Characterization of the Peri-Membrane Fluorescence Phenomenon Allowing the Detection of Urothelial Tumor Cells in Urine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cytological Slides
2.2. Cell Line Culture
2.3. Cytological Slides with Cell Line
2.4. Phalloidin Staining
2.5. Quantification Software
2.6. Animals
2.7. Experimental Protocol
2.8. Permeabilization Assays
2.9. Induction of Cellular Stress
2.10. Western Blot
2.11. Cells Death Assay and Flow Cytometry
2.12. Statistical Analysis
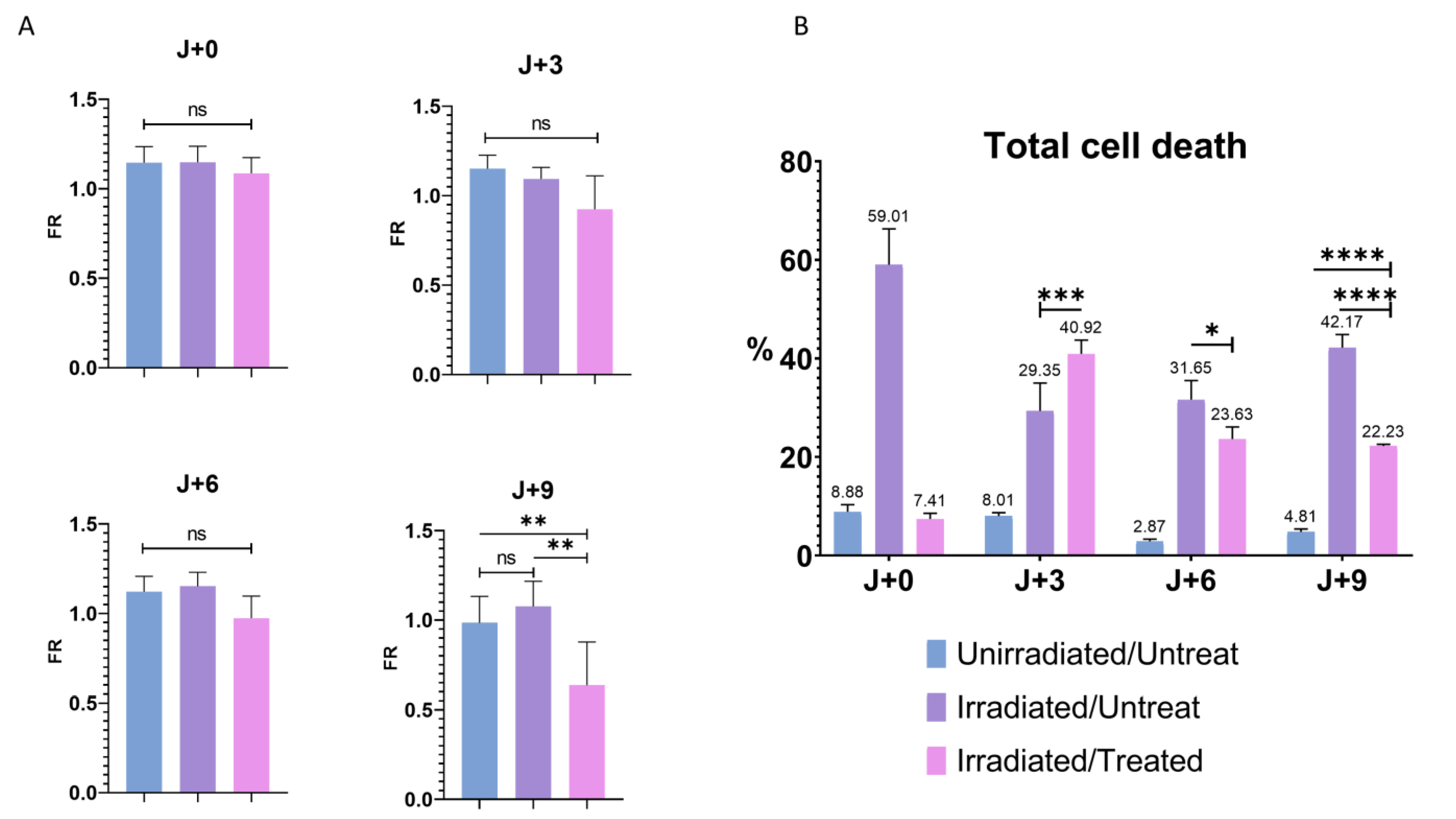
3. Results
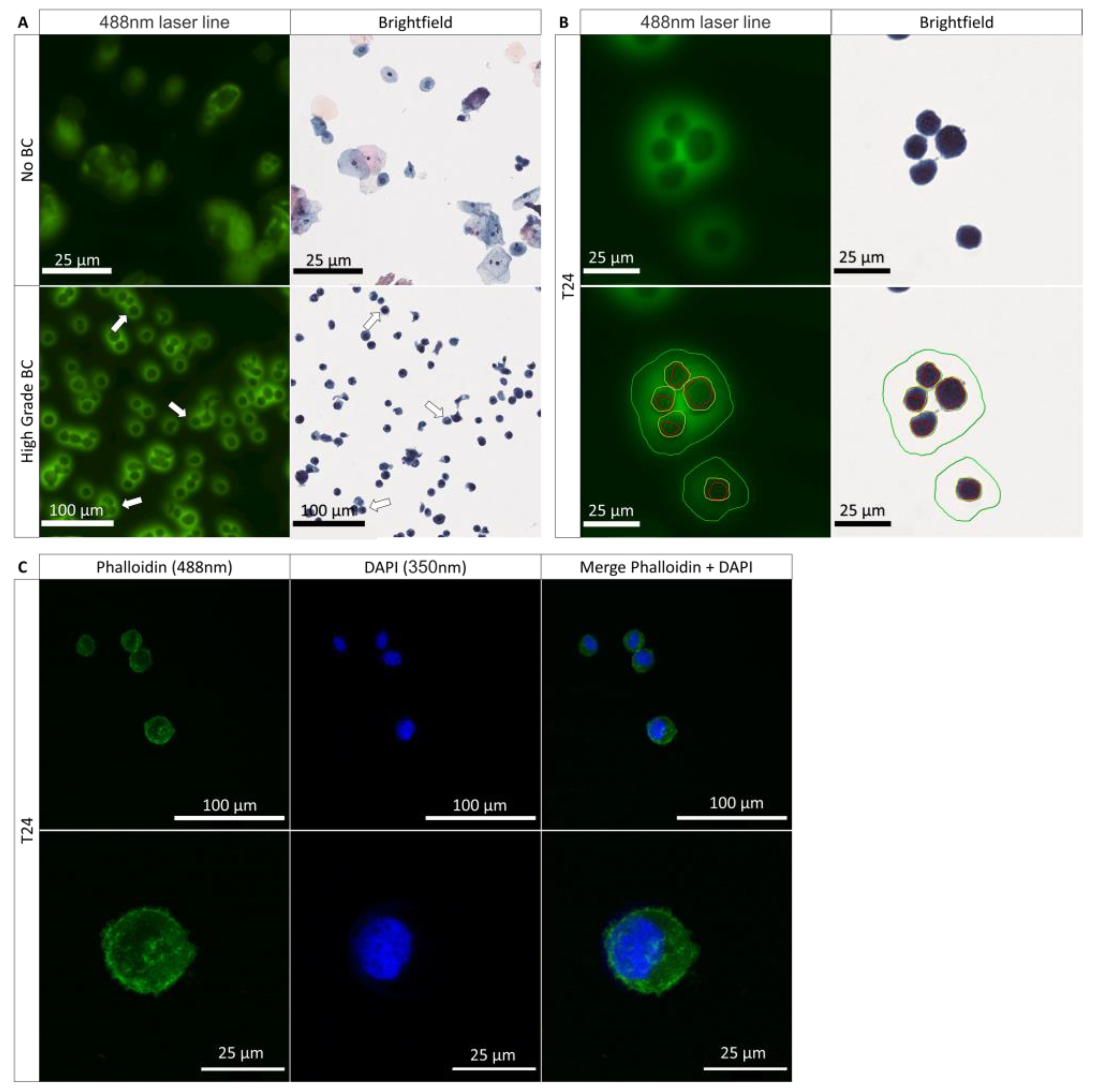
3.1. Role of the Nucleus and the Cytoplasm in the PMF Phenomenon
3.2. PMF Expressed on Cells Line from Various Origins
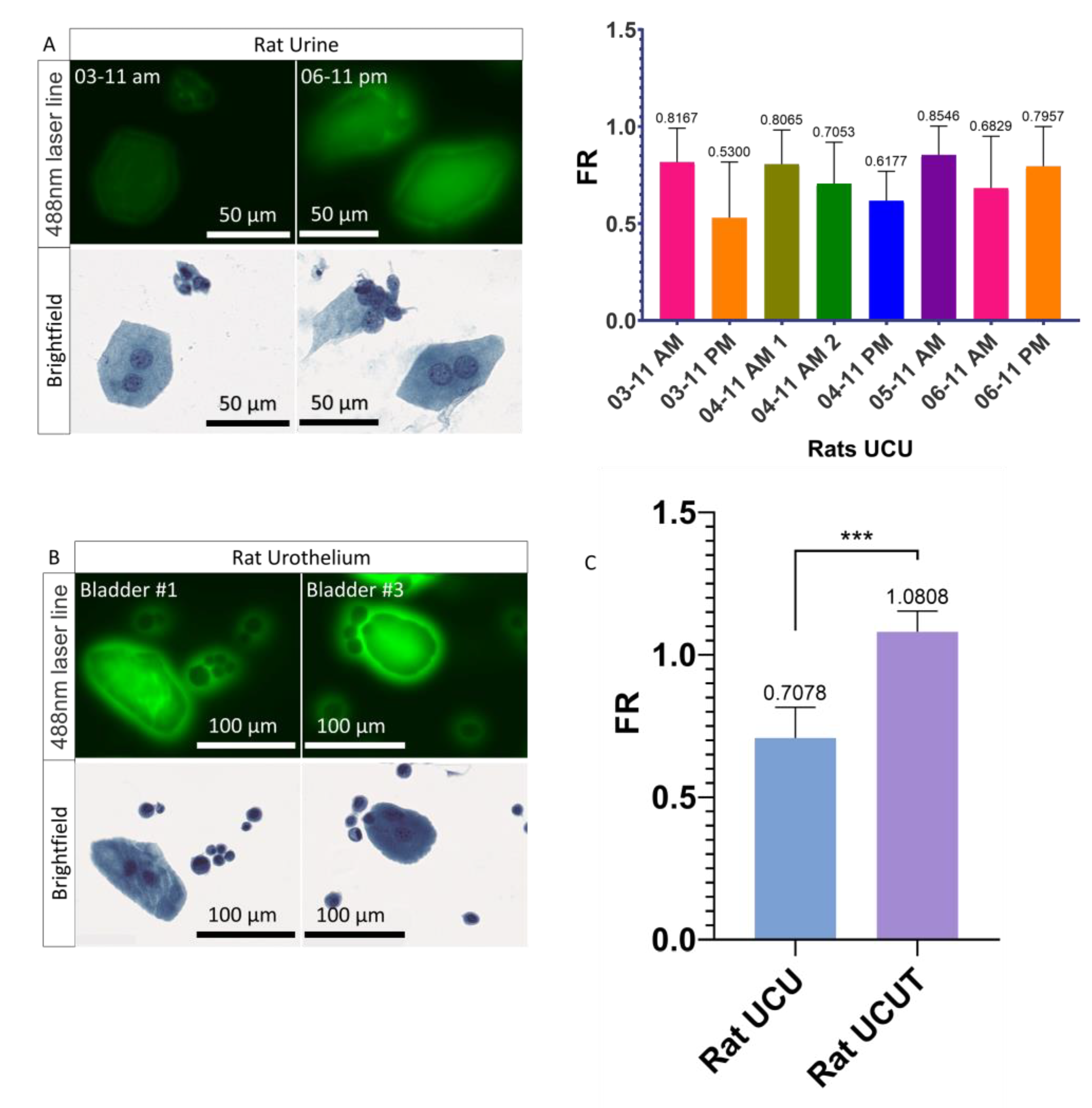
3.3. Urothelial Cells Collected in the Urine versus the Urothelial Cells Collected in the Urothelium
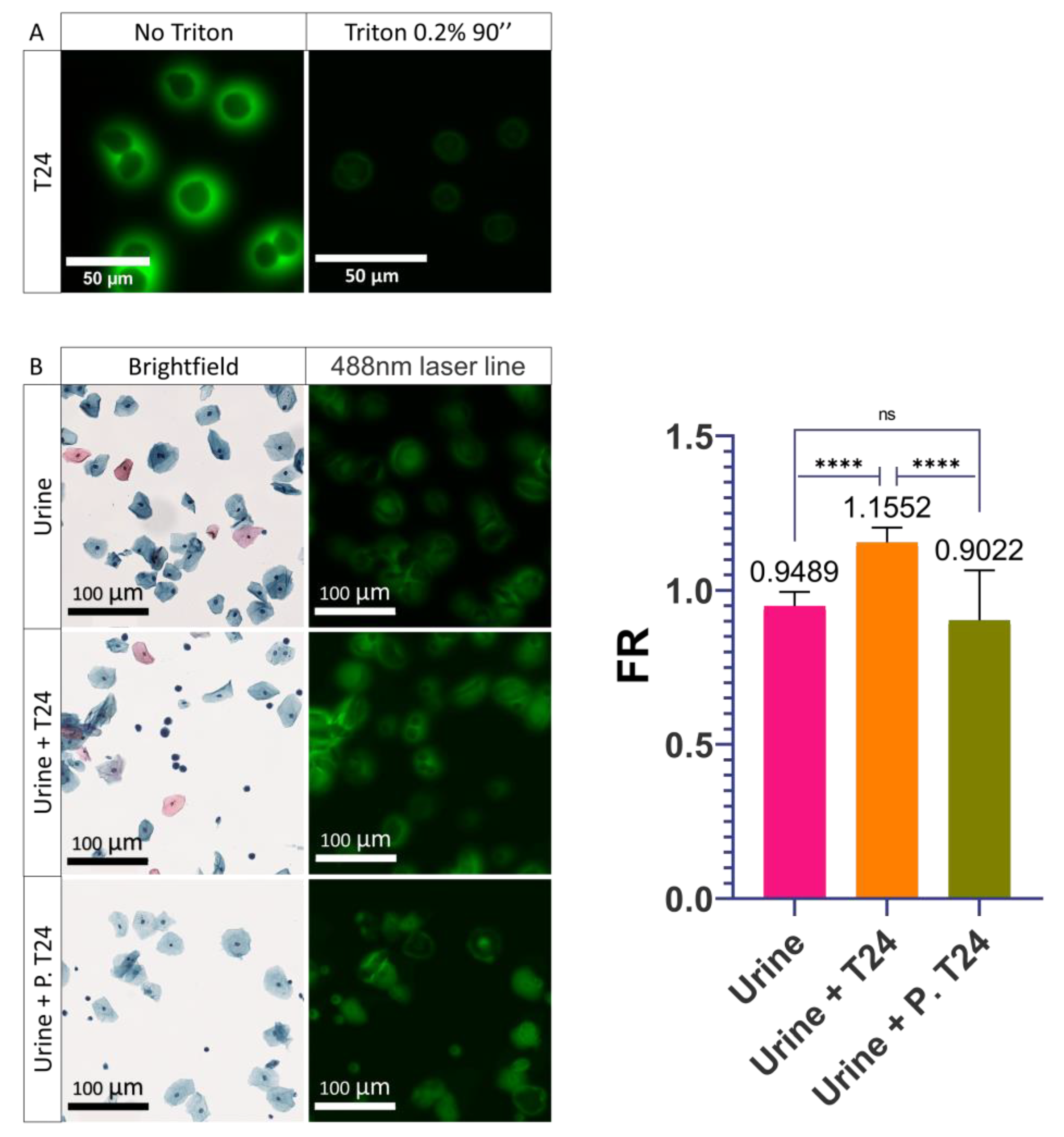
3.4. Role of the Plasma Membrane Integrity on the PMF
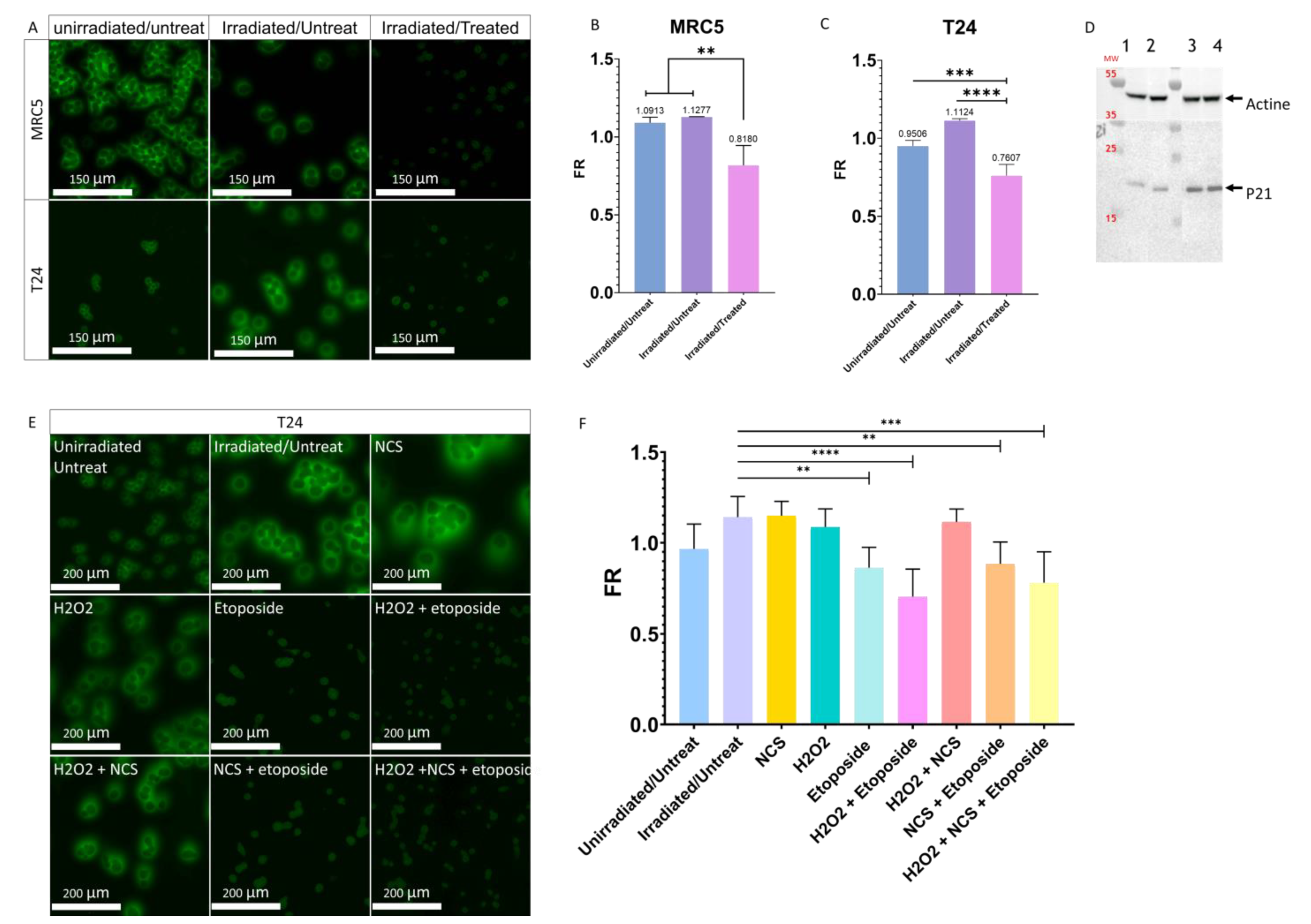
3.5. Influence of the Cellular Stresses on the PMF
3.6. Influence of the Cell Death in the Modulation of PMF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 14 October 2021).
- Barkan, G.A.; Wojcik, E.M.; Nayar, R.; Savic-Prince, S.; Quek, M.L.; Kurtycz, D.F.I.; Rosenthal, D.L. The Paris System for Reporting Urinary Cytology: The Quest to Develop a Standardized Terminology. Acta Cytol. 2016, 60, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, M.; Gutierrez, C.; Guillaudeux, T.; Verhoest, G.; Pedeux, R. Noninvasive Urine-Based Tests to Diagnose or Detect Recurrence of Bladder Cancer. Cancers 2021, 13, 1650. [Google Scholar] [CrossRef] [PubMed]
- Farling, K.B. Bladder Cancer: Risk Factors, Diagnosis, and Management. Nurse Pract. 2017, 42, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Planz, B.; Jochims, E.; Deix, T.; Caspers, H.P.; Jakse, G.; Boecking, A. The Role of Urinary Cytology for Detection of Bladder Cancer. Eur. J. Surg. Oncol. 2005, 31, 304–308. [Google Scholar] [CrossRef]
- Birkó, Z.; Nagy, B.; Klekner, Á.; Virga, J. Novel Molecular Markers in Glioblastoma-Benefits of Liquid Biopsy. Int. J. Mol. Sci. 2020, 21, E7522. [Google Scholar] [CrossRef]
- Pisapia, P.; Malapelle, U.; Troncone, G. Liquid Biopsy and Lung Cancer. Acta Cytol. 2019, 63, 489–496. [Google Scholar] [CrossRef]
- Alimirzaie, S.; Bagherzadeh, M.; Akbari, M.R. Liquid Biopsy in Breast Cancer: A Comprehensive Review. Clin. Genet. 2019, 95, 643–660. [Google Scholar] [CrossRef]
- The Finn Bladder Group; Raitanen, M.-P.; Kaasinen, E.; Rintala, E.; Hansson, E.; Nieminen, P.; Aine, R.; Tammela, T.L.J. Prognostic Utility of Human Complement Factor H Related Protein Test (The BTA Stat®Test). Br. J. Cancer 2001, 85, 552–556. [Google Scholar] [CrossRef]
- Kinders, R.; Jones, T.; Root, R.; Bruce, C.; Murchison, H.; Corey, M.; Williams, L.; Enfield, D.; Hass, G.M. Complement Factor H or a Related Protein Is a Marker for Transitional Cell Cancer of the Bladder. Clin. Cancer Res. 1998, 4, 2511–2520. [Google Scholar]
- Miyanaga, N.; Akaza, H.; Tsukamoto, T.; Ishikawa, S.; Noguchi, R.; Ohtani, M.; Kawabe, K.; Kubota, Y.; Fujita, K.; Obata, K.; et al. Urinary Nuclear Matrix Protein 22 as a New Marker for the Screening of Urothelial Cancer in Patients with Microscopic Hematuria. Int. J. Urol. 1999, 6, 173–177. [Google Scholar] [CrossRef]
- Balci, M.; Tuncel, A.; Guzel, O.; Aslan, Y.; Sezgin, T.; Bilgin, O.; Senel, C.; Atan, A. Use of the Nuclear Matrix Protein 22 Bladder Chek TestTM in the Diagnosis of Residual Urothelial Cancer before a Second Transurethral Resection of Bladder Cancer. Int. Urol. Nephrol. 2015, 47, 473–477. [Google Scholar] [CrossRef]
- Bergeron, A.; LaRue, H.; Fradet, Y. Identification of a Superficial Bladder Tumor-Associated Glycoform of the Carcinoembryonic Antigen by Monoclonal Antibody 19A211. Cancer Res. 1996, 56, 908–915. [Google Scholar]
- Bergeron, A.; Champetier, S.; LaRue, H.; Fradet, Y. MAUB Is a New Mucin Antigen Associated with Bladder Cancer∗. J. Biol. Chem. 1996, 271, 6933–6940. [Google Scholar] [CrossRef]
- Sokolova, I.A.; Halling, K.C.; Jenkins, R.B.; Burkhardt, H.M.; Meyer, R.G.; Seelig, S.A.; King, W. The Development of a Multitarget, Multicolor Fluorescence in Situ Hybridization Assay for the Detection of Urothelial Carcinoma in Urine. J. Mol. Diagn. 2000, 2, 116–123. [Google Scholar] [CrossRef]
- van Valenberg, F.J.P.; Strauss-Ayali, D.; Agmon-Gerstein, Y.; Friedman, A.; Arentsen, H.C.; Schaafsma, H.E.; Witjes, J.A.; Oosterwijk, E. Assessment of the Efficacy of Repeated Instillations of Mitomycin C Mixed with a Thermosensitive Hydrogel in an Orthotopic Rat Bladder Cancer Model. Adv. Urol. 2018, 10, 213–221. [Google Scholar] [CrossRef]
- Pichler, R.; Tulchiner, G.; Fritz, J.; Schaefer, G.; Horninger, W.; Heidegger, I. Urinary UBC Rapid and NMP22 Test for Bladder Cancer Surveillance in Comparison to Urinary Cytology: Results from a Prospective Single-Center Study. Int. J. Med. Sci. 2017, 14, 811–819. [Google Scholar] [CrossRef]
- Shariat, S.F.; Karam, J.A.; Lotan, Y.; Karakiewizc, P.I. Critical Evaluation of Urinary Markers for Bladder Cancer Detection and Monitoring. Rev. Urol. 2008, 10, 120–135. [Google Scholar]
- Oeyen, E.; Hoekx, L.; De Wachter, S.; Baldewijns, M.; Ameye, F.; Mertens, I. Bladder Cancer Diagnosis and Follow-Up: The Current Status and Possible Role of Extracellular Vesicles. Int. J. Mol. Sci. 2019, 20, E821. [Google Scholar] [CrossRef]
- Kassouf, W.; Traboulsi, S.L.; Schmitz-Dräger, B.; Palou, J.; Witjes, J.A.; van Rhijn, B.W.G.; Grossman, H.B.; Kiemeney, L.A.; Goebell, P.J.; Kamat, A.M. Follow-up in Non–Muscle-Invasive Bladder Cancer—International Bladder Cancer Network Recommendations. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 460–468. [Google Scholar] [CrossRef]
- Steenkeste, K.; Lécart, S.; Deniset, A.; Pernot, P.; Eschwège, P.; Ferlicot, S.; Lévêque-Fort, S.; Briandet, R.; Fontaine-Aupart, M.-P. Ex Vivo Fluorescence Imaging of Normal and Malignant Urothelial Cells to Enhance Early Diagnosis. Photochem. Photobiol. 2007, 83, 1157–1166. [Google Scholar] [CrossRef]
- Olivo, M.; Lau, W.; Manivasager, V.; Bhuvaneswari, R.; Wei, Z.; Soo, K.C.; Cheng, C.; Tan, P.H. Novel Photodynamic Diagnosis of Bladder Cancer: Ex Vivo Fluorescence Cytology Using Hypericin. Int. J. Oncol. 2003, 23, 1501–1504. [Google Scholar] [CrossRef]
- Tauber, S.; Schneede, P.; Liedl, B.; Liesmann, F.; Zaak, D.; Hofstetter, A. Fluorescence Cytology of the Urinary Bladder. Urology 2003, 61, 1067–1071. [Google Scholar] [CrossRef]
- McCart, E.A.; Thangapazham, R.L.; Lombardini, E.D.; Mog, S.R.; Panganiban, R.A.M.; Dickson, K.M.; Mansur, R.A.; Nagy, V.; Kim, S.-Y.; Selwyn, R.; et al. Accelerated Senescence in Skin in a Murine Model of Radiation-Induced Multi-Organ Injury. J. Radiat. Res. 2017, 58, 636–646. [Google Scholar] [CrossRef]
- Veranič, P.; Jezernik, K. Succession of Events in Desquamation of Superficial Urothelial Cells as a Response to Stress Induced by Prolonged Constant Illumination. Tissue Cell 2001, 33, 280–285. [Google Scholar] [CrossRef]
- Fabre, M.; Ferlicot, S.; Fontaine Aupart, M.-P.; Steenkeste, K.; Eschwege, P. Method of Detecting Tumour Cells by Fluorescence Signals 2011, France. METHOD OF DETECTING TUMOUR CELLS BY FLUORESCENCE SIGNALS. International Application No.: PCT/EP2010/059324. Publication Number: WO/2011/000894. Publication Date 06.01.2011 World Intellectual Property Organization. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2011000894 (accessed on 13 March 2022).
- Papanicolaou, G.N. A new procedure for staining vaginal smears. Science 1942, 95, 438–439. [Google Scholar] [CrossRef]
- Pedeux, R.; Sengupta, S.; Shen, J.C.; Demidov, O.N.; Saito, S.; Onogi, H.; Kumamoto, K.; Wincovitch, S.; Garfield, S.H.; McMenamin, M.; et al. ING2 Regulates the Onset of Replicative Senescence by Induction of P300-Dependent P53 Acetylation. Mol. Cell. Biol. 2005, 25, 6639–6648. [Google Scholar] [CrossRef]
- NDP.View2.8.24 16/06/2020 Viewing Software, version U12388-01. 2020 Hamamatsu Photonics K.K; Available online: https://www.hamamatsu.com/eu/en/product/type/U12388-01/index.html (accessed on 26 January 2022).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- IDDN Certificate. Available online: https://secure2.iddn.org/app.server/certificate/?sn=2021180016000&key=8b289ffa692106d98093aecd8c7eaf6edcc158f9405c5598d1fd9a5819f1358b&lang=fr (accessed on 26 January 2022).
- Kloskowski, T.; Uzarska, M.; Gurtowska, N.; Olkowska, J.; Joachimiak, R.; Bajek, A.; Gagat, M.; Grzanka, A.; Bodnar, M.; Marszałek, A.; et al. How to Isolate Urothelial Cells? Comparison of Four Different Methods and Literature Review. Hum. Cell 2014, 27, 85–93. [Google Scholar] [CrossRef]
- Correia-Melo, C.; Marques, F.D.M.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria Are Required for Pro-Ageing Features of the Senescent Phenotype. EMBO J. 2016, 35, 724–742. [Google Scholar] [CrossRef]
- Alsibai, K.D.; Daste, G.; Ferlicot, S.; Fabre, M.; Steenkeste, K.; Salleron, J.; Hammoudi, Y.; Fontaine-Aupart, M.-P.; Eschwege, P. Fluorescence Emitted by Papanicolaou-Stained Urothelial Cells Improves Sensitivity of Urinary Conventional Cytology for Detection of Urothelial Tumors. World J. Oncol. 2020, 11, 204–215. [Google Scholar] [CrossRef]
- The Paris System for Reporting Urinary Cytology; Rosenthal, D.L.; Wojcik, E.M.; Kurtycz, D.F.I. (Eds.) Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-22863-1.
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef] [PubMed]
- Yamany, T.; Van Batavia, J.; Mendelsohn, C. Formation and Regeneration of the Urothelium. Curr. Opin. Organ Transplant. 2014, 19, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Malagobadan, S.; Nagoor, N.H. Anoikis. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2017; pp. 75–84. ISBN 978-0-12-801238-3. [Google Scholar]
- Gilmore, A.P. Anoikis. Cell Death Differ. 2005, 12, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma Membrane Changes during Programmed Cell Deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Tekpli, X.; Holme, J.A.; Sergent, O.; Lagadic-Gossmann, D. Role for Membrane Remodeling in Cell Death: Implication for Health and Disease. Toxicology 2013, 304, 141–157. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple Functions of P21 in Cell Cycle, Apoptosis and Transcriptional Regulation after DNA Damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Karpinich, N.O.; Tafani, M.; Rothman, R.J.; Russo, M.A.; Farber, J.L. The Course of Etoposide-Induced Apoptosis from Damage to DNA and P53 Activation to Mitochondrial Release of Cytochromec. J. Biol. Chem. 2002, 277, 16547–16552. [Google Scholar] [CrossRef]
- van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-Affinity Assay: A Review on an Apoptosis Detection System Based on Phosphatidylserine Exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Sloan, F.A.; Yashkin, A.P.; Akushevich, I.; Inman, B.A. The Cost to Medicare of Bladder Cancer Care. Eur. Urol. Oncol. 2020, 3, 515–522. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The Global Burden of Urinary Bladder Cancer: An Update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Ng, K.; Stenzl, A.; Sharma, A.; Vasdev, N. Urinary Biomarkers in Bladder Cancer: A Review of the Current Landscape and Future Directions. Urol. Oncol. 2021, 39, 41–51. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Ting, H.-N.; Ng, K.-H.; Ong, T.-A. A Review on the Accuracy of Bladder Cancer Detection Methods. J. Cancer 2019, 10, 4038–4044. [Google Scholar] [CrossRef]
- McClintock, G.; Wong, E.; Mancuso, P.; Lalak, N.; Gassner, P.; Haghighi, K.; Rathore, P.; McAulay, L.; Jeffery, N. Music during Flexible Cystoscopy for Pain and Anxiety—A Patient-Blinded Randomised Control Trial. BJU Int. 2021, 128 (Suppl. S1), 27–32. [Google Scholar] [CrossRef]
- Schrag, D.; Hsieh, L.J.; Rabbani, F.; Bach, P.B.; Herr, H.; Begg, C.B. Adherence to Surveillance among Patients with Superficial Bladder Cancer. J. Natl. Cancer Inst. 2003, 95, 588–597. [Google Scholar] [CrossRef]
- Poropatich, K.; Yang, J.C.; Goyal, R.; Parini, V.; Yang, X.J. Nuclear Size Measurement for Distinguishing Urothelial Carcinomas from Reactive Urothelium on Tissue Sections. Diagn. Pathol. 2016, 11, 57. [Google Scholar] [CrossRef][Green Version]
- Bailey, R.W.; Nguyen, T.; Robertson, L.; Gibbons, E.; Nelson, J.; Christensen, R.E.; Bell, J.P.; Judd, A.M.; Bell, J.D. Sequence of Physical Changes to the Cell Membrane During Glucocorticoid-Induced Apoptosis in S49 Lymphoma Cells. Biophys. J. 2009, 96, 2709–2718. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis Molecular Pathways and Its Role in Cancer Progression. Biochim. Biophys. Acta 2013, 1833, 3481–3498. [Google Scholar] [CrossRef]
- Simpson, C.D.; Anyiwe, K.; Schimmer, A.D. Anoikis Resistance and Tumor Metastasis. Cancer Lett. 2008, 272, 177–185. [Google Scholar] [CrossRef]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Mutoh, M. Induction of Anoikis in Human Colorectal Cancer Cells by Fucoxanthinol. Nutr. Cancer 2017, 69, 1043–1052. [Google Scholar] [CrossRef]
- Furse, S.; de Kroon, A.I.P.M. Phosphatidylcholine’s Functions beyond That of a Membrane Brick. Mol. Membr. Biol. 2015, 32, 117–119. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, C.; Pinson, X.; Jarnouen, K.; Charpentier, M.; Pineau, R.; Lallement, L.; Pedeux, R. Characterization of the Peri-Membrane Fluorescence Phenomenon Allowing the Detection of Urothelial Tumor Cells in Urine. Cancers 2022, 14, 2171. https://doi.org/10.3390/cancers14092171
Gutierrez C, Pinson X, Jarnouen K, Charpentier M, Pineau R, Lallement L, Pedeux R. Characterization of the Peri-Membrane Fluorescence Phenomenon Allowing the Detection of Urothelial Tumor Cells in Urine. Cancers. 2022; 14(9):2171. https://doi.org/10.3390/cancers14092171
Chicago/Turabian StyleGutierrez, Charly, Xavier Pinson, Kathleen Jarnouen, Marine Charpentier, Raphael Pineau, Laëtitia Lallement, and Rémy Pedeux. 2022. "Characterization of the Peri-Membrane Fluorescence Phenomenon Allowing the Detection of Urothelial Tumor Cells in Urine" Cancers 14, no. 9: 2171. https://doi.org/10.3390/cancers14092171
APA StyleGutierrez, C., Pinson, X., Jarnouen, K., Charpentier, M., Pineau, R., Lallement, L., & Pedeux, R. (2022). Characterization of the Peri-Membrane Fluorescence Phenomenon Allowing the Detection of Urothelial Tumor Cells in Urine. Cancers, 14(9), 2171. https://doi.org/10.3390/cancers14092171





