Labile Heme and Heme Oxygenase-1 Maintain Tumor-Permissive Niche for Endometriosis-Associated Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Material
2.2. IHC and Analysis
2.3. Mouse Model of ID8 Metastatic Ovarian Cancer
2.4. Real-Time PCR
2.5. Cell Culture and Treatments
2.6. Reagents
2.7. Soft Agar Colony Assay
2.8. Benzidine Staining
2.9. Immunoblotting
2.10. Geo Profiles
2.11. Statistics
3. Results
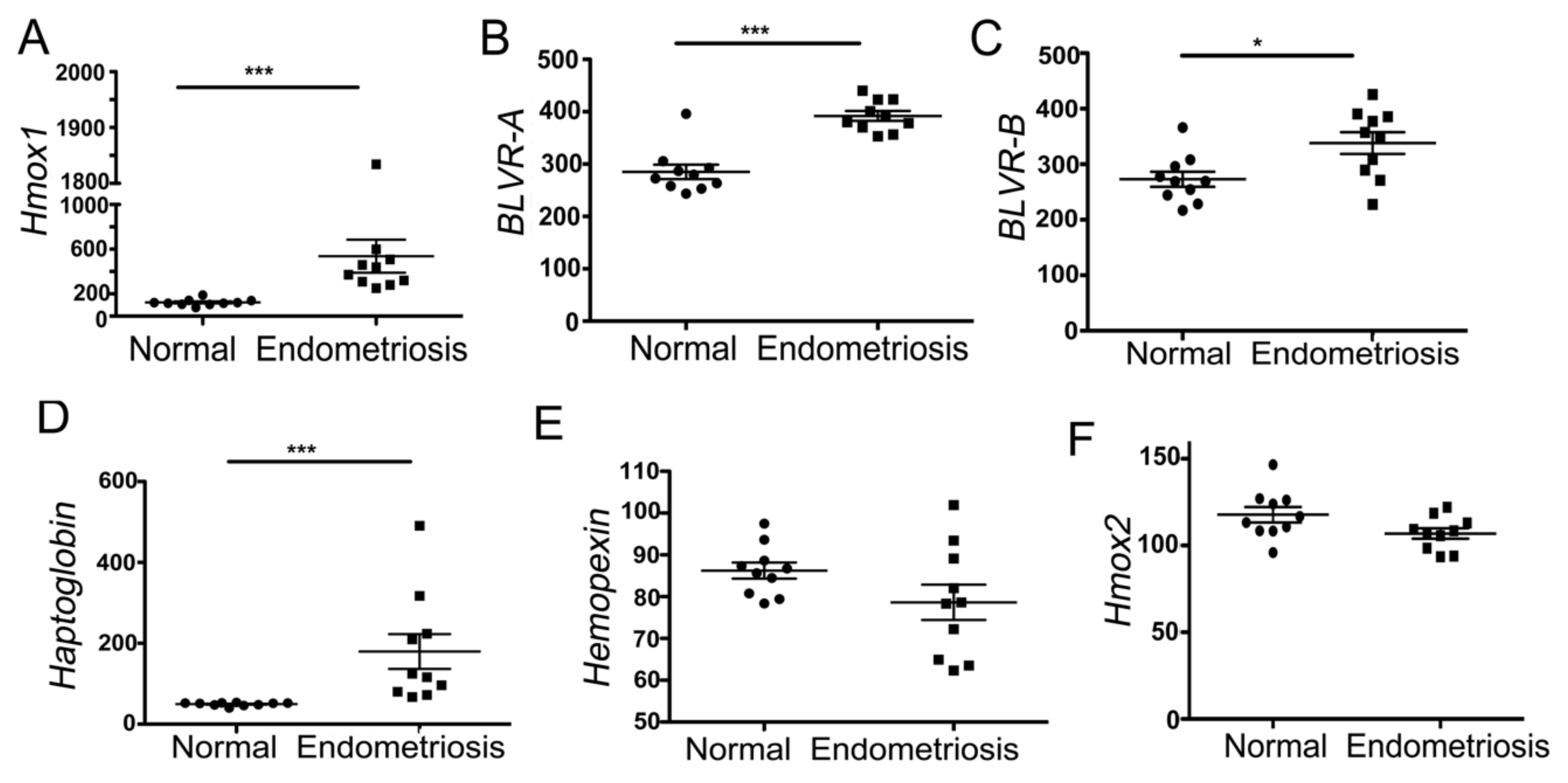
3.1. Heme-Associated Scavenging Proteins Are Elevated in Endometriosis
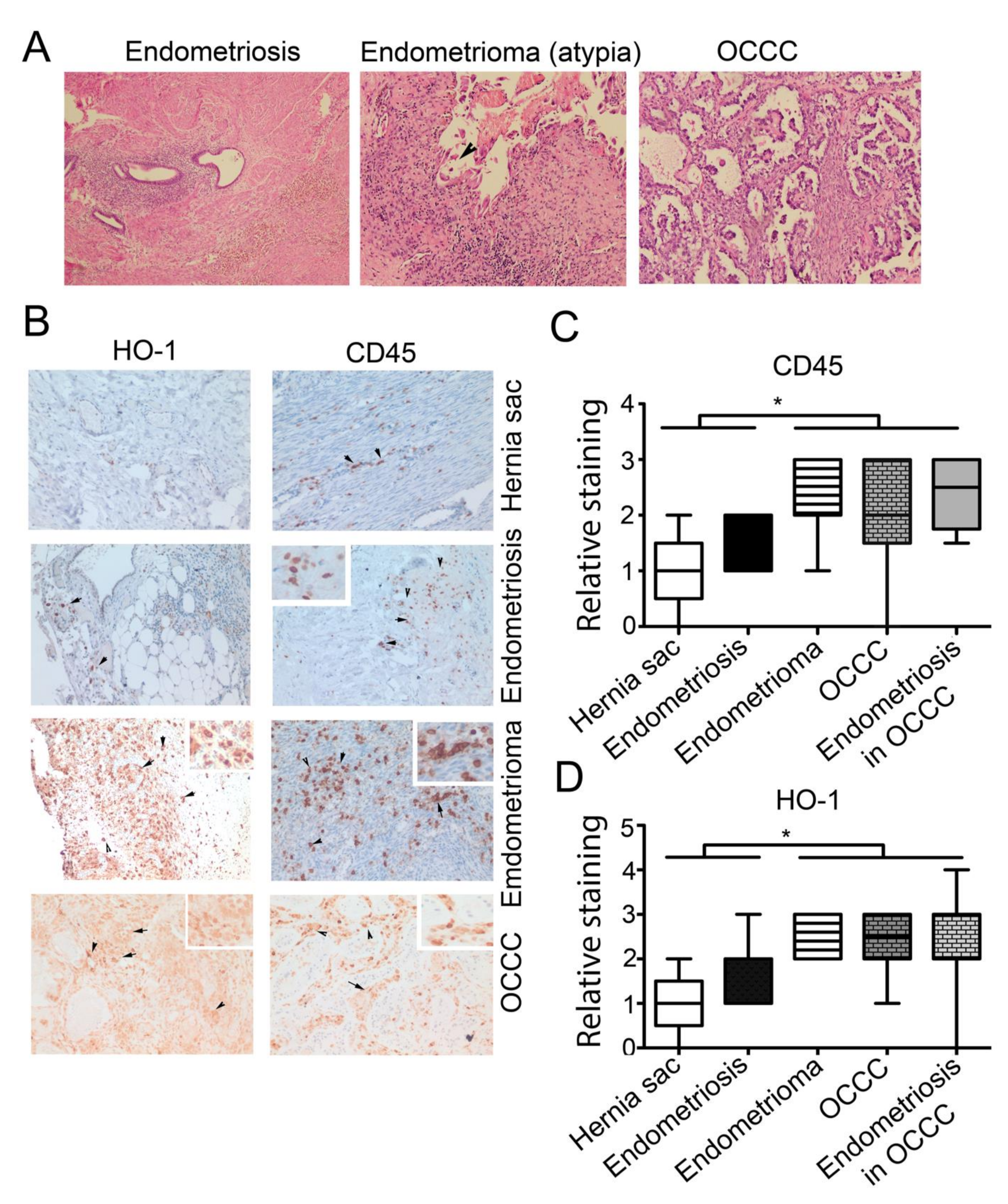
3.2. High Expression of CD45+ and HO-1+ Cells Infiltration in Endometrioma and Associated EAOC
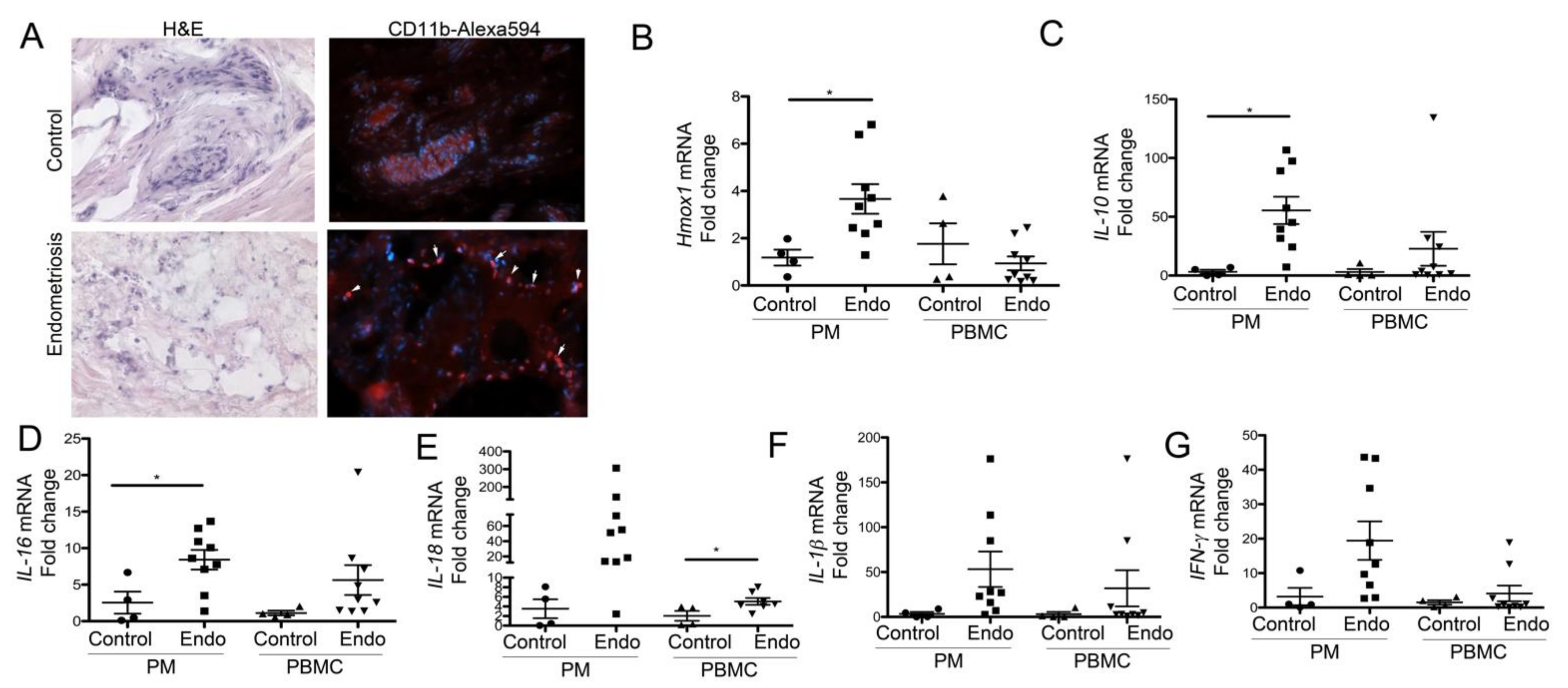
3.3. Peritoneal Macrophages from Women with Endometriosis Express High Levels of HO-1 and IL-10, Suggesting a Stromal M2-Like Phenotype
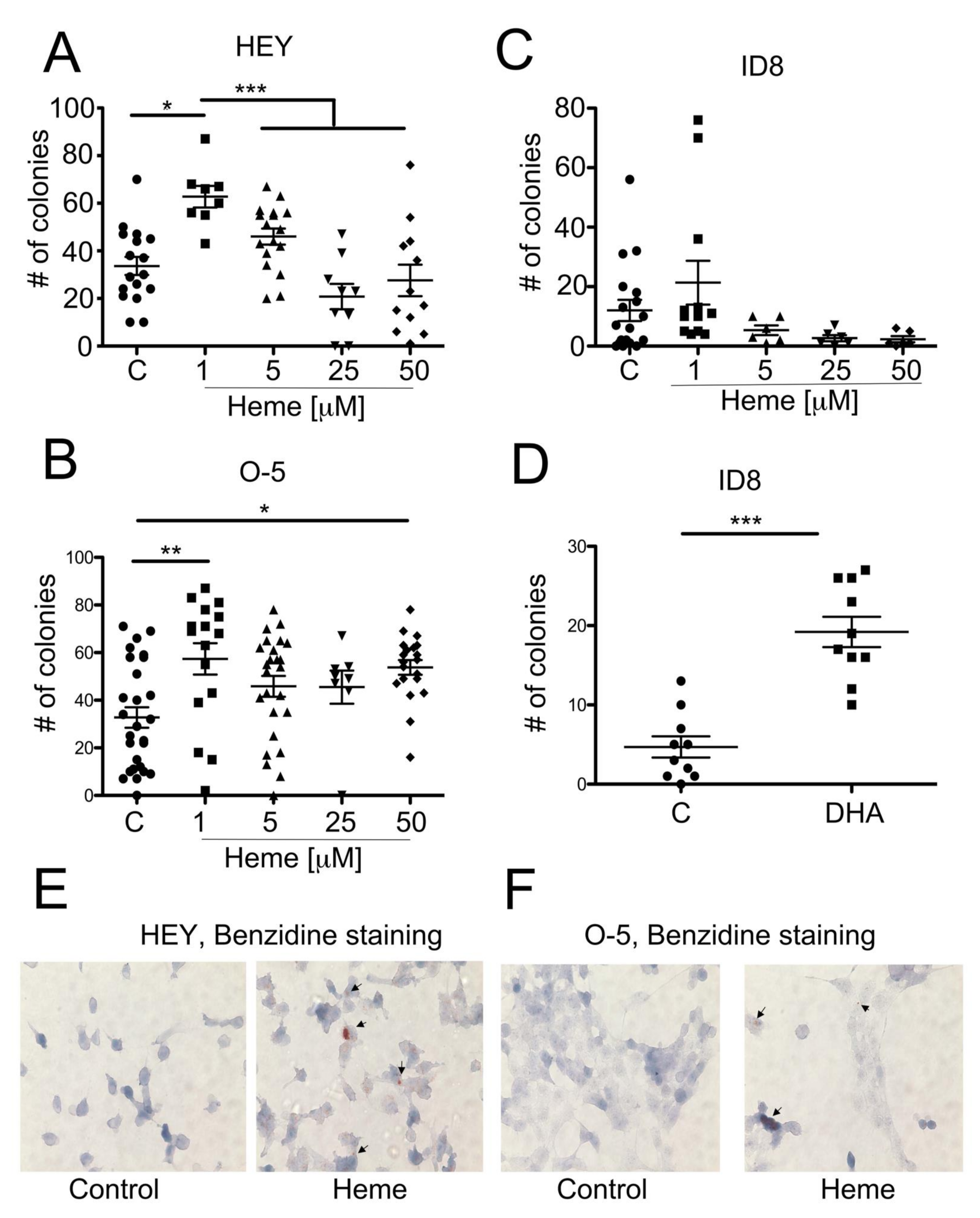
3.4. Heme Modulates the Growth of Ovarian Cancer Colonies in Soft Agar
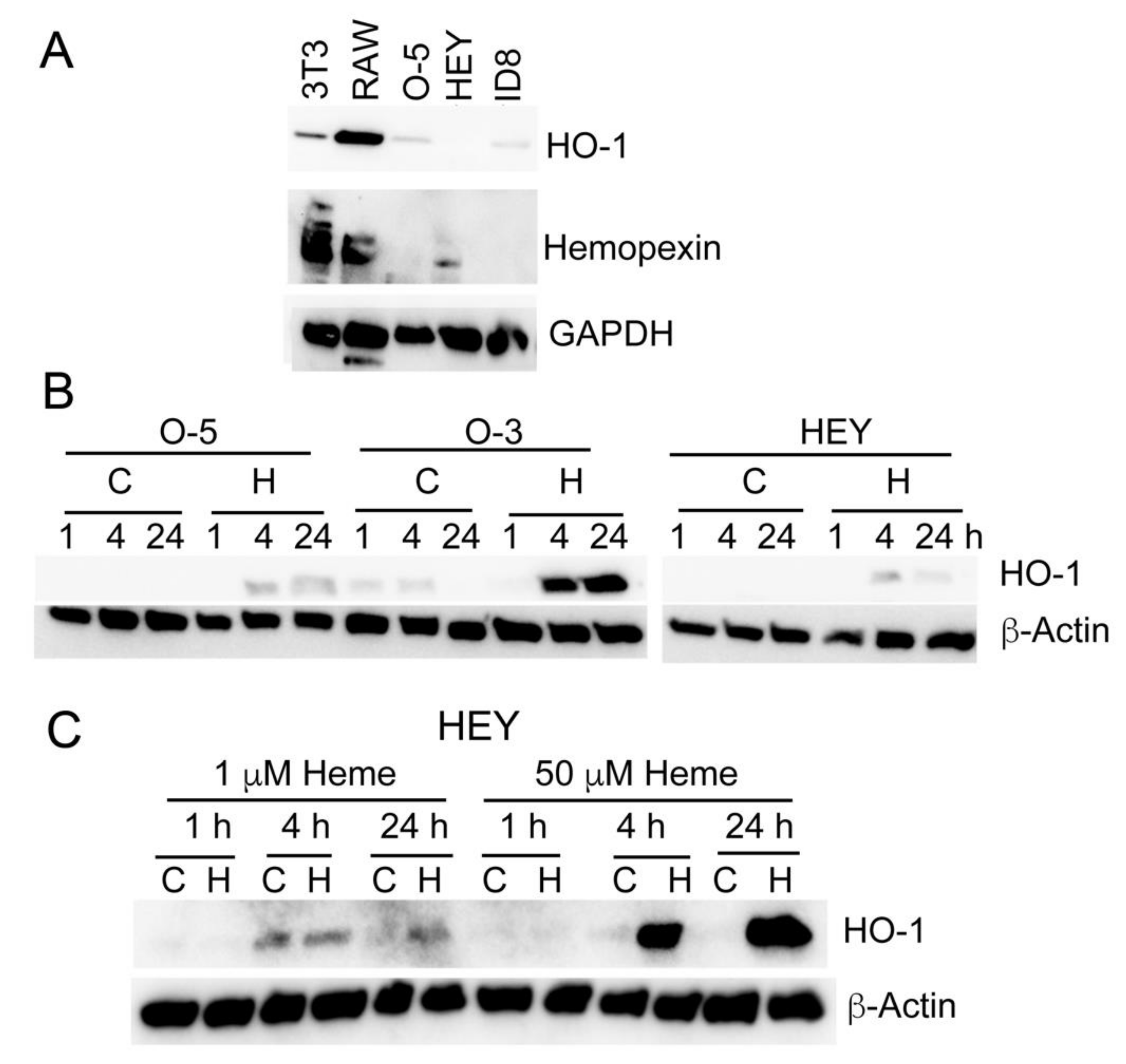
3.5. Heme Induces HO-1 in Ovarian Cancer Cell Lines
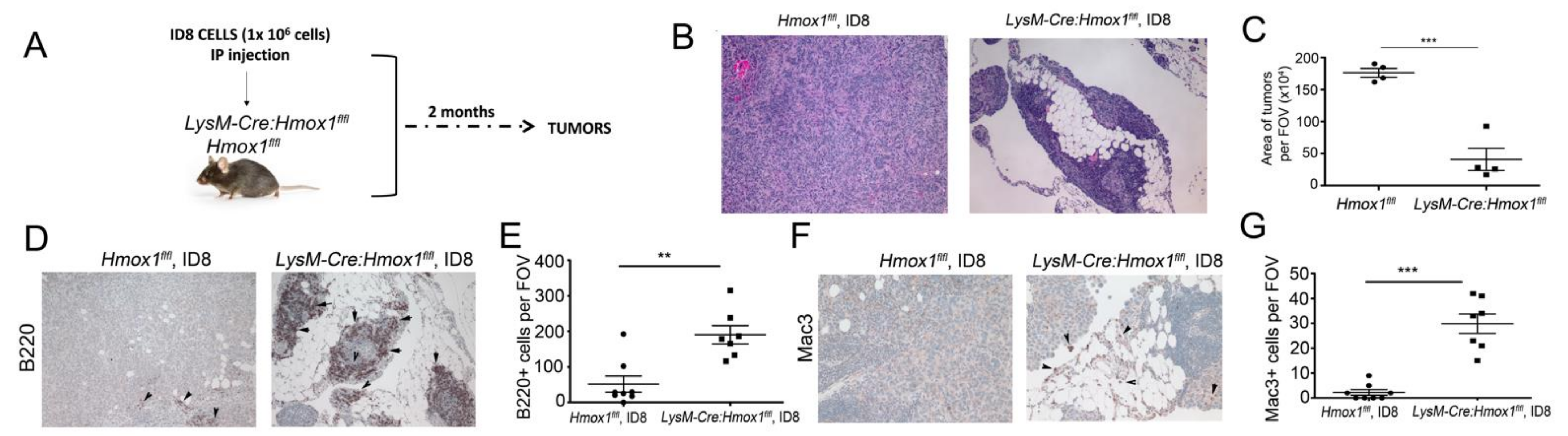
3.6. Lack of HO-1 in Macrophages Results in Attenuated Growth of ID8 Tumors In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lac, V.; Nazeran, T.M.; Tessier-Cloutier, B.; Aguirre-Hernandez, R.; Albert, A.; Lum, A.; Khattra, J.; Praetorius, T.; Mason, M.; Chiu, D.; et al. Oncogenic mutations in histologically normal endometrium: The new normal? J. Pathol. 2019, 249, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Munksgaard, P.S.; Blaakaer, J. The association between endometriosis and ovarian cancer: A review of histological, genetic and molecular alterations. Gynecol. Oncol. 2012, 124, 164–169. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H. Potential scenarios leading to ovarian cancer arising from endometriosis. Redox Rep. 2016, 21, 119–126. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil. Steril. 2002, 78, 712–718. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [Green Version]
- Canesin, G.; Hejazi, S.M.; Swanson, K.D.; Wegiel, B. Heme-Derived Metabolic Signals Dictate Immune Responses. Front. Immunol. 2020, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Canesin, G.; Di Ruscio, A.; Li, M.; Ummarino, S.; Hedblom, A.; Choudhury, R.; Krzyzanowska, A.; Csizmadia, E.; Palominos, M.; Stiehm, A.; et al. Scavenging of Labile Heme by Hemopexin Is a Key Checkpoint in Cancer Growth and Metastases. Cell Rep. 2020, 32, 108181. [Google Scholar] [CrossRef]
- Wegiel, B.; Gallo, D.; Csizmadia, E.; Harris, C.; Belcher, J.; Vercellotti, G.M.; Penacho, N.; Seth, P.; Sukhatme, V.; Ahmed, A.; et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013, 73, 7009–7021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacca, P.; Meiss, R.; Casas, G.; Mazza, O.; Calvo, J.C.; Navone, N.; Vazquez, E. Nuclear translocation of haeme oxygenase-1 is associated to prostate cancer. Br. J. Cancer 2007, 97, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Barbagallo, I.; Giallongo, C.; La Cava, P.; Parrinello, N.; Vanella, L.; Stagno, F.; Palumbo, G.A.; Li Volti, G.; Di Raimondo, F. Nuclear translocation of heme oxygenase-1 confers resistance to imatinib in chronic myeloid leukemia cells. Curr. Pharm. Des. 2013, 19, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F.; Yeh, C.T.; Sun, Y.J.; Chiang, M.T.; Lan, W.M.; Li, F.A.; Lee, W.H.; Chau, L.Y. Signal peptide peptidase-mediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene 2015, 34, 2410–2411. [Google Scholar] [CrossRef]
- Lin, Q.; Weis, S.; Yang, G.; Weng, Y.H.; Helston, R.; Rish, K.; Smith, A.; Bordner, J.; Polte, T.; Gaunitz, F.; et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007, 282, 20621–20633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Sumimoto, K.; Moniwa, N.; Imai, M.; Takakura, K.; Kuromaki, T.; Morioka, E.; Arisawa, K.; Terao, T. Risk of developing ovarian cancer among women with ovarian endometrioma: A cohort study in Shizuoka, Japan. Int. J. Gynecol. Cancer 2007, 17, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Rovere-Querini, P. Endometriosis, a disease of the macrophage. Front. Immunol. 2013, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Wolfler, M.M.; Meinhold-Heerlein, I.M.; Henkel, C.; Rath, W.; Neulen, J.; Maass, N.; Brautigam, K. Reduced hemopexin levels in peritoneal fluid of patients with endometriosis. Fertil. Steril. 2013, 100, 777–781. [Google Scholar] [CrossRef]
- Tai, C.S.; Lin, Y.R.; Teng, T.H.; Lin, P.Y.; Tu, S.J.; Chou, C.H.; Huang, Y.R.; Huang, W.C.; Weng, S.L.; Huang, H.D.; et al. Haptoglobin expression correlates with tumor differentiation and five-year overall survival rate in hepatocellular carcinoma. PLoS ONE 2017, 12, e0171269. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nouso, K.; Kinugasa, H.; Takeuchi, Y.; Tomoda, T.; Miyahara, K.; Hagihara, H.; Kuwaki, K.; Onishi, H.; Nakamura, S.; et al. Clinical utility of serum fucosylated hemopexin in Japanese patients with hepatocellular carcinoma. Hepatol. Res. 2012, 42, 1187–1195. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.K.; Lai, Y.H.; Chu, Y.; Lee, M.C.; Huang, C.Y.; Wu, S. Haptoglobin is a serological biomarker for adenocarcinoma lung cancer by using the ProteomeLab PF2D combined with mass spectrometry. Am. J. Cancer Res. 2016, 6, 1828–1836. [Google Scholar] [PubMed]
- Bisht, K.; Canesin, G.; Cheytan, T.; Li, M.; Nemeth, Z.; Csizmadia, E.; Woodruff, T.M.; Stec, D.E.; Bulmer, A.C.; Otterbein, L.E.; et al. Deletion of Biliverdin Reductase A in Myeloid Cells Promotes Chemokine Expression and Chemotaxis in Part via a Complement C5a--C5aR1 Pathway. J. Immunol. 2019, 202, 2982–2990. [Google Scholar] [CrossRef]
- Hedblom, A.; Hejazi, S.M.; Canesin, G.; Choudhury, R.; Hanafy, K.A.; Csizmadia, E.; Persson, J.L.; Wegiel, B. Heme detoxification by heme oxygenase-1 reinstates proliferative and immune balances upon genotoxic tissue injury. Cell Death Dis. 2019, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Hever, A.; Roth, R.B.; Hevezi, P.; Marin, M.E.; Acosta, J.A.; Acosta, H.; Rojas, J.; Herrera, R.; Grigoriadis, D.; White, E.; et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc. Natl. Acad. Sci. USA 2007, 104, 12451–12456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otterbein, L.E.; Soares, M.P.; Yamashita, K.; Bach, F.H. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003, 24, 449–455. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Mitsopoulou, A.; Iliopoulou, S.M.; Daniilidis, A.; Samartzis, E.P.; Economopoulos, K.P. Association between endometriosis and gynecological cancers: A critical review of the literature. Arch. Gynecol. Obstet. 2020, 301, 355–367. [Google Scholar] [CrossRef]
- Grandi, G.; Toss, A.; Cortesi, L.; Botticelli, L.; Volpe, A.; Cagnacci, A. The Association between Endometriomas and Ovarian Cancer: Preventive Effect of Inhibiting Ovulation and Menstruation during Reproductive Life. Biomed. Res. Int. 2015, 2015, 751571. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.H.; Khalaj, K.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Immune-inflammation gene signatures in endometriosis patients. Fertil. Steril. 2016, 106, 1420–1431.e7. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Yan, Y.; Liu, Z.; Wang, Y. Inflammation and endometriosis. Front. Biosci (Landmark Ed.) 2016, 21, 941–948. [Google Scholar]
- Leenen, S.; Hermens, M.; de Vos van Steenwijk, P.J.; Bekkers, R.L.M.; van Esch, E.M.G. Immunologic factors involved in the malignant transformation of endometriosis to endometriosis-associated ovarian carcinoma. Cancer Immunol. Immunother. 2021, 70, 1821–1829. [Google Scholar] [CrossRef]
- Wendel, J.R.H.; Wang, X.; Hawkins, S.M. The Endometriotic Tumor Microenvironment in Ovarian Cancer. Cancers 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, K.; Osuga, Y.; Yoshino, O.; Hirota, Y.; Yano, T.; Tsutsumi, O.; Taketani, Y. Elevated interleukin-16 levels in the peritoneal fluid of women with endometriosis may be a mechanism for inflammatory reactions associated with endometriosis. Fertil. Steril. 2005, 83, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Nakamura, K.; Kageyama, S.; Lawal, A.O.; Gong, K.W.; Bhetraratana, M.; Fujii, T.; Sulaiman, D.; Hirao, H.; Bolisetty, S.; et al. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight 2018, 3, e120596. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. B cells and macrophages in cancer: Yin and yang. Nat. Med. 2011, 17, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Kikutani, H.; Kishimoto, T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc. Natl. Acad. Sci. USA 1994, 91, 2305–2309. [Google Scholar] [CrossRef] [Green Version]
- Deshane, J.; Chen, S.; Caballero, S.; Grochot-Przeczek, A.; Was, H.; Li Calzi, S.; Lach, R.; Hock, T.D.; Chen, B.; Hill-Kapturczak, N.; et al. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J. Exp. Med. 2007, 204, 605–618. [Google Scholar] [CrossRef]
- Watanabe-Matsui, M.; Muto, A.; Matsui, T.; Itoh-Nakadai, A.; Nakajima, O.; Murayama, K.; Yamamoto, M.; Ikeda-Saito, M.; Igarashi, K. Heme regulates B-cell differentiation, antibody class switch, and heme oxygenase-1 expression in B cells as a ligand of Bach2. Blood 2011, 117, 5438–5448. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.M.; Dafferner, A.J.; O'Connell, K.A.; Mehla, K.; Britigan, B.E.; Hollingsworth, M.A.; Abdalla, M.Y. Heme Oxygenase-1 Inhibition Potentiates the Effects of Nab-Paclitaxel-Gemcitabine and Modulates the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 2264. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Spentzos, D.; Fountzilas, E.; Francoeur, N.; Sanisetty, S.; Grammatikos, A.P.; Hecht, J.L.; Cannistra, S.A. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011, 71, 5081–5089. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hecht, J.L.; Janikova, M.; Choudhury, R.; Liu, F.; Canesin, G.; Janovicova, L.; Csizmadia, E.; Jorgensen, E.M.; Esselen, K.M.; Celec, P.; et al. Labile Heme and Heme Oxygenase-1 Maintain Tumor-Permissive Niche for Endometriosis-Associated Ovarian Cancer. Cancers 2022, 14, 2242. https://doi.org/10.3390/cancers14092242
Hecht JL, Janikova M, Choudhury R, Liu F, Canesin G, Janovicova L, Csizmadia E, Jorgensen EM, Esselen KM, Celec P, et al. Labile Heme and Heme Oxygenase-1 Maintain Tumor-Permissive Niche for Endometriosis-Associated Ovarian Cancer. Cancers. 2022; 14(9):2242. https://doi.org/10.3390/cancers14092242
Chicago/Turabian StyleHecht, Jonathan L., Monika Janikova, Reeham Choudhury, Fong Liu, Giacomo Canesin, Lubica Janovicova, Eva Csizmadia, Elisa M. Jorgensen, Katharine M. Esselen, Peter Celec, and et al. 2022. "Labile Heme and Heme Oxygenase-1 Maintain Tumor-Permissive Niche for Endometriosis-Associated Ovarian Cancer" Cancers 14, no. 9: 2242. https://doi.org/10.3390/cancers14092242
APA StyleHecht, J. L., Janikova, M., Choudhury, R., Liu, F., Canesin, G., Janovicova, L., Csizmadia, E., Jorgensen, E. M., Esselen, K. M., Celec, P., Swanson, K. D., & Wegiel, B. (2022). Labile Heme and Heme Oxygenase-1 Maintain Tumor-Permissive Niche for Endometriosis-Associated Ovarian Cancer. Cancers, 14(9), 2242. https://doi.org/10.3390/cancers14092242







