loose Stool | 7 (14%) | 9 (18%) | 12 (24%) | 0.48 |
| Flatulence | 3 (6%) | 7 (14%) | 10 (20%) | 0.21 |
| Nausea | 1 (2%) | 0 (0%) | 1 (2%) | NA |
| Vomiting | 0 (0%) | 1 (2%) | 1 (2%) | NA |
| Weight Loss | 0(0%) | 1 (2%) | 1 (2%) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauman, J.E.; Hsu, C.-H.; Centuori, S.; Guillen-Rodriguez, J.; Garland, L.L.; Ho, E.; Padi, M.; Bageerathan, V.; Bengtson, L.; Wojtowicz, M.; et al. Randomized Crossover Trial Evaluating Detoxification of Tobacco Carcinogens by Broccoli Seed and Sprout Extract in Current Smokers. Cancers 2022, 14, 2129. https://doi.org/10.3390/cancers14092129
Bauman JE, Hsu C-H, Centuori S, Guillen-Rodriguez J, Garland LL, Ho E, Padi M, Bageerathan V, Bengtson L, Wojtowicz M, et al. Randomized Crossover Trial Evaluating Detoxification of Tobacco Carcinogens by Broccoli Seed and Sprout Extract in Current Smokers. Cancers. 2022; 14(9):2129. https://doi.org/10.3390/cancers14092129
Chicago/Turabian StyleBauman, Julie E., Chiu-Hsieh Hsu, Sara Centuori, Jose Guillen-Rodriguez, Linda L. Garland, Emily Ho, Megha Padi, Vignesh Bageerathan, Lisa Bengtson, Malgorzata Wojtowicz, and et al. 2022. "Randomized Crossover Trial Evaluating Detoxification of Tobacco Carcinogens by Broccoli Seed and Sprout Extract in Current Smokers" Cancers 14, no. 9: 2129. https://doi.org/10.3390/cancers14092129
APA StyleBauman, J. E., Hsu, C.-H., Centuori, S., Guillen-Rodriguez, J., Garland, L. L., Ho, E., Padi, M., Bageerathan, V., Bengtson, L., Wojtowicz, M., Szabo, E., & Chow, H.-H. S. (2022). Randomized Crossover Trial Evaluating Detoxification of Tobacco Carcinogens by Broccoli Seed and Sprout Extract in Current Smokers. Cancers, 14(9), 2129. https://doi.org/10.3390/cancers14092129