Surgery after Neoadjuvant Chemotherapy: A Clip-Based Technique to Improve Surgical Outcomes, a Single-Center Experience
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Indications to Breast and Axillary Conservative Management
1.2. Different Marking Techniques
2. Materials and Methods
2.1. Selection of Patients
2.2. Choice of Tissue Marker
2.3. Pre-Operative Evaluation
- No clinical axillary involvement at diagnosis: sentinel lymph node biopsy after NACT was performed.
- Clinical axillary involvement at diagnosis and still evident after NACT: axillary dissection was performed.
- Clinical axillary involvement at diagnosis with a radiological response after NACT: modified sentinel lymph nodes biopsy was performed (based on the retrieval of at least 3 lymph nodes, identified with a dual tracer technique, and the excision of the clipped lymph node—which was biopsied with positive results before NACT).
3. Results
3.1. Population
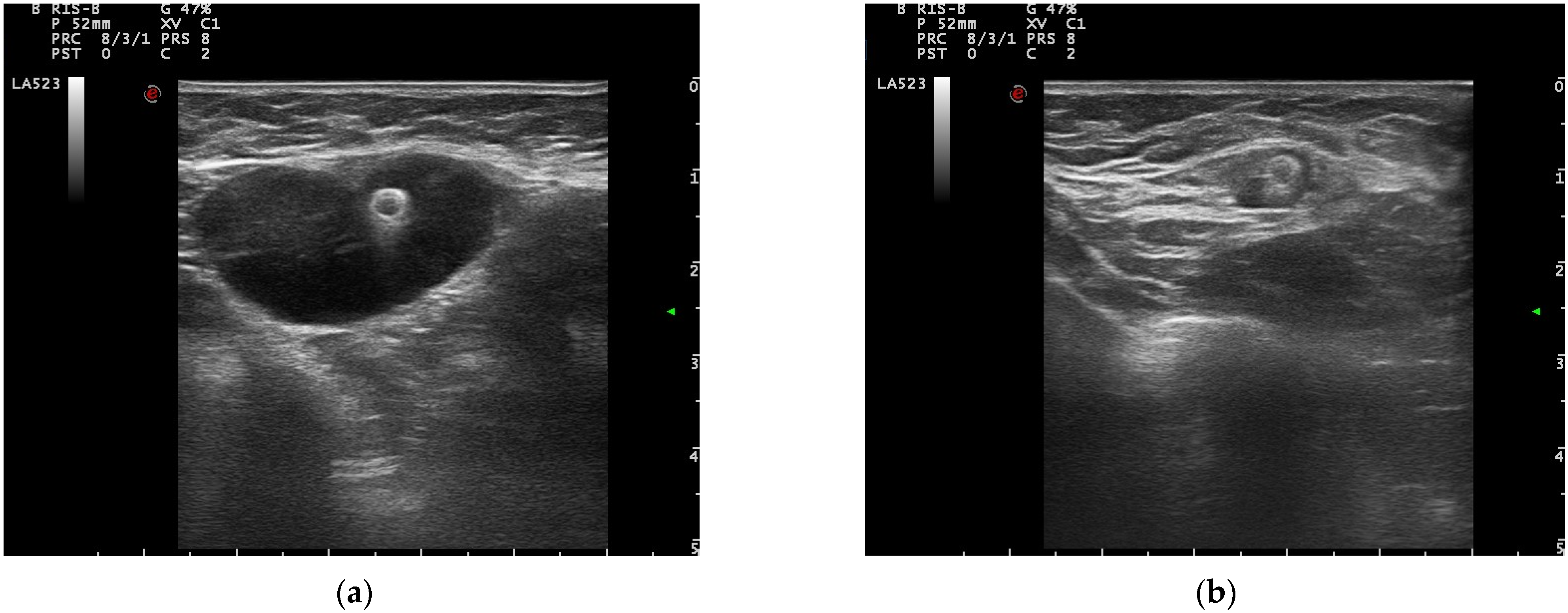
3.2. Pre-Operative Clip Visibility, Localization, and Surgical Management
4. Discussion
4.1. Selection of Patients
4.2. Clip Positioning and Visibility
4.3. Pre-Operative Evaluation and Surgical Management
5. Limitations and Strengths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Age Standardized (World) Incidence Rates, Breast, All Ages., in Breast Cancer, Globocan, WHO 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf (accessed on 21 December 2021).
- Breast-Cancer Screening—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2015, 373, 1478–1479. [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.Gov Website. Available online: https://Clinicaltrials.Gov/Ct2/Show/NCT02945579.2017 (accessed on 7 January 2022).
- Rüland, A.M.; Hagemann, F.; Reinisch, M.; Holtschmidt, J.; Kümmel, A.; Dittmer-Grabowski, C.; Stöblen, F.; Rotthaus, H.; Dreesmann, V.; Blohmer, J.-U.; et al. Using a New Marker Clip System in Breast Cancer: Tumark Vision® Clip-Feasibility Testing in Everyday Clinical Practice. Breast Care 2018, 13, 114–118. [Google Scholar] [CrossRef]
- Oh, J.L.; Nguyen, G.; Whitman, G.J.; Hunt, K.K.; Yu, T.-K.; Woodward, W.A.; Tereffe, W.; Strom, E.A.; Perkins, G.H.; Buchholz, T.A. Placement of Radiopaque Clips for Tumor Localization in Patients Undergoing Neoadjuvant Chemotherapy and Breast Conservation Therapy. Cancer 2007, 110, 2420–2427. [Google Scholar] [CrossRef] [Green Version]
- Rashmi Kumar, N.; Burns, J.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Blair, S.L.; Burstein, H.J.; Chew, H.; et al. NCCN Guidelines Version 2.2022 Breast Cancer; National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients with Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Boughey, J.C.; Ballman, K.V.; Le-Petross, H.T.; McCall, L.M.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Feliberti, E.C.; Hunt, K.K. Identification and Resection of Clipped Node Decreases the False-Negative Rate of Sentinel Lymph Node Surgery in Patients Presenting with Node-Positive Breast Cancer (T0–T4, N1–N2) Who Receive Neoadjuvant Chemotherapy. Ann. Surg. 2016, 263, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Mamtani, A.; Barrio, A.V.; King, T.A.; van Zee, K.J.; Plitas, G.; Pilewskie, M.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.S.; Sclafani, L.M.; et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients with Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann. Surg. Oncol. 2016, 23, 3467–3474. [Google Scholar] [CrossRef] [Green Version]
- Banys-Paluchowski, M.; Gasparri, M.; de Boniface, J.; Gentilini, O.; Stickeler, E.; Hartmann, S.; Thill, M.; Rubio, I.; di Micco, R.; Bonci, E.-A.; et al. Surgical Management of the Axilla in Clinically Node-Positive Breast Cancer Patients Converting to Clinical Node Negativity through Neoadjuvant Chemotherapy: Current Status, Knowledge Gaps, and Rationale for the EUBREAST-03 AXSANA Study. Cancers 2021, 13, 1565. [Google Scholar] [CrossRef]
- Choy, N.; Lipson, J.; Porter, C.; Ozawa, M.; Kieryn, A.; Pal, S.; Kao, J.; Trinh, L.; Wheeler, A.; Ikeda, D.; et al. Initial Results with Preoperative Tattooing of Biopsied Axillary Lymph Nodes and Correlation to Sentinel Lymph Nodes in Breast Cancer Patients. Ann. Surg. Oncol. 2015, 22, 377–382. [Google Scholar] [CrossRef]
- Patel, R.; MacKerricher, W.; Tsai, J.; Choy, N.; Lipson, J.; Ikeda, D.; Pal, S.; de Martini, W.; Allison, K.H.; Wapnir, I.L. Pretreatment Tattoo Marking of Suspicious Axillary Lymph Nodes: Reliability and Correlation with Sentinel Lymph Node. Ann. Surg. Oncol. 2019, 26, 2452–2458. [Google Scholar] [CrossRef]
- Donker, M.; Straver, M.E.; Wesseling, J.; Loo, C.E.; Schot, M.; Drukker, C.A.; van Tinteren, H.; Sonke, G.S.; Rutgers, E.J.; Vrancken Peeters, M.-J.T.F.D. Marking Axillary Lymph Nodes with Radioactive Iodine Seeds for Axillary Staging After Neoadjuvant Systemic Treatment in Breast Cancer Patients. Ann. Surg. 2015, 261, 378–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitsamer, R.; Peintinger, F.; Forsthuber, E.; Sir, A. The Applicability of Magseed® for Targeted Axillary Dissection in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Breast 2021, 57, 113–117. [Google Scholar] [CrossRef]
- Woods, R.W.; Camp, M.S.; Durr, N.J.; Harvey, S.C. A Review of Options for Localization of Axillary Lymph Nodes in the Treatment of Invasive Breast Cancer. Acad. Radiol. 2019, 26, 805–819. [Google Scholar] [CrossRef]
- Murthy, V.; Young, J.; Tokumaru, Y.; Quinn, M.; Edge, S.B.; Takabe, K. Options to Determine Pathological Response of Axillary Lymph Node Metastasis after Neoadjuvant Chemotherapy in Advanced Breast Cancer. Cancers 2021, 13, 4167. [Google Scholar] [CrossRef]
- Carmon, M.; Olsha, O.; Gekhtman, D.; Nikitin, I.; Cohen, Y.; Messing, M.; Lioubashevsky, N.; Abu Dalo, R.; Hadar, T.; Golomb, E. Detectability of Hygroscopic Clips Used in Breast Cancer Surgery. J. Ultrasound Med. 2017, 36, 401–408. [Google Scholar] [CrossRef]
- Koo, J.H.; Kim, E.-K.; Moon, H.J.; Yoon, J.H.; Park, V.Y.; Kim, M.J. Comparison of Breast Tissue Markers for Tumor Localization in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Ultrasonography 2019, 38, 336–344. [Google Scholar] [CrossRef]
- Kim, E.Y.; Byon, W.S.; Lee, K.H.; Yun, J.-S.; Park, Y.L.; Park, C.H.; Youn, I.Y.; Choi, S.H.; Choi, Y.J.; Kook, S.H.; et al. Feasibility of Preoperative Axillary Lymph Node Marking with a Clip in Breast Cancer Patients Before Neoadjuvant Chemotherapy: A Preliminary Study. World J. Surg. 2018, 42, 582–589. [Google Scholar] [CrossRef]
- Kuemmel, S.; Heil, J.; Rueland, A.; Seiberling, C.; Harrach, H.; Schindowski, D.; Lubitz, J.; Hellerhoff, K.; Ankel, C.; Graßhoff, S.-T.; et al. A Prospective, Multicenter Registry Study to Evaluate the Clinical Feasibility of Targeted Axillary Dissection (TAD) in Node-Positive Breast Cancer Patients. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Malter, W.; Eichler, C.; Hanstein, B.; Mallmann, P.; Holtschmidt, J. First Reported Use of Radiofrequency Identification (RFID) Technique for Targeted Excision of Suspicious Axillary Lymph Nodes in Early Stage Breast Cancer–Evaluation of Feasibility and Review of Current Recommendations. In Vivo 2020, 34, 1207–1213. [Google Scholar] [CrossRef]
- Pinkney, D.M.; Mychajlowycz, M.; Shah, B.A. A Prospective Comparative Study to Evaluate the Displacement of Four Commercially Available Breast Biopsy Markers. Br. J. Radiol. 2016, 89, 20160149. [Google Scholar] [CrossRef] [Green Version]
- Youn, I.; Choi, S.H.; Kook, S.H.; Choi, Y.J.; Park, C.H.; Park, Y.L.; Kim, D.H. Ultrasonography-Guided Surgical Clip Placement for Tumor Localization in Patients Undergoing Neoadjuvant Chemotherapy for Breast Cancer. J. Breast Cancer 2015, 18, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz-Wendtland, R.; Dankerl, P.; Bani, M.; Fasching, P.; Heusinger, K.; Lux, M.; Jud, S.; Rauh, C.; Bayer, C.; Schrauder, M.; et al. Evaluation of a Marker Clip System in Sonographically Guided Core Needle Biopsy for Breast Cancer Localization Before and After Neoadjuvant Chemotherapy. Geburtshilfe Frauenheilkd 2017, 77, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Reimer, T.; Gerber, B.; Stubert, J.; Stengel, B.; Stachs, A. Wire Localization of Clip-Marked Axillary Lymph Nodes in Breast Cancer Patients Treated with Primary Systemic Therapy. Eur. J. Surg. Oncol. 2018, 44, 1307–1311. [Google Scholar] [CrossRef]
- del Mastro, L. LINEE GUIDA AIOM 2021 Neoplasie Della Mammella; Associazione Italiana di Oncologia Medica: Milan, Italy, 2021. [Google Scholar]
- UltraCor Twirl clip BARD ® Breast Tissue Marker. Available online: https://www.bd.com/assets/documents/PDH/BD_BARD_BAW1464200R0-UltraCor-Twirl-IFU_EN.pdf (accessed on 23 March 2020).
- Garetto, A.; Garrone, O.; Lauro, C.; Montemurro, F.; Zanon, E.; Abbona, G.; Ala, A.; Bagnato, R.; Barone, C.; Bruna, P.; et al. Intervallo Ottimale Tra La Fine Della Chemioterapia Neoadiuvante Ed Intervento Chirurgico Gruppo Di Studio Sui Tumori Della Mammella Version 1; Rete Oncologica di Piemonte e Valle d’Aosta: Turin, Italy, 2018. [Google Scholar]
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [Green Version]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Pinkney, D.M.; Shah, B.A. Prospective Comparative Study to Evaluate the Sonographic Visibility of Five Commercially Available Breast Biopsy Markers. J. Diagn. Med. Sonogr. 2013, 29, 151–158. [Google Scholar] [CrossRef]
- Portnow, L.H.; Kwak, E.; Senapati, G.M.; Kwait, D.C.; Denison, C.M.; Giess, C.S. Ultrasound Visibility of Select Breast Biopsy Markers for Targeted Axillary Node Localization Following Neoadjuvant Treatment: Simulation Using Animal Tissue Models. Breast Cancer Res. Treat. 2020, 184, 185–192. [Google Scholar] [CrossRef]
- Lim, G.H.; Teo, S.Y.; Gudi, M.; Ng, R.P.; Pang, J.; Tan, Y.S.; Lee, Y.S.; Allen, J.C.; Leong, L.C.H. Initial Results of a Novel Technique of Clipped Node Localization in Breast Cancer Patients Postneoadjuvant Chemotherapy: Skin Mark Clipped Axillary Nodes Removal Technique (SMART Trial). Cancer Med. 2020, 9, 1978–1985. [Google Scholar] [CrossRef]
- Plecha, D.; Bai, S.; Patterson, H.; Thompson, C.; Shenk, R. Improving the Accuracy of Axillary Lymph Node Surgery in Breast Cancer with Ultrasound-Guided Wire Localization of Biopsy Proven Metastatic Lymph Nodes. Ann. Surg. Oncol. 2015, 22, 4241–4246. [Google Scholar] [CrossRef]
- Weber, J.J.; Jochelson, M.S.; Eaton, A.; Zabor, E.C.; Barrio, A.V.; Gemignani, M.L.; Pilewskie, M.; van Zee, K.J.; Morrow, M.; El-Tamer, M. MRI and Prediction of Pathologic Complete Response in the Breast and Axilla after Neoadjuvant Chemotherapy for Breast Cancer. J. Am. Coll. Surg. 2017, 225, 740–746. [Google Scholar] [CrossRef]
- Houssami, N.; Macaskill, P.; von Minckwitz, G.; Marinovich, M.L.; Mamounas, E. Meta-Analysis of the Association of Breast Cancer Subtype and Pathologic Complete Response to Neoadjuvant Chemotherapy. Eur. J. Cancer 2012, 48, 3342–3354. [Google Scholar] [CrossRef]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-Term Outcomes for Neoadjuvant versus Adjuvant Chemotherapy in Early Breast Cancer: Meta-Analysis of Individual Patient Data from Ten Randomised Trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, V.; Ribeiro Fontana, S.K.; Maisonneuve, P.; Steccanella, F.; Vento, A.R.; Intra, M.; Naninato, P.; Caldarella, P.; Iorfida, M.; Colleoni, M.; et al. Sentinel Node Biopsy after Neoadjuvant Treatment in Breast Cancer: Five-Year Follow-up of Patients with Clinically Node-Negative or Node-Positive Disease before Treatment. Eur. J. Surg. Oncol. 2016, 42, 361–368. [Google Scholar] [CrossRef]
- Flores-Funes, D.; Aguilar-Jiménez, J.; Martínez-Gálvez, M.; Ibáñez-Ibáñez, M.J.; Carrasco-González, L.; Gil-Izquierdo, J.I.; Chaves-Benito, M.A.; Ayala-De La Peña, F.; Nieto-Olivares, A.; Aguayo-Albasini, J.L. Validation of the Targeted Axillary Dissection Technique in the Axillary Staging of Breast Cancer after Neoadjuvant Therapy: Preliminary Results. Surg. Oncol. 2019, 30, 52–57. [Google Scholar] [CrossRef]
- Gentile, L.F.; Plitas, G.; Zabor, E.C.; Stempel, M.; Morrow, M.; Barrio, A.V. Tumor Biology Predicts Pathologic Complete Response to Neoadjuvant Chemotherapy in Patients Presenting with Locally Advanced Breast Cancer. Ann. Surg. Oncol. 2017, 24, 3896–3902. [Google Scholar] [CrossRef]


| Breast + axillary clips | 30 (54.5%) |
| Only in breast | 19 (34.5%) |
| Only in lymph node | 6 (11%) |
| Clips placed in breast lesions | 34 (61.8%) |
| Clips placed in lymph nodes | 21 (38.2%) |
| Total number of clips | 55 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minella, C.; Villasco, A.; D’Alonzo, M.; Cellini, L.; Accomasso, F.; Actis, S.; Biglia, N. Surgery after Neoadjuvant Chemotherapy: A Clip-Based Technique to Improve Surgical Outcomes, a Single-Center Experience. Cancers 2022, 14, 2229. https://doi.org/10.3390/cancers14092229
Minella C, Villasco A, D’Alonzo M, Cellini L, Accomasso F, Actis S, Biglia N. Surgery after Neoadjuvant Chemotherapy: A Clip-Based Technique to Improve Surgical Outcomes, a Single-Center Experience. Cancers. 2022; 14(9):2229. https://doi.org/10.3390/cancers14092229
Chicago/Turabian StyleMinella, Carola, Andrea Villasco, Marta D’Alonzo, Lisa Cellini, Francesca Accomasso, Silvia Actis, and Nicoletta Biglia. 2022. "Surgery after Neoadjuvant Chemotherapy: A Clip-Based Technique to Improve Surgical Outcomes, a Single-Center Experience" Cancers 14, no. 9: 2229. https://doi.org/10.3390/cancers14092229






