Skin Cancer-Associated S. aureus Strains Can Induce DNA Damage in Human Keratinocytes by Downregulating DNA Repair and Promoting Oxidative Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Sampling of Skin Cancer and Precancerous Lesions
2.2. Isolation of Staphylococcus Clinical Strains
2.3. Species Identification
2.4. Collection of Bacterial Secretome Samples
2.5. Characterization of S. aureus Isolates
2.6. Culture of Primary Human Keratinocytes
2.7. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide) Assay
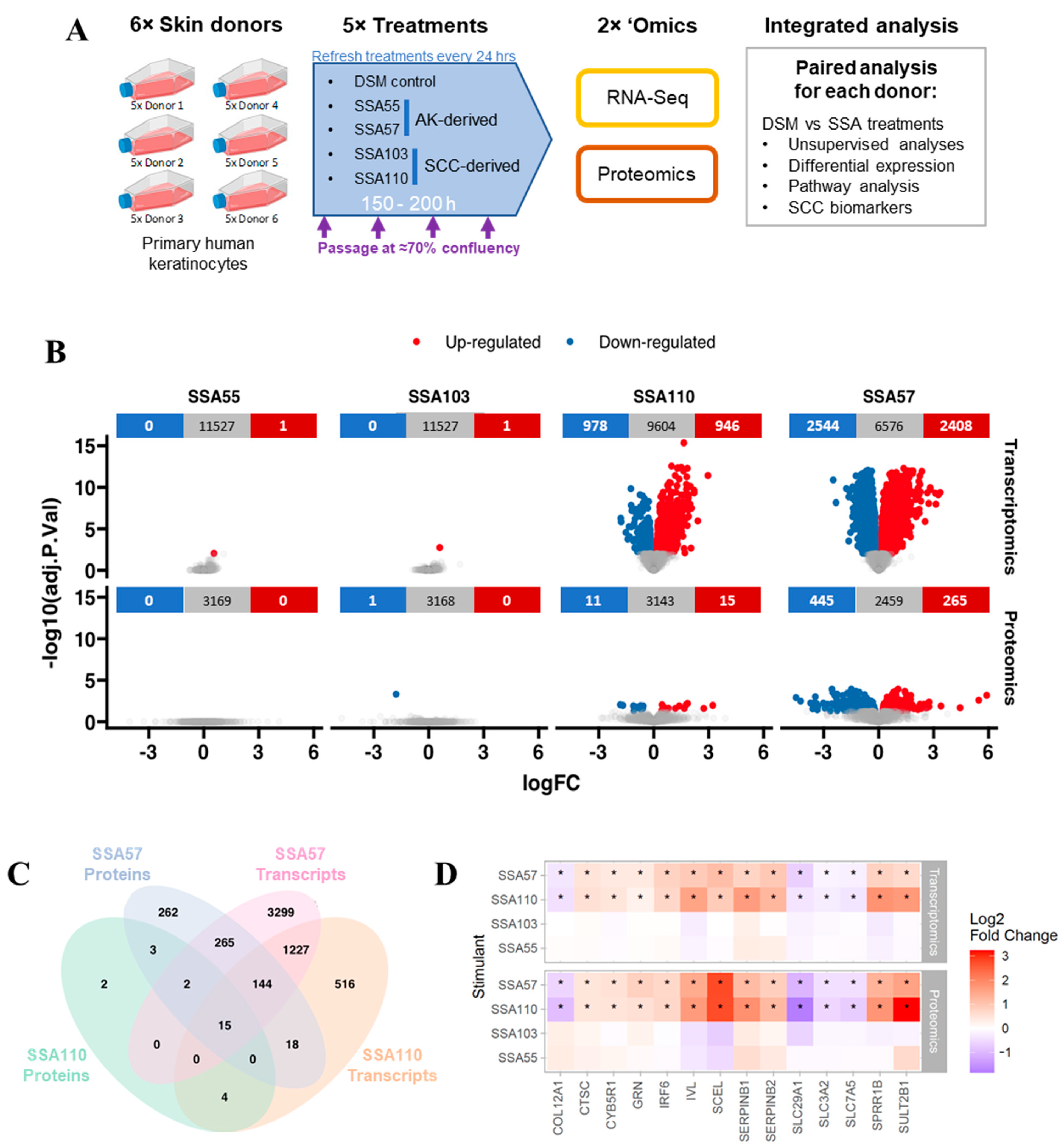
2.8. Keratinocyte Transcriptomics and Proteomics Experiments
2.9. RNA Sequencing and Bioinformatics Analysis
2.10. Shotgun Proteomics on Keratinocyte Cell Lysates
2.11. Measurement of Intracellular ROS
2.12. Assessment of Histone H2A.X Phosphorylation via Immunofluorescence Staining
2.13. 8-Hydroxy-Deoxyguanosine (8-OHdG) ELISA
2.14. Quantification of Phenol-Soluble Modulin (PSM) Toxins
3. Results
3.1. RNA-Seq and Proteomics on Human Keratinocytes after Challenge with S. aureus Secretomes
3.2. Gene and Protein Expression in Keratinocytes Is Strongly Altered by Some S. aureus Secretomes
3.3. S. aureus Mediates Upregulation of Several SCC Biomarkers in Primary Human Keratinocytes
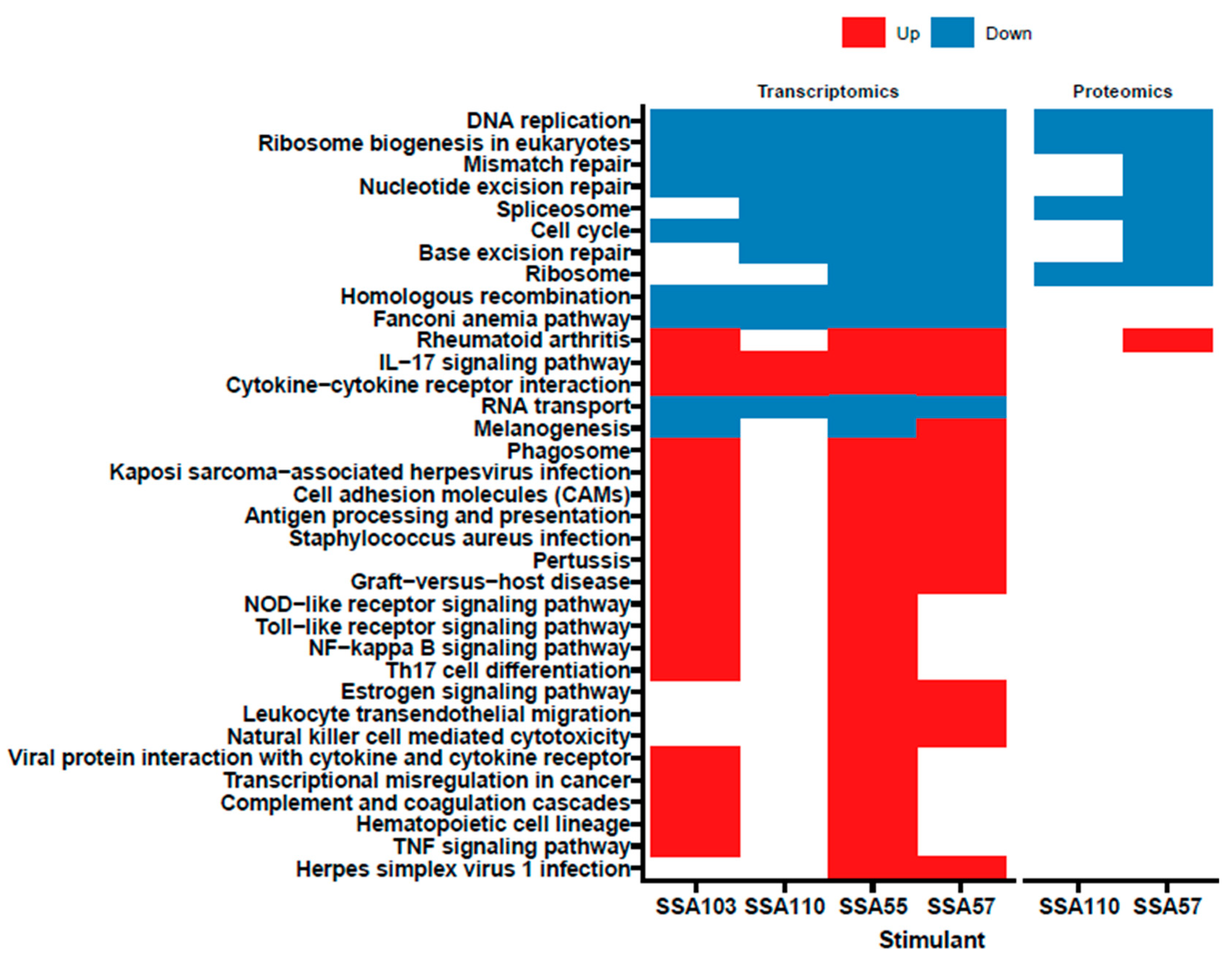
3.4. S. aureus Secretome Downregulates Cell Cycle and DNA Repair and Induces Oxidative Stress Markers in Primary Human Keratinocytes
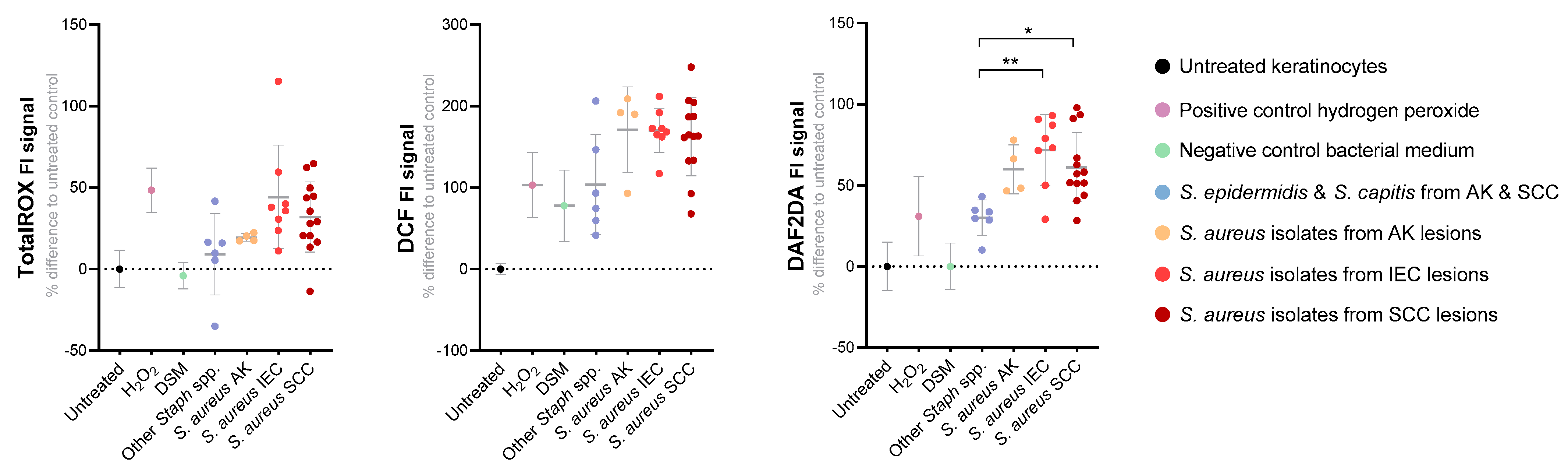
3.5. S. aureus Secreted Factors Trigger Oxidative Stress in Primary Human Keratinocytes
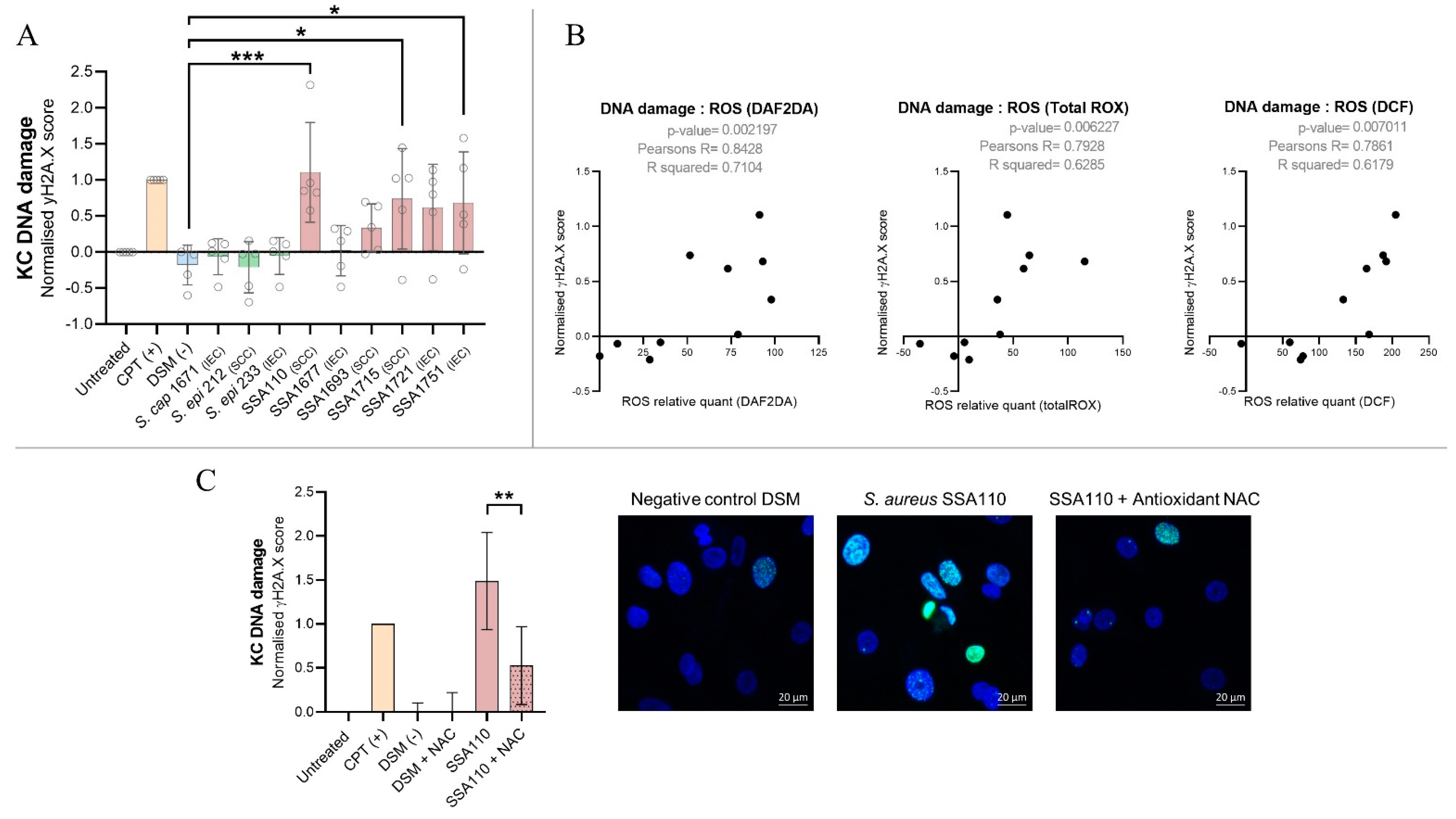
3.6. S. aureus Products Compromise the Integrity of DNA in Human Keratinocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.B.; Young, V.B.; Skufca, J.; Ginty, F.; Testerman, T.; Pearson, A.T.; Macklin, P.; Mitchell, A.; Shmulevich, I.; Xie, L.; et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 2020, 6, 192–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, inflammation and cancer. Cancer J. 2014, 20, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.-R.; Chang, Y.-F.; Ma, J.; Chiu, C.-H.; Kuo, M.-L.; Lai, C.-H. From DNA Damage to Cancer Progression: Potential Effects of Cytolethal Distending Toxin. Front. Immunol. 2021, 12, 760451. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2019, 17, 50–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, G.; Gulati, A.K.; Shukla, V.K. Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J. Gastroenterol. WJG 2010, 16, 5395. [Google Scholar] [CrossRef]
- Krueger, A.; Zaugg, J.; Lachner, N.; Bialasiewicz, S.; Lin, L.L.; Gabizon, S.; Sobarun, P.; Morrison, M.; Soyer, H.P.; Hugenholtz, P.; et al. Changes in the skin microbiome associated with squamous cell carcinoma in transplant recipients. ISME Commun. 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Andersson, T.; Bergdahl, G.E.; Saleh, K.; Magnúsdóttir, H.; Stødkilde, K.; Andersen, C.B.F.; Lundqvist, K.; Jensen, A.; Brüggemann, H.; Lood, R. Common skin bacteria protect their host from oxidative stress through secreted antioxidant RoxP. Sci. Rep. 2019, 9, 3596. [Google Scholar] [CrossRef] [Green Version]
- Kullander, J.; Forslund, O.; Dillner, J. Staphylococcus aureus and Squamous Cell Carcinoma of the Skin. Cancer Epidemiol. Biomark. Prev. 2009, 18, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Madhusudhan, N.; Pausan, M.R.; Halwachs, B.; Durdević, M.; Windisch, M.; Kehrmann, J.; Gorkiewicz, G. Molecular Profiling of Keratinocyte Skin Tumors Links Staphylococcus aureus Overabundance and Increased Human β-Defensin-2 Expression to Growth Promotion of Squamous Cell Carcinoma. Cancers 2020, 12, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, D.L.A.; Lachner, N.; Tan, J.-M.; Tang, S.; Angel, N.; Laino, A.; Linedale, R.; Cao, K.-A.L.; Morrison, M.; Frazer, I.H.; et al. A Natural History of Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Microbiomes. mBio 2018, 9, e01432-18. [Google Scholar] [CrossRef] [Green Version]
- Krueger, A.; Zaugg, J.; Chisholm, S.; Linedale, R.; Lachner, N.; Teoh, S.M.; Tuong, Z.K.; Lukowski, S.W.; Morrison, M.; Soyer, H.P.; et al. Secreted Toxins from Staphylococcus aureus Strains Isolated from Keratinocyte Skin Cancers Mediate Pro-tumorigenic Inflammatory Responses in the Skin. Front. Microbiol. 2022, 12, 789042. [Google Scholar] [CrossRef] [PubMed]
- Reuschenbach, M.; Tran, T.; Faulstich, F.; Hartschuh, W.; Vinokurova, S.; Kloor, M.; Krautkramer, E.; Zeier, M.; Doeberitz, M.V.K.; Sommerer, C. High-risk human papillomavirus in non-melanoma skin lesions from renal allograft recipients and immunocompetent patients. Br. J. Cancer 2011, 104, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 28 March 2022).
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Välikangas, T.; Suomi, T.; Elo, L.L. A comprehensive evaluation of popular proteomics software workflows for label-free proteome quantification and imputation. Briefings Bioinform. 2017, 19, 1344–1355. [Google Scholar] [CrossRef] [Green Version]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Xin, K.; Li, X.; Guo, Y.; Zhong, Y.; Wang, J.; Yang, H.; Zhao, J.; Guo, C.; Huang, Y.; Lei, Z.; et al. A Monochromophoric Approach to Succinct Ratiometric Fluorescent Probes without Probe-Product Crosstalk. CCS Chem. 2021, 3, 2307–2315. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Peschel, A.; Otto, M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.R.; Jiang, J.-H.; Hassan, K.A.; Elbourne, L.D.H.; Tuck, K.L.; Paulsen, I.T.; Peleg, A.Y. Insights on virulence from the complete genome of Staphylococcus capitis. Front. Microbiol. 2015, 6, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.J.; Vuong, L.; Gonzalez, I.S.; Keller, N.; McGrosso, D.; Hwang, J.H.; Nizet, V. Phenol soluble modulin (PSM) variants of community-associated methicillin-resistant Staphylococcus aureus (MRSA) captured using mass spectrometry-based molecular networking. Mol. Cell Proteomics 2014, 13, 1262–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.J.; Okumura, C.Y.; Hollands, A.; Kersten, R.; Akong-Moore, K.; Pence, M.A.; Malone, C.L.; Derieux, J.; Moore, B.S.; Horswill, A.R.; et al. Novel Phenol-soluble Modulin Derivatives in Community-associated Methicillin-resistant Staphylococcus aureus Identified through Imaging Mass Spectrometry. J. Biol. Chem. 2012, 287, 13889–13898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, H.-S.; Cheung, G.Y.; Otto, M. Antimicrobial Activity of Community-associated Methicillin-resistant Staphylococcus aureus Is Caused by Phenol-soluble Modulin Derivatives. J. Biol. Chem. 2011, 286, 8933–8940. [Google Scholar] [CrossRef] [Green Version]
- Pino, L.; Searle, B.; Bollinger, J.G.; Nunn, B.; MacLean, B.; MacCoss, M.J. The Skyline ecosystem: Informatics for quantitative mass spectrometry proteomics. Mass Spectrom. Rev. 2017, 39, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Ziebandt, A.-K.; Kusch, H.; Degner, M.; Jaglitz, S.; Sibbald, M.J.J.B.; Arends, J.P.; Chlebowicz, M.A.; Albrecht, D.; Pantucek, R.; Doškar, J.; et al. Proteomics uncovers extreme heterogeneity in the Staphylococcus aureus exoproteome due to genomic plasticity and variant gene regulation. Proteomics 2010, 10, 1634–1644. [Google Scholar] [CrossRef]
- Ruffell, B.; Affara, N.I.; Cottone, L.; Junankar, S.; Johansson, M.; DeNardo, D.G.; Korets, L.; Reinheckel, T.; Sloane, B.F.; Bogyo, M.; et al. Cathepsin C is a tissue-specific regulator of squamous carcinogenesis. Genes Dev. 2013, 27, 2086–2098. [Google Scholar] [CrossRef] [Green Version]
- Hameetman, L.; Commandeur, S.; Bavinck, J.N.B.; Wisgerhof, H.C.; De Gruijl, F.R.; Willemze, R.; Mullenders, L.; Tensen, C.P.; Vrieling, H. Molecular profiling of cutaneous squamous cell carcinomas and actinic keratoses from organ transplant recipients. BMC Cancer 2013, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [Green Version]
- Kerkelä, E.; Saarialho-Kere, U. Matrix metalloproteinases in tumor progression: Focus on basal and squamous cell skin cancer. Exp. Dermatol. 2003, 12, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Klucky, B.; Mueller, R.; Vogt, I.; Teurich, S.; Hartenstein, B.; Breuhahn, K.; Flechtenmacher, C.; Angel, P.; Hess, J. Kallikrein 6 Induces E-Cadherin Shedding and Promotes Cell Proliferation, Migration, and Invasion. Cancer Res. 2007, 67, 8198–8206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, D.-D.; Xu, D.; Deng, Y.-Y.; Wu, W.-J.; Zhang, J.; Huang, L.; He, L. Identification of key genes in cutaneous squamous cell carcinoma: A transcriptome sequencing and bioinformatics profiling study. Ann. Transl. Med. 2021, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Tuong, Z.K.; Lewandowski, A.; Bridge, J.A.; Cruz, J.L.G.; Yamada, M.; Lambie, D.; Lewandowski, R.; Steptoe, R.J.; Leggatt, G.R.; Simpson, F.; et al. Cytokine/chemokine profiles in squamous cell carcinoma correlate with precancerous and cancerous disease stage. Sci. Rep. 2019, 9, 17754. [Google Scholar] [CrossRef] [Green Version]
- DePianto, D.; Kerns, M.L.; Dlugosz, A.A.; Coulombe, P.A. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat. Genet. 2010, 42, 910–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J. Oxidative DNA damage & repair: An introduction. Free. Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar] [CrossRef]
- Palovcak, A.; Liu, W.; Yuan, F.; Zhang, Y. Maintenance of genome stability by Fanconi anemia proteins. Cell Biosci. 2017, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Deplanche, M.; Mouhali, N.; Nguyen, M.-T.; Cauty, C.; Ezan, F.; Diot, A.; Raulin, L.; Dutertre, S.; Langouet, S.; Legembre, P.; et al. Staphylococcus aureus induces DNA damage in host cell. Sci. Rep. 2019, 9, 7694. [Google Scholar] [CrossRef]
- Chebassier, N.; Leroy, S.; Tenaud, I.; Knol, A.C.; Dreno, B. Overexpression of MMP-2 and MMP-9 in squamous cell carcinomas of immunosuppressed patients. Arch. Dermatol. Res. 2002, 294, 124–126. [Google Scholar] [CrossRef]
- Nauroy, P.; Nyström, A. Kallikreins: Essential epidermal messengers for regulation of the skin microenvironment during homeostasis, repair and disease. Matrix Biol. Plus 2019, 6, 100019. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Nakatsuji, T.; Sanford, J.; Vrbanac, A.F.; Gallo, R.L. Staphylococcus aureus Induces Increased Serine Protease Activity in Keratinocytes. J. Investig. Dermatol. 2016, 137, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darido, C.; Georgy, S.R.; Jane, S.M. The role of barrier genes in epidermal malignancy. Oncogene 2016, 35, 5705–5712. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Chéreau, C.; Raingeaud, J.; Nicco, C.; Weill, B.; Dupin, N.; Batteux, F. Production of Superoxide Anions by Keratinocytes Initiates P. acnes-Induced Inflammation of the Skin. PLoS Pathog. 2009, 5, e1000527. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.R.; Aiassa, V.; Sola, C.; Becerra, M.C. Oxidative stress response in reference and clinical Staphylococcus aureus strains under Linezolid exposure. J. Glob. Antimicrob. Resist. 2020, 22, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., II; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and quantification of nitric oxide–derived oxidants in biological systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef] [Green Version]
- Forsman, H.; Christenson, K.; Bylund, J.; Dahlgren, C. Receptor-Dependent and -Independent Immunomodulatory Effects of Phenol-Soluble Modulin Peptides from Staphylococcus aureus on Human Neutrophils Are Abrogated through Peptide Inactivation by Reactive Oxygen Species. Infect. Immun. 2012, 80, 1987–1995. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, W.; Domann, E.; Chakraborty, T.; Mannala, G.; Lips, K.S.; Heiss, C.; Schnettler, R.; Alt, V. TLR9 mediates S. aureus killing inside osteoblasts via induction of oxidative stress. BMC Microbiol. 2016, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Campos, A.; Clemente-Blanco, A. Cell Cycle and DNA Repair Regulation in the Damage Response: Protein Phosphatases Take Over the Reins. Int. J. Mol. Sci. 2020, 21, 446. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.-T.; Deplanche, M.; Nega, M.; Le Loir, Y.; Peisl, L.; Götz, F.; Berkova, N. Staphylococcus aureus Lpl lipoproteins delay G2/M phase transition in HeLa cells. Front. Cell. Infect. Microbiol. 2016, 6, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deplanche, M.; Filho, R.A.E.A.; Alekseeva, L.; Ladier, E.; Jardin, J.; Henry, G.; Berkova, N. Phenol-soluble modulin α induces G2/M phase transition delay in eukaryotic HeLa cells. FASEB J. 2015, 29, 1950–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodille, E.; Alekseeva, L.; Berkova, N.; Serrier, A.; Badiou, C.; Gilquin, B.; Brun, V.; Vandenesch, F.; Terman, D.S.; Lina, G. Staphylococcal Enterotoxin O Exhibits Cell Cycle Modulating Activity. Front. Microbiol. 2016, 7, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alekseeva, L.; Rault, L.; Almeida, S.; Legembre, P.; Edmond, V.; Azevedo, V.; Berkova, N. Staphylococcus aureus-induced G2/M phase transition delay in host epithelial cells increases bacterial infective efficiency. PLoS ONE 2013, 8, e63279. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, N.M.; 23andMe Research Team; Wang, W.; Furlotte, N.A.; Hinds, D.A.; Bustamante, C.D.; Jorgenson, E.; Asgari, M.M.; Whittemore, A.S. Gene expression imputation identifies candidate genes and susceptibility loci associated with cutaneous squamous cell carcinoma. Nat. Commun. 2018, 9, 4264. [Google Scholar] [CrossRef]
- Rass, K. UV damage and DNA repair in basal cell and squamous cell carcinomas. In Molecular Mechanisms of Basal Cell and Squamous Cell Carcinomas; Springer: Berlin/Heidelberg, Germany, 2006; pp. 18–30. [Google Scholar]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Sharma, V.; Eckels, J.; Schilling, B.; Ludwig, C.; Jaffe, J.D.; MacCoss, M.J.; MacLean, B. Panorama Public: A Public Repository for Quantitative Data Sets Processed in Skyline. Mol. Cell. Proteom. 2018, 17, 1239–1244. [Google Scholar] [CrossRef] [Green Version]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef]

| Variable I | Variable II | Pearson’s R | p-Value |
|---|---|---|---|
| δ-toxin | γH2A.X | 0.94 | <0.0001 |
| PSMα1 | γH2A.X | 0.75 | 0.012 |
| PSMα2 | γH2A.X | 0.77 | 0.0089 |
| PSMα3 | γH2A.X | 0.82 | 0.0038 |
| PSMα4 | γH2A.X | 0.7 | 0.024 |
| δ-toxin | TotalRox | 0.79 | 0.0062 |
| PSMα1 | TotalRox | 0.88 | 0.00089 |
| PSMα2 | TotalRox | 0.88 | 0.00088 |
| PSMα3 | TotalRox | 0.9 | 0.00035 |
| PSMα4 | TotalRox | 0.9 | 0.00033 |
| δ-toxin | DAF2DA | 0.7 | 0.024 |
| PSMα1 | DAF2DA | 0.84 | 0.0026 |
| PSMα2 | DAF2DA | 0.83 | 0.0028 |
| PSMα3 | DAF2DA | 0.83 | 0.0031 |
| PSMα4 | DAF2DA | 0.84 | 0.0022 |
| δ-toxin | DCF | 0.79 | 0.007 |
| PSMα1 | DCF | 0.74 | 0.014 |
| PSMα2 | DCF | 0.76 | 0.012 |
| PSMα3 | DCF | 0.79 | 0.0065 |
| PSMα4 | DCF | 0.78 | 0.0072 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krueger, A.; Mohamed, A.; Kolka, C.M.; Stoll, T.; Zaugg, J.; Linedale, R.; Morrison, M.; Soyer, H.P.; Hugenholtz, P.; Frazer, I.H.; et al. Skin Cancer-Associated S. aureus Strains Can Induce DNA Damage in Human Keratinocytes by Downregulating DNA Repair and Promoting Oxidative Stress. Cancers 2022, 14, 2143. https://doi.org/10.3390/cancers14092143
Krueger A, Mohamed A, Kolka CM, Stoll T, Zaugg J, Linedale R, Morrison M, Soyer HP, Hugenholtz P, Frazer IH, et al. Skin Cancer-Associated S. aureus Strains Can Induce DNA Damage in Human Keratinocytes by Downregulating DNA Repair and Promoting Oxidative Stress. Cancers. 2022; 14(9):2143. https://doi.org/10.3390/cancers14092143
Chicago/Turabian StyleKrueger, Annika, Ahmed Mohamed, Cathryn M. Kolka, Thomas Stoll, Julian Zaugg, Richard Linedale, Mark Morrison, H. Peter Soyer, Philip Hugenholtz, Ian H. Frazer, and et al. 2022. "Skin Cancer-Associated S. aureus Strains Can Induce DNA Damage in Human Keratinocytes by Downregulating DNA Repair and Promoting Oxidative Stress" Cancers 14, no. 9: 2143. https://doi.org/10.3390/cancers14092143
APA StyleKrueger, A., Mohamed, A., Kolka, C. M., Stoll, T., Zaugg, J., Linedale, R., Morrison, M., Soyer, H. P., Hugenholtz, P., Frazer, I. H., & Hill, M. M. (2022). Skin Cancer-Associated S. aureus Strains Can Induce DNA Damage in Human Keratinocytes by Downregulating DNA Repair and Promoting Oxidative Stress. Cancers, 14(9), 2143. https://doi.org/10.3390/cancers14092143







