The SARS-CoV-2 Pandemic and Cancer Trials Ireland: Impact, Resolution and Legacy †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
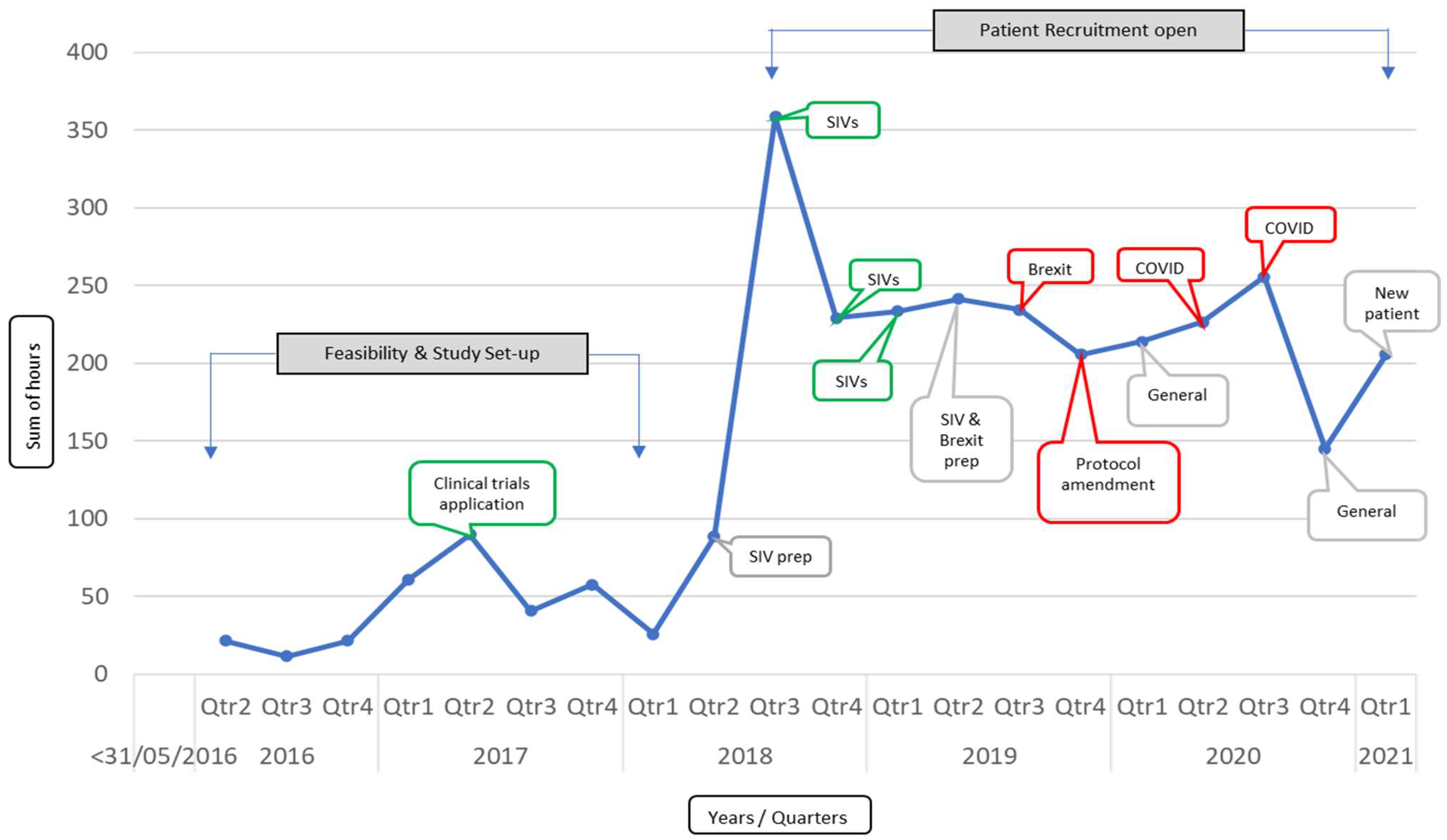
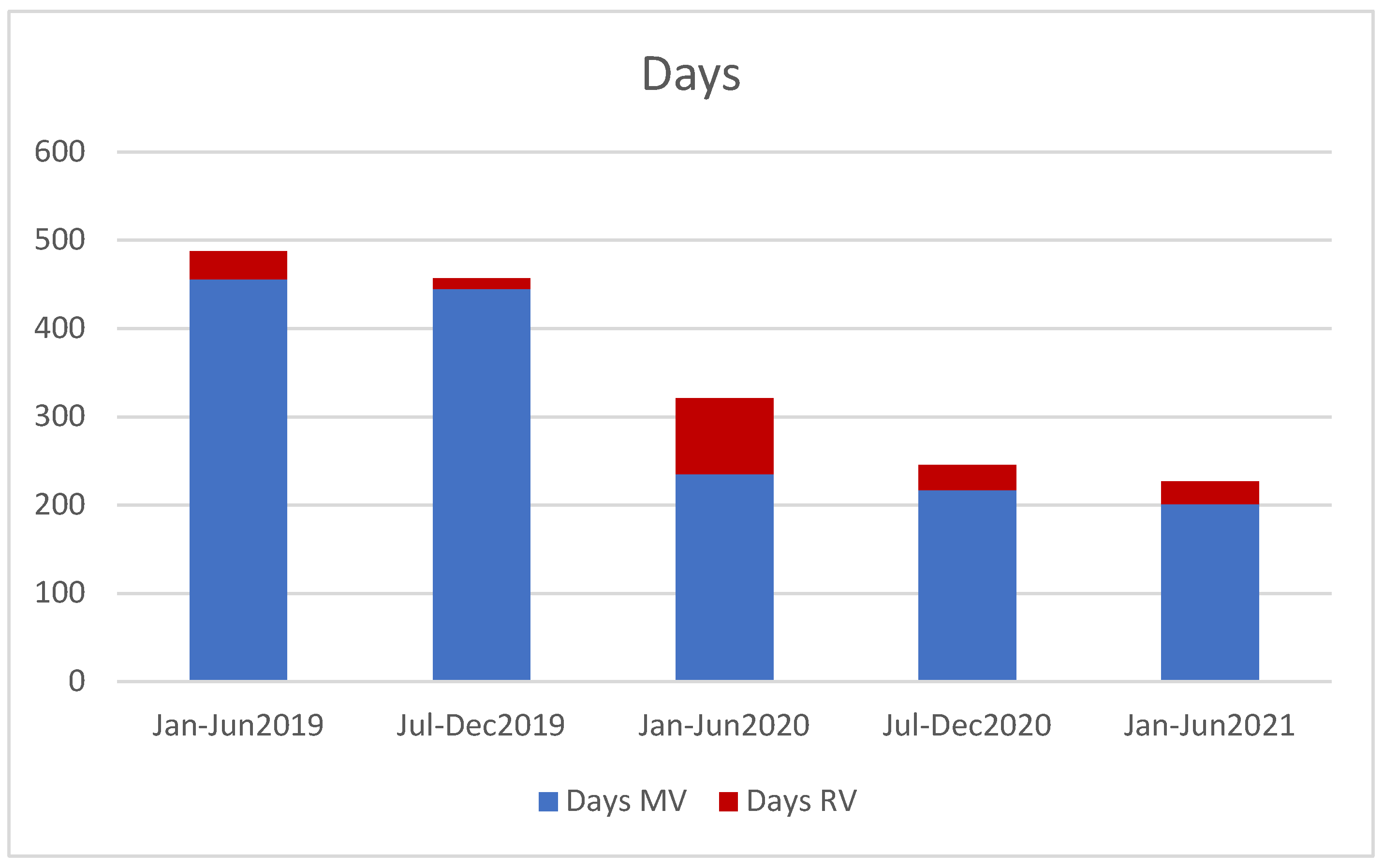
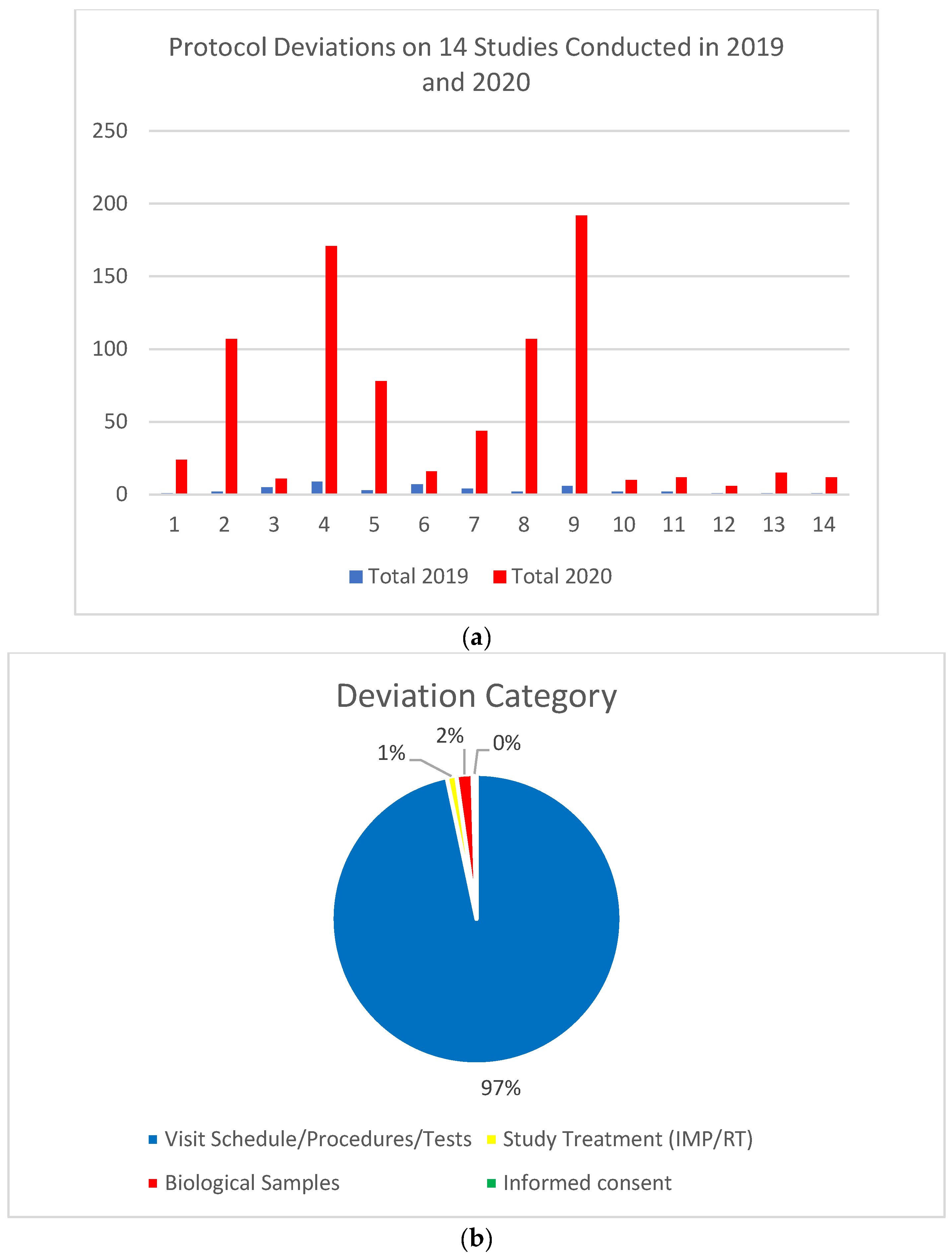
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLaughlin, R.A.; Madigan, V.; O’Grady, M.; Korpanty, G.; Coate, L. The impact of the COVID-19 pandemic on oncology clinical trials. Ir. Med. J. 2020, 113, 1–7. [Google Scholar]
- Burke, S.; Parker, S.; Fleming, P.; Barry, S.; Thomas, S. Building health system resilience through policy development in response to COVID-19 in Ireland: From shock to reform. Lancet Reg. Health-Eur. 2021, 9, 100223. [Google Scholar] [CrossRef] [PubMed]
- Cazelles, B.; Nguyen-Van-Yen, B.; Champagne, C.; Comiskey, C. Dynamics of the COVID-19 epidemic in Ireland under mitigation. BMC Infect. Dis. 2021, 21, 735. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, B.; O’Callaghan, M.; Coughlan, D.; Cullinan, J.; Doherty, E.; Glynn, L.; Moloney, E.; Queally, M. The COVID-19 pandemic in Ireland: An overview of the health service and economic policy response. Health Policy Technol. 2020, 9, 419–429. [Google Scholar] [CrossRef]
- Fitzpatrick, O.; Ni Dhonaill, R.; Linehan, A.; Coyne, Z.; Hennessy, M.; Clarke, M.; McGee, E.; Barrett, F.; O’Doherty, D.; Matassa, C.; et al. Delivery of systemic anticancer therapy during the COVID-19 pandemic. Ir. J. Med. Sci. 2021, 191, 559–562. [Google Scholar] [CrossRef]
- Linehan, A.; Fitzpatrick, O.; Cowzer, D.; Hennessy, M.A.; Coyne, Z.L.; Nolan, A.; Clarke, M.; Ni Dhonaill, R.; Hennessy, B.T.; Morris, P.G.; et al. COVID-19-related mortality in cancer patients in an Irish setting. Ir. J. Med Sci. 2021. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Jacob, S.; Barrett, P.M.; Gallagher, J. COVID-19 presentation among symptomatic healthcare workers in Ireland. Occup. Med. 2021, 71, 95–98. [Google Scholar] [CrossRef]
- Cancer Care during the COVID-19 Pandemic. Royal College of Physicians in Ireland College of Pathology. 2021. Available online: https://www.rcpi.ie/news/releases/rcpi-faculty-of-pathology-launches-new-report-on-cancer-care-during-COVID-19-pandemic (accessed on 3 December 2021).
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; De Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Phillips, N. The coronavirus is here tostay—Here’s what that means. Nature 2021, 590, 382–384. [Google Scholar] [CrossRef]
- Waterhouse, D.M.; Harvey, R.D.; Hurley, P.; Levit, L.A.; Kim, E.S.; Klepin, H.D.; Finch Mileham, K.; Nowakowski, G.; Schenkel, C.; Davis, C.; et al. Early Impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: Findings from an American Society of Clinical Oncology Survey. JCO Oncol. Pract. 2020, 16, 417–421. [Google Scholar] [CrossRef]
- Report Arising from Ireland’s Inaugural Cancer Retreat 2021. Available online: https://www.cancertrials.ie/2021/08/cancer-retreat-report (accessed on 28 April 2022).
- Al-Quieimat, O.M.; Amer, A.M. The impact of the COVID-19 pandemic on cancer patients. Am. J. Clin. Oncol. 2020, 43, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, A.; Park, K.U. The impact of COVID-19 on National Clinical Trials Network Breast Cancer Clinical Trials. Curr. Breast Cancer Rep. 2021, 13, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lara Gongora, A.B.; Werutsky, G.; Jardim, D.L.; Nogueira-Rodrigues, A.; Barrios, C.H.; Mathias, C.; Maluf, F.; Riechelmann, R.; Fraga, M.; Gomes, H.; et al. Impact of the COVID-19 pandemic on oncology clinical research in Latin America (LACOG 0420). JCO Glob. Oncol. 2021, 7, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; A Lydon, C.; Li, T.; Dai, J.; Standring, A.; A Legor, K.; Caparrotta, C.M.; Schenker, M.P.; I Glazer, D.; Tayob, N.; et al. The Impact of COVID-19 on Clinical Trial Execution at the Dana-Farber Cancer Institute. J. Natl. Cancer Inst. 2020, 113, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Marcum, M.; Kurtzweil, N.; Vollmer, C.; Schmid, L.; Vollmer, A.; Kastl, A.; Acker, K.; Gulati, S.; Grover, P.; Herzog, T.J.; et al. COVID-19 pandemic and impact on cancer clinical trials: An academic medical center perspective. Cancer Med. 2020, 9, 6141–6146. [Google Scholar] [CrossRef]
- Gerber, D.E.; Sheffield, T.Y.; Beg, M.S.; Williams, E.L.; Clark, V.L.; Xie, Y.; Holbein, M.E.B.; Skinner, C.S.; Lee, S.J.C. Experience, Perceptions, and Recommendations Concerning COVID-19–Related Clinical Research Adjustments. J. Natl. Compr. Cancer Netw. 2021, 19, 505–512. [Google Scholar] [CrossRef]
- Clark, S.; McGrane, A.; Boyle, N.; Joksimovic, N.; Burke, L.; Rock, N.; Sullivan, K.O. You’re a teacher you’re a mother, you’re a worker: Gender inequality during COVID-19 in Ireland. Gender Work Organ. 2020, 28, 1352–1362. [Google Scholar] [CrossRef]
- Farooq, A.R.; Iqbal, S.; Abdulaziz, N.; O’Brien, T.; Peters, N.; Collins, D.C. Professional and personal opinions of doctors in training during the first wave of the COVID-19 pandemic. Ir. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Kijowski, F.; Moore, S.; Iqbal SCronin, J.; Milewski, L.; Woods, N.; O’Reilly, S. Financial resilience among doctors in training and the COVID-19 pandemic. Ir. Med. J. 2021, 114, 380. [Google Scholar]
- Lim, K.H.J.; Murali, K.; Thome, E.; Punie, K.; Kamposioras, K.; Oing, C.; O’Connor, M.; Elez, E.; Amaral, T.; Garrido, P.; et al. The impact of the COVID-19 on oncology professionals—One year on, lessons learnt from the ESMO Resilience Task Force survey series. ESMO Open 2022, 7, 100374. [Google Scholar] [CrossRef]
- Delany, C.; Milch, V.; Keefe, D.; Wong, Z.W. COVID-19 recovery: Implications for cancer care clinicians. Support Care Cancer 2022, 30, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Wooston, C. Scientists count the career costs of COVID. Nature 2021, 599, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Ireland: Coronavirus Pandemic Country Profile. Available online: www.ourworldindata.org/coronavirus/country/ireland (accessed on 11 March 2022).
- Goshen Lago, T.; Waldhorn, I.; Holland, R.; Szwarcwort-Cohen, M.; Reiner-Benaim, A.; Shachor-Meyouhas, Y.; Hussein, K.; Fahoum, L.; Baruch, M.; Peer, A.; et al. Serological status and toxic effects of SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol 2021, 7, 1507. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Suffiotti, M.; Pellaton, C.; Bouchaab, H.; Cairoli, A.; Salvadé, V.; Stevenel, C.; Hottinger, R.; Pythoud, C.; Coutechier, L.; et al. Humoral responses against variants of concern by COVID-19 mRNA vaccines in immunocompromised patients. JAMA Oncol. 2022, 10, e220446. [Google Scholar] [CrossRef] [PubMed]
- Cagnazzo, C.; Besse, M.-G.; Manfellotto, D.; Minghetti, P.; Cazzaniga, S.; Cottini, L.; Fontanella, A.; Maruti, I.; Stabile, S.; Testoni, S.; et al. Lessons learned from COVID-19 for clinical research operations in Italy: What have we learned and what can we apply in the future. Tumori J. 2021, 107, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Ray-Cocquard, I.; Oaknin, A.; Banerjee, S. Clinical research disruption in the post COVID-19 era: Will the pandemic lead to change. ESMO Open 2020, 5, e000924. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Gordon, A.C.; Bauchner, H. Emerging lessons from COVID-19 for the US Clinical Research Enterprise. JAMA 2021, 325, 1159–1161. [Google Scholar] [CrossRef]
- Bailey, C.; Black, J.R.M.; Swanton, C. Cancer Research: The lessons to learn from COVID-19. Cancer Disc. 2020, 10, 1263–1266. [Google Scholar] [CrossRef]
- Edge, R.; Meyers, J.; Tiernan, G.; Li, Z.; Schiavuzzi, A.; Chan, P.; Vassallo, A.; Morrow, A.; Mazariego, C.; Wakefiled, C.E.; et al. Cancer care disruption and reorganisation during the COVID-19 pandemic in Australia: A patient, carer and healthcare worker perspective. PLoS ONE 2021, 16, e0257420. [Google Scholar] [CrossRef]
- Fuks, A.; Weijer, C.; Freedman, B.; Shapiro, S.; Skrutkowska, M.; Riaz, A. A study in contrasts: Eligibility criteria in a twenty year sample of NSABP and POG clinical trials. National Surgical Adjuvant Breast and Bowel Program. Pediatric Oncology Group. J. Clin. Epidemiol. 1998, 51, 69–79. [Google Scholar] [CrossRef]
- Garcia, S.; Bisen, A.; Yan, J.; Xie, X.J.; Ramalingam, S.; Schiller, J.H.; Johnson, D.H.; Gerber, D.E. Thoracic oncology clinical trial eligibility criteria and requirements continue to increase in number and complexity. J. Thorac. Oncol. 2017, 12, 1489–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Association of Cancer Research. AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care. Available online: https://www.AACR.org/COVIDReport (accessed on 1 February 2022).
- Williams, E.L.; Pierre, D.L.; Martin, M.E.; Beg, M.S.; Gerber, D.E. Taking tele behind the scenes: Remote clinical trial monitoring comes of age during the COVID-19 pandemic. JCO Oncol. Pract. 2021, 17, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, K.T.; Asaad, M.; Adlakha, E.; Overman, M.J.; Checka, C.M.; Offodile, A.C., 2nd. Virtual Clinical Trials in Oncology—Overview, Challenges, Policy Considerations, and Future Directions. JCO Clin. Cancer Inform. 2021, 5, 421–425. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised controlled, open label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin NBrightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in hospitalised patients with COVID-19. N. Eng. J. Med. 2021, 384, 693–704. [Google Scholar]
- Horowitz, J. Italy’s Health Care System Groans under Coronavirus—A Warning to the World. New York Times. 12 March 2020. Available online: www.nytimes.com (accessed on 2 March 2022).
- O’Leary, N.; Kingston, L.; Griffin, A.; Morrissey, A.M.; Noonan, M.; Kelly, D.; Doody, O.; Niranjan, V.; Gallagher, A.; O’riordan, C.; et al. COVID-19 healthcare policies in Ireland: A rapid review of the initial pandemic response. Scand. J. Pub. Health 2021, 49, 713–720. [Google Scholar] [CrossRef]
- Doroshow, J.H.; Prindiville, S.; McCaskill-Stevens, W.; Mooney, M.; Loehrer, P. COVID-19, social justice, and clinical cancer research. J. Natl. Cancer Inst. 2020, 113, 1281–1284. [Google Scholar] [CrossRef]
- Malagon, T.; Yong, J.H.E.; Tope, P.; Miller, W.H., Jr.; Franco, E.L. Predicted long term impact of COVID-19 pandemic related care delays on cancer mortality in Canada. Int. J. Cancer 2022, 150, 1244–1254. [Google Scholar] [CrossRef]
- Cutler, D.M. Challenges for the beleaguered health care workforce during COVID-19. JAMA Health Forum 2022, 3, e220143. [Google Scholar] [CrossRef]

| Activity | Heading | Activity | Heading |
|---|---|---|---|
| Staff reassignment | 41% | In person clinic visits reduced | 88% |
| Diagnostics impacted | 71% | Telehealth visits increased | 71% |
| Accrual suspended (full) | 56% | Onsite monitoring reduced | 100% |
| Accrual suspended (partial) | 71% | Physical site initiations of trials reduced | 94% |
| Accrual to trials impacted | 81% | Remote monitoring adopted | 53% |
| Research ethics decisions delayed | 67% | Remote working adopted | 65% |
| Risk management delayed | 31% | Virtual site initiations adopted | 44% |
| 1. Clinical research must be embedded into wider healthcare planning. |
| 2. Development of a funding stream for translational research. |
| 3. Increase of Public and Patient Involvement (PPI) in cancer trials. |
| 4. Analysis of the future effects of the pandemic on cancer diagnosis. |
| Challenges | Solutions |
|---|---|
| Reassignment of research staff | Research unit specific contracts of employment, e.g., involvement in clinical trials is part of the contract |
| Limited networking opportunities | Regular topic specific virtual meetings with in-person meetings when public health guideline acceptable |
| Recruitment and retention in a virtual workplace | Assigned mentorship by organisation leadership |
| Homeworking for clinical trial staff | Cyber secure clinical trials information technology access, e.g., providing IT equipment, which can be used securely outside the hospital |
| In-person hospital visits for trial assessments and product delivery | Integration of telehealth visits into protocols in-lieu of attendance with home delivery of trial products |
| Trial schedule disruption due to COVID-19 related absences | Flexibility of trial assessments without hampering treatment safety |
| Burnout and exhaustion among investigators | Health and wellbeing support, reassignment of tasks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Reilly, S.; Murphy, V.; Mulroe, E.; Tucker, L.; Carragher, F.; Marron, J.; Shannon, A.M.; Rogan, K.; Connolly, R.M.; Hennessy, B.T.; et al. The SARS-CoV-2 Pandemic and Cancer Trials Ireland: Impact, Resolution and Legacy. Cancers 2022, 14, 2247. https://doi.org/10.3390/cancers14092247
O’Reilly S, Murphy V, Mulroe E, Tucker L, Carragher F, Marron J, Shannon AM, Rogan K, Connolly RM, Hennessy BT, et al. The SARS-CoV-2 Pandemic and Cancer Trials Ireland: Impact, Resolution and Legacy. Cancers. 2022; 14(9):2247. https://doi.org/10.3390/cancers14092247
Chicago/Turabian StyleO’Reilly, Seamus, Verena Murphy, Eibhlin Mulroe, Lisa Tucker, Fiona Carragher, Jacinta Marron, Aoife M. Shannon, Ken Rogan, Roisin M. Connolly, Bryan T. Hennessy, and et al. 2022. "The SARS-CoV-2 Pandemic and Cancer Trials Ireland: Impact, Resolution and Legacy" Cancers 14, no. 9: 2247. https://doi.org/10.3390/cancers14092247
APA StyleO’Reilly, S., Murphy, V., Mulroe, E., Tucker, L., Carragher, F., Marron, J., Shannon, A. M., Rogan, K., Connolly, R. M., Hennessy, B. T., & McDermott, R. S. (2022). The SARS-CoV-2 Pandemic and Cancer Trials Ireland: Impact, Resolution and Legacy. Cancers, 14(9), 2247. https://doi.org/10.3390/cancers14092247






