Hippo in Gastric Cancer: From Signalling to Therapy
Abstract
:Simple Summary
Abstract
1. The Hippo Pathway
1.1. Overview
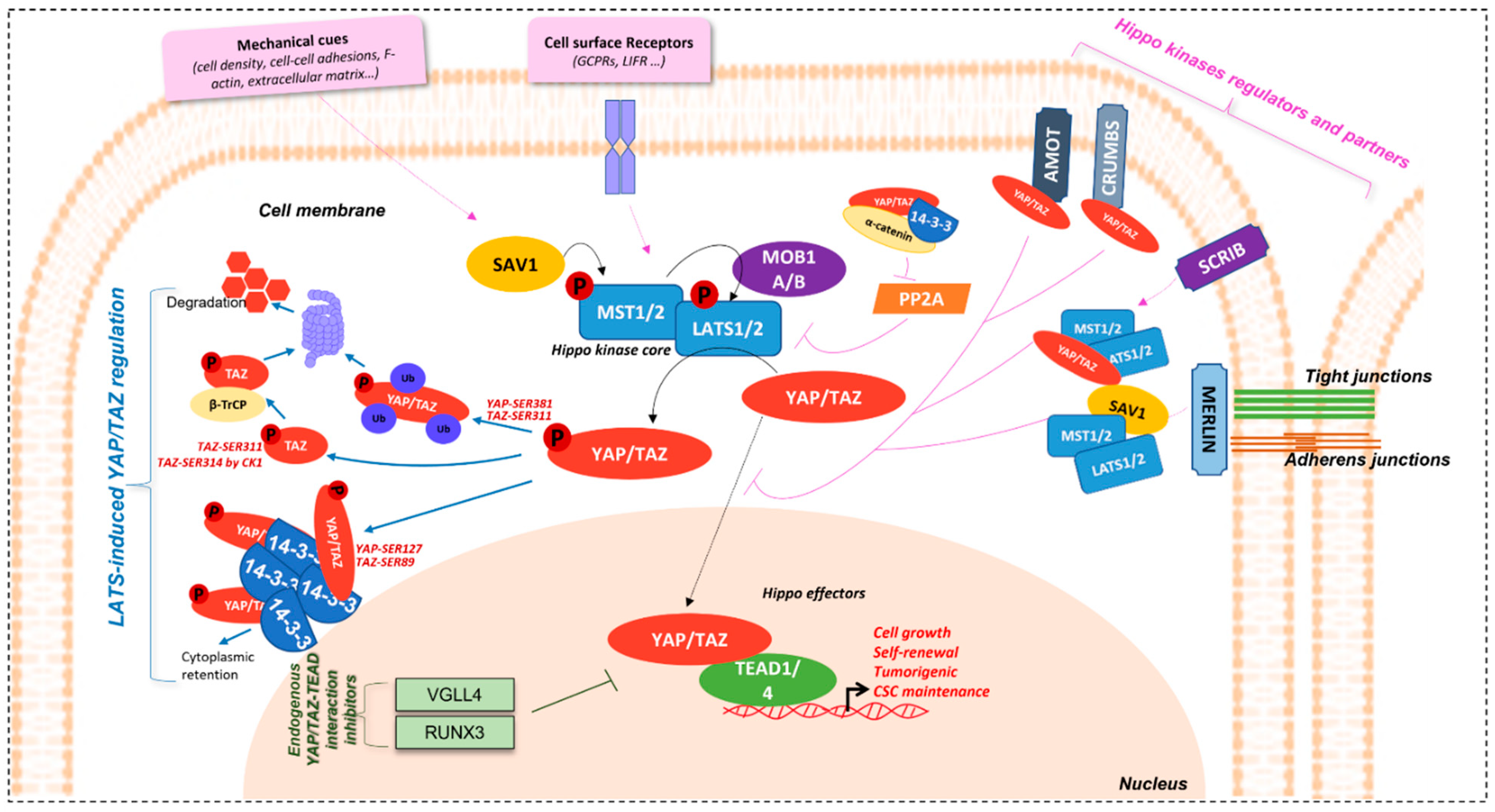
1.2. Regulation Mechanisms
1.2.1. YAP/TAZ Effectors Regulation
1.2.2. Hippo Kinase Core and Upstream Partners Regulation
1.2.3. Hippo Pathway Crosstalk with/and Regulation by Other Signalling Pathways
- ○
- AKT/p73 related pathway
- ○
- JNK/AP1/c-Jun/p73 pathway
- ○
- Src/c-Abl/p73 pathway
- ○
- Wnt/β-catenin pathway
- ○
- TGFβ/BMP/Smad pathway
- ○
- PI3K/AKT/mTOR pathway
- ○
- Pax8/TFF-1 pathway
- ○
- EGFR/RAF/MEK/ERK pathway
- ○
- AMPK pathway
- ○
- EMT/ZEB1/SNAIL/SLUG pathway
1.3. Hippo and Cancer
2. Hippo Pathway in Gastric Carcinogenesis
2.1. Hippo Pathway in Helicobacter-Mediated Gastric Carcinogenesis
2.2. Hippo Pathway in GC and CSCs
2.3. Hippo Pathway in GC Resistance to Therapy
3. Anti-GC Strategies Involving the Hippo Pathway
3.1. Downstream Strategies: Targetting Oncogenic YAP/TAZ-TEAD Signaling
3.2. Upstream Strategies: Hippo Kinases and/or Side-Pathways Stimulation
4. Hippo Pathway-Aiming Strategies, Not Tested in GC
4.1. Downstream Strategies: Targetting Oncogenic YAP/TAZ-TEAD Signaling
4.2. Upstream Strategies: Hippo Kinases and/or Side-Pathways Stimulation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Justice, R.W.; Zilian, O.; Woods, D.F.; Noll, M.; Bryant, P.J. The Drosophila Tumor Suppressor Gene Warts Encodes a Homolog of Human Myotonic Dystrophy Kinase and Is Required for the Control of Cell Shape and Proliferation. Genes Dev. 1995, 9, 534–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Wang, W.; Zhang, S.; Stewart, R.A.; Yu, W. Identifying Tumor Suppressors in Genetic Mosaics: The Drosophila Lats Gene Encodes a Putative Protein Kinase. Dev. Camb. Engl. 1995, 121, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Udan, R.S.; Kango-Singh, M.; Nolo, R.; Tao, C.; Halder, G. Hippo Promotes Proliferation Arrest and Apoptosis in the Salvador/Warts Pathway. Nat. Cell Biol. 2003, 5, 914–920. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo Signaling Pathway Coordinately Regulates Cell Proliferation and Apoptosis by Inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP Oncoprotein by the Hippo Pathway Is Involved in Cell Contact Inhibition and Tissue Growth Control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S. Role of YAP/TAZ in Cell-Matrix Adhesion-Mediated Signalling and Mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef]
- Camargo, F.D.; Gokhale, S.; Johnnidis, J.B.; Fu, D.; Bell, G.W.; Jaenisch, R.; Brummelkamp, T.R. YAP1 Increases Organ Size and Expands Undifferentiated Progenitor Cells. Curr. Biol. 2007, 17, 2054–2060. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a Universal Size-Control Mechanism in Drosophila and Mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-Y.; Zha, Z.-Y.; Zhou, X.; Zhang, H.; Huang, W.; Zhao, D.; Li, T.; Chan, S.W.; Lim, C.J.; Hong, W.; et al. The Hippo Tumor Pathway Promotes TAZ Degradation by Phosphorylating a Phosphodegron and Recruiting the SCFβ-TrCP E3 Ligase. J. Biol. Chem. 2010, 285, 37159. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Zhang, Y.; Wu, H.; Barry, E.; Yin, Y.; Lawrence, E.; Dawson, D.; Willis, J.E.; Markowitz, S.D.; Camargo, F.D.; et al. Mst1 and Mst2 Protein Kinases Restrain Intestinal Stem Cell Proliferation and Colonic Tumorigenesis by Inhibition of Yes-Associated Protein (Yap) Overabundance. Proc. Natl. Acad. Sci. USA 2011, 108, E1312–E1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanconato, F.; Battilana, G.; Cordenonsi, M.; Piccolo, S. YAP/TAZ as Therapeutic Targets in Cancer. Curr. Opin. Pharmacol. 2016, 29, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, S.W.; Meng, Z.; Lin, K.C.; Lin, B.; Hong, A.W.; Chun, J.V.; Guan, K.-L. Characterization of Hippo Pathway Components by Gene Inactivation. Mol. Cell 2016, 64, 993–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudol, M. Yes-Associated Protein (YAP65) Is a Proline-Rich Phosphoprotein That Binds to the SH3 Domain of the Yes Proto-Oncogene Product. Oncogene 1994, 9, 2145–2152. [Google Scholar] [PubMed]
- Sudol, M.; Chen, H.I.; Bougeret, C.; Einbond, A.; Bork, P. Characterization of a Novel Protein-Binding Module--the WW Domain. FEBS Lett. 1995, 369, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Yagi, R.; Chen, L.F.; Shigesada, K.; Murakami, Y.; Ito, Y. A WW Domain-Containing Yes-Associated Protein (YAP) Is a Novel Transcriptional Co-Activator. EMBO J. 1999, 18, 2551–2562. [Google Scholar] [CrossRef] [Green Version]
- Han, Y. Analysis of the Role of the Hippo Pathway in Cancer. J. Transl. Med. 2019, 17, 116. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.-Y.; Chinnaiyan, A.M.; et al. TEAD Mediates YAP-Dependent Gene Induction and Growth Control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef] [Green Version]
- Heallen, T.; Morikawa, Y.; Leach, J.; Tao, G.; Willerson, J.T.; Johnson, R.L.; Martin, J.F. Hippo Signaling Impedes Adult Heart Regeneration. Dev. Camb. Engl. 2013, 140, 4683–4690. [Google Scholar] [CrossRef] [Green Version]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo Pathway Inhibits Wnt Signaling to Restrain Cardiomyocyte Proliferation and Heart Size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Murakami, M.; Qi, X.; McAnally, J.; Porrello, E.R.; Mahmoud, A.I.; Tan, W.; Shelton, J.M.; et al. Hippo Pathway Effector Yap Promotes Cardiac Regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13839–13844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Gise, A.; Lin, Z.; Schlegelmilch, K.; Honor, L.B.; Pan, G.M.; Buck, J.N.; Ma, Q.; Ishiwata, T.; Zhou, B.; Camargo, F.D.; et al. YAP1, the Nuclear Target of Hippo Signaling, Stimulates Heart Growth through Cardiomyocyte Proliferation but Not Hypertrophy. Proc. Natl. Acad. Sci. USA 2012, 109, 2394–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Q.-Y.; Zhang, H.; Zhao, B.; Zha, Z.-Y.; Bai, F.; Pei, X.-H.; Zhao, S.; Xiong, Y.; Guan, K.-L. TAZ Promotes Cell Proliferation and Epithelial-Mesenchymal Transition and Is Inhibited by the Hippo Pathway. Mol. Cell. Biol. 2008, 28, 2426–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, F.; Zhang, L.; Jiang, J. Hippo Signaling Regulates Yorkie Nuclear Localization and Activity through 14-3-3 Dependent and Independent Mechanisms. Dev. Biol. 2010, 337, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.-Y.; Guan, K.-L. A Coordinated Phosphorylation by Lats and CK1 Regulates YAP Stability through SCFβ-TRCP. Genes Dev. 2010, 24, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Zygulska, A.L.; Krzemieniecki, K.; Pierzchalski, P. Hippo Pathway—Brief Overview of Its Relevance in Cancer. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2017, 68, 311–335. [Google Scholar]
- Oka, T.; Remue, E.; Meerschaert, K.; Vanloo, B.; Boucherie, C.; Gfeller, D.; Bader, G.D.; Sidhu, S.S.; Vandekerckhove, J.; Gettemans, J.; et al. Functional Complexes between YAP2 and ZO-2 Are PDZ Domain-Dependent, and Regulate YAP2 Nuclear Localization and Signalling. Biochem. J. 2010, 432, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Remue, E.; Meerschaert, K.; Oka, T.; Boucherie, C.; Vandekerckhove, J.; Sudol, M.; Gettemans, J. TAZ Interacts with Zonula Occludens-1 and-2 Proteins in a PDZ-1 Dependent Manner. FEBS Lett. 2010, 584, 4175–4180. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, H.; Cao, Y.; Zohrabian, S.; Zhou, P.; Ma, Q.; VanDusen, N.; Guo, Y.; Zhang, J.; Stevens, S.M.; et al. Acetylation of VGLL4 Regulates Hippo-YAP Signaling and Postnatal Cardiac Growth. Dev. Cell 2016, 39, 466–479. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Lin, S.J.; Chen, Y.; Voon, D.C.-C.; Zhu, F.; Chuang, L.S.H.; Wang, T.; Tan, P.; Lee, S.C.; Yeoh, K.G.; et al. RUNX3 Is a Novel Negative Regulator of Oncogenic TEAD–YAP Complex in Gastric Cancer. Oncogene 2016, 35, 2664–2674. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-X.; Zhao, B.; Guan, K.-L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokowski, R.P.; Cox, D.R. Functional Analysis of the Neurofibromatosis Type 2 Protein by Means of Disease-Causing Point Mutations. Am. J. Hum. Genet. 2000, 66, 873–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, C.; Troutman, S.; Fera, D.; Stemmer-Rachamimov, A.; Avila, J.L.; Christian, N.; Persson, N.L.; Shimono, A.; Speicher, D.W.; Marmorstein, R.; et al. A Tight Junction-Associated Merlin-Angiomotin Complex Mediates Merlin’s Regulation of Mitogenic Signaling and Tumor Suppressive Functions. Cancer Cell 2011, 19, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, F.; Yu, J.; Zheng, Y.; Chen, Q.; Zhang, N.; Pan, D. Spatial Organization of Hippo Signaling at the Plasma Membrane Mediated by the Tumor Suppressor Merlin/NF2. Cell 2013, 154, 1342–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhamouche, S.; Curto, M.; Saotome, I.; Gladden, A.B.; Liu, C.-H.; Giovannini, M.; McClatchey, A.I. Nf2/Merlin Controls Progenitor Homeostasis and Tumorigenesis in the Liver. Genes Dev. 2010, 24, 1718–1730. [Google Scholar] [CrossRef] [Green Version]
- Cordenonsi, M.; Zanconato, F.; Azzolin, L.; Forcato, M.; Rosato, A.; Frasson, C.; Inui, M.; Montagner, M.; Parenti, A.R.; Poletti, A.; et al. The Hippo Transducer TAZ Confers Cancer Stem Cell-Related Traits on Breast Cancer Cells. Cell 2011, 147, 759–772. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Chen, J. Angiomotin-like Proteins Associate with and Negatively Regulate YAP1. J. Biol. Chem. 2011, 286, 4364–4370. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Li, L.; Lu, Q.; Wang, L.H.; Liu, C.-Y.; Lei, Q.; Guan, K.-L. Angiomotin Is a Novel Hippo Pathway Component That Inhibits YAP Oncoprotein. Genes Dev. 2011, 25, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 Acts Downstream of α-Catenin to Control Epidermal Proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Silvis, M.R.; Kreger, B.T.; Lien, W.-H.; Klezovitch, O.; Rudakova, G.M.; Camargo, F.D.; Lantz, D.M.; Seykora, J.T.; Vasioukhin, V. α-Catenin Is a Tumor Suppressor That Controls Cell Accumulation by Regulating the Localization and Activity of the Transcriptional Coactivator Yap1. Sci. Signal. 2011, 4, ra33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Sun, Y.; Wei, Y.; Zhang, P.; Rezaeian, A.H.; Teruya-Feldstein, J.; Gupta, S.; Liang, H.; Lin, H.-K.; Hung, M.-C.; et al. LIFR Is a Breast Cancer Metastasis Suppressor Upstream of the Hippo-YAP Pathway and a Prognostic Marker. Nat. Med. 2012, 18, 1511–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeneevassen, L.; Giraud, J.; Molina-Castro, S.; Sifré, E.; Tiffon, C.; Beauvoit, C.; Staedel, C.; Mégraud, F.; Lehours, P.; Martin, O.C.B.; et al. Leukaemia Inhibitory Factor (LIF) Inhibits Cancer Stem Cells Tumorigenic Properties through Hippo Kinases Activation in Gastric Cancer. Cancers 2020, 12, 2011. [Google Scholar] [CrossRef]
- Dai, X.; She, P.; Chi, F.; Feng, Y.; Liu, H.; Jin, D.; Zhao, Y.; Guo, X.; Jiang, D.; Guan, K.-L.; et al. Phosphorylation of Angiomotin by Lats1/2 Kinases Inhibits F-Actin Binding, Cell Migration, and Angiogenesis. J. Biol. Chem. 2013, 288, 34041–34051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, P.S.; Josué, F.; Wepf, A.; Wehr, M.C.; Rinner, O.; Kelly, G.; Tapon, N.; Gstaiger, M. Combined Functional Genomic and Proteomic Approaches Identify a PP2A Complex as a Negative Regulator of Hippo Signaling. Mol. Cell 2010, 39, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Stiewe, T.; Pützer, B.M. P73 in Apoptosis. Apoptosis Int. J. Program. Cell Death 2001, 6, 447–452. [Google Scholar] [CrossRef]
- Strano, S.; Munarriz, E.; Rossi, M.; Castagnoli, L.; Shaul, Y.; Sacchi, A.; Oren, M.; Sudol, M.; Cesareni, G.; Blandino, G. Physical Interaction with Yes-Associated Protein Enhances P73 Transcriptional Activity. J. Biol. Chem. 2001, 276, 15164–15173. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Totty, N.F.; Irwin, M.S.; Sudol, M.; Downward, J. Akt Phosphorylates the Yes-Associated Protein, YAP, to Induce Interaction with 14-3-3 and Attenuation of P73-Mediated Apoptosis. Mol. Cell 2003, 11, 11–23. [Google Scholar] [CrossRef]
- Danovi, S.A.; Rossi, M.; Gudmundsdottir, K.; Yuan, M.; Melino, G.; Basu, S. Yes-Associated Protein (YAP) Is a Critical Mediator of c-Jun-Dependent Apoptosis. Cell Death Differ. 2008, 15, 217–219. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, V.; Gudmundsdottir, K.; Luong, P.; Leung, K.-Y.; Knebel, A.; Basu, S. JNK Phosphorylates Yes-Associated Protein (YAP) to Regulate Apoptosis. Cell Death Dis. 2010, 1, e29. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.; Adamovich, Y.; Reuven, N.; Shaul, Y. Yap1 Phosphorylation by C-Abl Is a Critical Step in Selective Activation of Proapoptotic Genes in Response to DNA Damage. Mol. Cell 2008, 29, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Azzolin, L.; Panciera, T.; Soligo, S.; Enzo, E.; Bicciato, S.; Dupont, S.; Bresolin, S.; Frasson, C.; Basso, G.; Guzzardo, V.; et al. YAP/TAZ Incorporation in the β-Catenin Destruction Complex Orchestrates the Wnt Response. Cell 2014, 158, 157–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzolin, L.; Zanconato, F.; Bresolin, S.; Forcato, M.; Basso, G.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Role of TAZ as Mediator of Wnt Signaling. Cell 2012, 151, 1443–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imajo, M.; Miyatake, K.; Iimura, A.; Miyamoto, A.; Nishida, E. A Molecular Mechanism That Links Hippo Signalling to the Inhibition of Wnt/β-Catenin Signalling. EMBO J. 2012, 31, 1109–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbluh, J.; Nijhawan, D.; Cox, A.G.; Li, X.; Neal, J.T.; Schafer, E.J.; Zack, T.I.; Wang, X.; Tsherniak, A.; Schinzel, A.C.; et al. β-Catenin-Driven Cancers Require a YAP1 Transcriptional Complex for Survival and Tumorigenesis. Cell 2012, 151, 1457–1473. [Google Scholar] [CrossRef] [Green Version]
- Varelas, X.; Miller, B.W.; Sopko, R.; Song, S.; Gregorieff, A.; Fellouse, F.A.; Sakuma, R.; Pawson, T.; Hunziker, W.; McNeill, H.; et al. The Hippo Pathway Regulates Wnt/Beta-Catenin Signaling. Dev. Cell 2010, 18, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, C.; Zaromytidou, A.-I.; Xi, Q.; Gao, S.; Yu, J.; Fujisawa, S.; Barlas, A.; Miller, A.N.; Manova-Todorova, K.; Macias, M.J.; et al. Nuclear CDKs Drive Smad Transcriptional Activation and Turnover in BMP and TGF-Beta Pathways. Cell 2009, 139, 757–769. [Google Scholar] [CrossRef] [Green Version]
- Ferrigno, O.; Lallemand, F.; Verrecchia, F.; L’hoste, S.; Camonis, J.; Atfi, A.; Mauviel, A. Yes-Associated Protein (YAP65) Interacts with Smad7 and Potentiates Its Inhibitory Activity against TGF-β/Smad Signaling. Oncogene 2002, 21, 4879–4884. [Google Scholar] [CrossRef] [Green Version]
- Varelas, X.; Samavarchi-Tehrani, P.; Narimatsu, M.; Weiss, A.; Cockburn, K.; Larsen, B.G.; Rossant, J.; Wrana, J.L. The Crumbs Complex Couples Cell Density Sensing to Hippo-Dependent Control of the TGF-β-SMAD Pathway. Dev. Cell 2010, 19, 831–844. [Google Scholar] [CrossRef]
- Varelas, X.; Sakuma, R.; Samavarchi-Tehrani, P.; Peerani, R.; Rao, B.M.; Dembowy, J.; Yaffe, M.B.; Zandstra, P.W.; Wrana, J.L. TAZ Controls Smad Nucleocytoplasmic Shuttling and Regulates Human Embryonic Stem-Cell Self-Renewal. Nat. Cell Biol. 2008, 10, 837–848. [Google Scholar] [CrossRef]
- Tumaneng, K.; Schlegelmilch, K.; Russell, R.; Yimlamai, D.; Basnet, H.; Mahadevan, N.; Fitamant, J.; Bardeesy, N.; Camargo, F.; Guan, K.-L. YAP Mediates Crosstalk between the Hippo and PI3K-TOR Pathways by Suppressing PTEN via MiR-29. Nat. Cell Biol. 2012, 14, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Dai, X.; Dai, X.; Xie, J.; Yin, S.; Zhu, J.; Wang, C.; Liu, Y.; Guo, J.; Wang, M.; et al. LATS Suppresses MTORC1 Activity to Directly Coordinate Hippo and MTORC1 Pathways in Growth Control. Nat. Cell Biol. 2020, 22, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Palma, T.D.; D’Andrea, B.; Liguori, G.; Liguoro, A.; Cristofaro, T.; de Prete, D.D.; Pappalardo, A.; Mascia, A.; Zannini, M. TAZ Is a Coactivator for Pax8 and TTF-1, Two Transcription Factors Involved in Thyroid Differentiation. Exp. Cell Res. 2009, 315, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Whitsett, J.A.; Di Palma, T.; Hong, J.-H.; Yaffe, M.B.; Zannini, M. TAZ Interacts with TTF-1 and Regulates Expression of Surfactant Protein-C. J. Biol. Chem. 2004, 279, 17384–17390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, B.; Yang, Y.-L.; Xu, Z.; Dai, Y.; Liu, S.; Mao, J.-H.; Tetsu, O.; Li, H.; Jablons, D.M.; You, L. Inhibition of ERK1/2 down-Regulates the Hippo/YAP Signaling Pathway in Human NSCLC Cells. Oncotarget 2015, 6, 4357–4368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Sun, L.; Zhu, X. Yes-Associated Protein Enhances Proliferation and Attenuates Sensitivity to Cisplatin in Human Gastric Cancer Cells. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 105, 1269–1275. [Google Scholar] [CrossRef]
- DeRan, M.; Yang, J.; Shen, C.-H.; Peters, E.C.; Fitamant, J.; Chan, P.; Hsieh, M.; Zhu, S.; Asara, J.M.; Zheng, B.; et al. Energy Stress Regulates Hippo-YAP Signaling Involving AMPK-Mediated Regulation of Angiomotin-like 1 Protein. Cell Rep. 2014, 9, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, W.; Mossmann, D.; Kleemann, J.; Mock, K.; Meisinger, C.; Brummer, T.; Herr, R.; Brabletz, S.; Stemmler, M.P.; Brabletz, T. ZEB1 Turns into a Transcriptional Activator by Interacting with YAP1 in Aggressive Cancer Types. Nat. Commun. 2016, 7, 10498. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, Y.; Ye, F.; Wang, W.; Lu, Q.; Kaplan, H.J.; Dean, D.C. Taz-Tead1 Links Cell-Cell Contact to Zeb1 Expression, Proliferation, and Dedifferentiation in Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3372–3378. [Google Scholar] [CrossRef]
- Tang, Y.; Feinberg, T.; Keller, E.T.; Li, X.-Y.; Weiss, S.J. Snail/Slug Binding Interactions with YAP/TAZ Control Skeletal Stem Cell Self-Renewal and Differentiation. Nat. Cell Biol. 2016, 18, 917–929. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Lamar, J.M.; Stern, P.; Liu, H.; Schindler, J.W.; Jiang, Z.-G.; Hynes, R.O. The Hippo Pathway Target, YAP, Promotes Metastasis through Its TEAD-Interaction Domain. Proc. Natl. Acad. Sci. USA 2012, 109, E2441–E2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plouffe, S.W.; Lin, K.C.; Moore, J.L.; Tan, F.E.; Ma, S.; Ye, Z.; Qiu, Y.; Ren, B.; Guan, K.-L. The Hippo Pathway Effector Proteins YAP and TAZ Have Both Distinct and Overlapping Functions in the Cell. J. Biol. Chem. 2018, 293, 11230–11240. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Chen, L.; Zhou, Y.; Wang, Q.; Zheng, Z.; Xu, B.; Wu, C.; Zhou, Q.; Hu, W.; Wu, C.; et al. A Novel Protein Encoded by a Circular RNA CircPPP1R12A Promotes Tumor Pathogenesis and Metastasis of Colon Cancer via Hippo-YAP Signaling. Mol. Cancer 2019, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Gilkes, D.M.; Hu, H.; Luo, W.; Bullen, J.W.; Liang, H.; Semenza, G.L. HIF-1α and TAZ Serve as Reciprocal Co-Activators in Human Breast Cancer Cells. Oncotarget 2015, 6, 11768–11778. [Google Scholar] [CrossRef] [Green Version]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Luzzati, A.; Perrucchini, G.; Desiderio, M.A. Hypoxia Inducible Factor-1 Is Activated by Transcriptional Co-Activator with PDZ-Binding Motif (TAZ) versus WWdomain-Containing Oxidoreductase (WWOX) in Hypoxic Microenvironment of Bone Metastasis from Breast Cancer. Eur. J. Cancer Oxf. Engl. 1990 2013, 49, 2608–2618. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Xing, Z.; Lin, A.; Liang, K.; Song, J.; Hu, Q.; Yao, J.; Chen, Z.; Park, P.K.; et al. A ROR1-HER3-LncRNA Signalling Axis Modulates the Hippo-YAP Pathway to Regulate Bone Metastasis. Nat. Cell Biol. 2017, 19, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhang, N.; Gray, R.S.; Li, H.; Ewald, A.J.; Zahnow, C.A.; Pan, D. A Temporal Requirement for Hippo Signaling in Mammary Gland Differentiation, Growth, and Tumorigenesis. Genes Dev. 2014, 28, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Nishio, M.; Hamada, K.; Kawahara, K.; Sasaki, M.; Noguchi, F.; Chiba, S.; Mizuno, K.; Suzuki, S.O.; Dong, Y.; Tokuda, M.; et al. Cancer Susceptibility and Embryonic Lethality in Mob1a/1b Double-Mutant Mice. J. Clin. Investig. 2012, 122, 4505–4518. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer-Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Liu, H.; Li, Z.; Zhong, C.; Fang, W. Down-Regulation of LATS2 in Non-Small Cell Lung Cancer Promoted the Growth and Motility of Cancer Cells. Tumor Biol. 2015, 36, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chang, Y.-L.; Chang, Y.-C.; Lin, J.-C.; Chen, C.-C.; Pan, S.-H.; Wu, C.-T.; Chen, H.-Y.; Yang, S.-C.; Hong, T.-M.; et al. MicroRNA-135b Promotes Lung Cancer Metastasis by Regulating Multiple Targets in the Hippo Pathway and LZTS1. Nat. Commun. 2013, 4, 1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurppa, K.J.; Liu, Y.; To, C.; Zhang, T.; Fan, M.; Vajdi, A.; Knelson, E.H.; Xie, Y.; Lim, K.; Cejas, P.; et al. Treatment-Induced Tumor Dormancy through YAP-Mediated Transcriptional Reprogramming of the Apoptotic Pathway. Cancer Cell 2020, 37, 104–122. [Google Scholar] [CrossRef]
- Lee, K.-P.; Lee, J.-H.; Kim, T.-S.; Kim, T.-H.; Park, H.-D.; Byun, J.-S.; Kim, M.-C.; Jeong, W.-I.; Calvisi, D.F.; Kim, J.-M.; et al. The Hippo-Salvador Pathway Restrains Hepatic Oval Cell Proliferation, Liver Size, and Liver Tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 8248–8253. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Yang, C.; Yang, S.; Cheng, F.; Rao, J.; Wang, X. MiR-665 Promotes Hepatocellular Carcinoma Cell Migration, Invasion, and Proliferation by Decreasing Hippo Signaling through Targeting PTPRB. Cell Death Dis. 2018, 9, 954. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.-H.; Kim, S.J.; Hyun, J. MicroRNAs Regulating Hippo-YAP Signaling in Liver Cancer. Biomedicines 2021, 9, 347. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, H.; Han, S. MiR-3910 Promotes the Growth and Migration of Cancer Cells in the Progression of Hepatocellular Carcinoma. Dig. Dis. Sci. 2017, 62, 2812–2820. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, H.-R.; Liu, T.-T.; Shen, X.-Z.; Zhu, J.-M. The Hippo Pathway in Hepatocellular Carcinoma: Non-Coding RNAs in Action. Cancer Lett. 2017, 400, 175–182. [Google Scholar] [CrossRef]
- Yang, X.; Yu, J.; Yin, J.; Xiang, Q.; Tang, H.; Lei, X. MiR-195 Regulates Cell Apoptosis of Human Hepatocellular Carcinoma Cells by Targeting LATS2. Die Pharm. Int. J. Pharm. Sci. 2012, 67, 645–651. [Google Scholar]
- Liu, Y.; Wang, X.; Yang, Y. Hepatic Hippo Signaling Inhibits Development of Hepatocellular Carcinoma. Clin. Mol. Hepatol. 2020, 26, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, L.; Weng, W.; Qiao, Y.; Zhang, Y.; He, J.; Wang, H.; Xiao, W.; Li, L.; Chu, Q.; et al. Mutual Interaction between YAP and CREB Promotes Tumorigenesis in Liver Cancer. Hepatology 2013, 58, 1011–1020. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Zhang, Y.; Zhang, Y.; Ma, L.; Weng, W.; Qiao, Y.; Xiao, W.; Wang, H.; Yu, W.; et al. MEK1 Promotes YAP and Their Interaction Is Critical for Tumorigenesis in Liver Cancer. FEBS Lett. 2013, 587, 3921–3927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yang, L.; Szeto, P.; Abali, G.K.; Zhang, Y.; Kulkarni, A.; Amarasinghe, K.; Li, J.; Vergara, I.A.; Molania, R.; et al. The Hippo Pathway Oncoprotein YAP Promotes Melanoma Cell Invasion and Spontaneous Metastasis. Oncogene 2020, 39, 5267–5281. [Google Scholar] [CrossRef]
- Goel, H.; Mathur, R.; Syeda, S.; Shrivastava, A.; Kumar Jha, A. Promoter Hypermethylation of LATS1 Gene in Oral Squamous Cell Carcinoma (OSCC) among North Indian Population. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 977–982. [Google Scholar] [CrossRef]
- Ladiz, M.A.R.; Najafi, M.; Kordi-Tamandani, D.M. Contribution of LATS1 and LATS2 Promoter Methylation in OSCC Development. J. Cell Commun. Signal. 2017, 11, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, D.D.; Xue, W.; Krall, E.B.; Bhutkar, A.; Piccioni, F.; Wang, X.; Schinzel, A.C.; Sood, S.; Rosenbluh, J.; Kim, J.W.; et al. KRAS and YAP1 Converge to Regulate EMT and Tumor Survival. Cell 2014, 158, 171–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Moujahed, A.; Brodowska, K.; Stryjewski, T.; Efstathiou, N.; Vasilikos, I.; Cichy, J.; Miller, J.; Gragoudas, E.; Vavvas, D. Verteporfin Inhibits Growth of Human Glioma in Vitro without Light Activation. Sci. Rep. 2017, 7, 7602. [Google Scholar] [CrossRef]
- Brodowska, K.; Al-Moujahed, A.; Marmalidou, A.; Meyer Zu Horste, M.; Cichy, J.; Miller, J.; Gragoudas, E.; Vavvas, D. The Clinically Used Photosensitizer Verteporfin (VP) Inhibits YAP-TEAD and Human Retinoblastoma Cell Growth in Vitro without Light Activation. Exp. Eye Res. 2014, 124, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Dasari, V.R.; Mazack, V.; Feng, W.; Nash, J.; Carey, D.J.; Gogoi, R. Verteporfin Exhibits YAP-Independent Anti-Proliferative and Cytotoxic Effects in Endometrial Cancer Cells. Oncotarget 2017, 8, 28628–28640. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Yi, C. YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 2019, 5, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Dong, J. The Hippo Signaling Pathway in Drug Resistance in Cancer. Cancers 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Lin, F.; Wu, W.; Liu, Y.; Huang, W. Verteporfin Inhibits YAP-Induced Bladder Cancer Cell Growth and Invasion via Hippo Signaling Pathway. Int. J. Med. Sci. 2018, 15, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janse van Rensburg, H.J.; Yang, X. The Roles of the Hippo Pathway in Cancer Metastasis. Cell. Signal. 2016, 28, 1761–1772. [Google Scholar] [CrossRef]
- Pan, Z.; Tian, Y.; Niu, G.; Cao, C. The Emerging Role of GC-MSCs in the Gastric Cancer Microenvironment: From Tumor to Tumor Immunity. Stem Cells Int. 2019, 2019, 8071842. [Google Scholar] [CrossRef] [Green Version]
- Molina-Castro, S.E.; Tiffon, C.; Giraud, J.; Boeuf, H.; Sifre, E.; Giese, A.; Belleannée, G.; Lehours, P.; Bessède, E.; Mégraud, F.; et al. The Hippo Kinase LATS2 Controls Helicobacter Pylori-Induced Epithelial-Mesenchymal Transition and Intestinal Metaplasia in Gastric Mucosa. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 257–276. [Google Scholar] [CrossRef] [Green Version]
- Tiffon, C.; Giraud, J.; Molina-Castro, S.E.; Peru, S.; Seeneevassen, L.; Sifré, E.; Staedel, C.; Bessède, E.; Dubus, P.; Mégraud, F.; et al. TAZ Controls Helicobacter Pylori-Induced Epithelial-Mesenchymal Transition and Cancer Stem Cell-Like Invasive and Tumorigenic Properties. Cells 2020, 9, 1462. [Google Scholar] [CrossRef]
- Seeneevassen, L.; Bessède, E.; Mégraud, F.; Lehours, P.; Dubus, P.; Varon, C. Gastric Cancer: Advances in Carcinogenesis Research and New Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 3418. [Google Scholar] [CrossRef]
- Varon, C.; Azzi-Martin, L.; Khalid, S.; Seeneevassen, L.; Ménard, A.; Spuul, P. Helicobacters and Cancer, Not Only Gastric Cancer? Semin. Cancer Biol. 2021, in press. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhu, J.S.; Zhang, Q.; Wang, X.Y. A Breakdown of the Hippo Pathway in Gastric Cancer. Hepatogastroenterology 2011, 58, 1611–1617. [Google Scholar] [CrossRef]
- Zhou, G.-X.; Li, X.-Y.; Zhang, Q.; Zhao, K.; Zhang, C.-P.; Xue, C.-H.; Yang, K.; Tian, Z.-B. Effects of the Hippo Signaling Pathway in Human Gastric Cancer. Asian Pac. J. Cancer Prev. 2013, 14, 5199–5205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
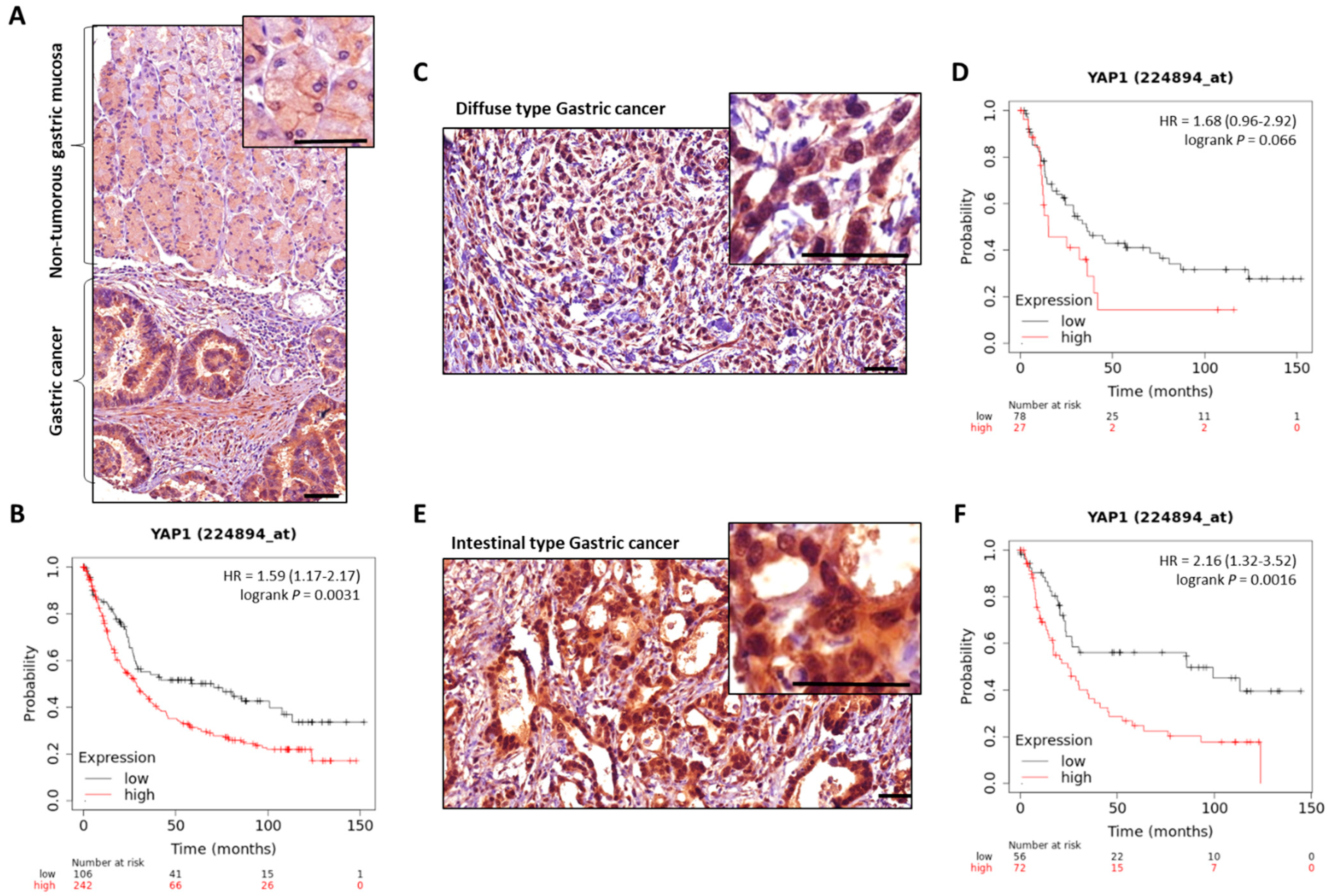
- Sun, D.; Li, X.; He, Y.; Li, W.; Wang, Y.; Wang, H.; Jiang, S.; Xin, Y. YAP1 Enhances Cell Proliferation, Migration, and Invasion of Gastric Cancer in Vitro and in Vivo. Oncotarget 2016, 7, 81062–81076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, W.; Tong, J.H.M.; Chan, A.W.H.; Lee, T.-L.; Lung, R.W.M.; Leung, P.P.S.; So, K.K.Y.; Wu, K.; Fan, D.; Yu, J.; et al. Yes-Associated Protein 1 Exhibits Oncogenic Property in Gastric Cancer and Its Nuclear Accumulation Associates with Poor Prognosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 2130–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Cheong, J.-H.; Kim, H.; Noh, S.H.; Kim, H. Nuclear Expression of Yes-Associated Protein 1 Correlates with Poor Prognosis in Intestinal Type Gastric Cancer. Anticancer Res. 2012, 32, 3827–3834. [Google Scholar] [PubMed]
- Song, M.; Rabkin, C.S.; Camargo, M.C. Gastric Cancer: An Evolving Disease. Curr. Treat. Options Gastroenterol. 2018, 16, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Wang, H.; Shi, Z.; Dong, A.; Zhang, W.; Song, X.; He, F.; Wang, Y.; Zhang, Z.; Wang, W.; et al. A Peptide Mimicking VGLL4 Function Acts as a YAP Antagonist Therapy against Gastric Cancer. Cancer Cell 2014, 25, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective Identification of Tumorigenic Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and Expansion of Human Colon-Cancer-Initiating Cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.W.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.K.; Shimada, Y.; Wang, T.C. Identification of Gastric Cancer Stem Cells Using the Cell Surface Marker CD44. Stem Cells Dayt. Ohio 2009, 27, 1006–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, K.; Saikawa, Y.; Ohashi, M.; Kumagai, K.; Kitajima, M.; Okano, H.; Matsuzaki, Y.; Kitagawa, Y. Tumor Initiating Potential of Side Population Cells in Human Gastric Cancer. Int. J. Oncol. 2009, 34, 1201–1207. [Google Scholar] [PubMed]
- Nguyen, P.H.; Giraud, J.; Chambonnier, L.; Dubus, P.; Wittkop, L.; Belleannée, G.; Collet, D.; Soubeyran, I.; Evrard, S.; Rousseau, B.; et al. Characterization of Biomarkers of Tumorigenic and Chemoresistant Cancer Stem Cells in Human Gastric Carcinoma. Clin. Cancer Res. 2017, 23, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessède, E.; Staedel, C.; Acuña Amador, L.A.; Nguyen, P.H.; Chambonnier, L.; Hatakeyama, M.; Belleannée, G.; Mégraud, F.; Varon, C. Helicobacter Pylori Generates Cells with Cancer Stem Cell Properties via Epithelial-Mesenchymal Transition-like Changes. Oncogene 2014, 33, 4123–4131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraud, J.; Molina-Castro, S.; Seeneevassen, L.; Sifré, E.; Izotte, J.; Tiffon, C.; Staedel, C.; Boeuf, H.; Fernandez, S.; Barthelemy, P.; et al. Verteporfin Targeting YAP1/TAZ-TEAD Transcriptional Activity Inhibits the Tumorigenic Properties of Gastric Cancer Stem Cells. Int. J. Cancer 2019, 146, 2255–2267. [Google Scholar] [CrossRef]
- Fujimoto, D.; Ueda, Y.; Hirono, Y.; Goi, T.; Yamaguchi, A. PAR1 Participates in the Ability of Multidrug Resistance and Tumorigenesis by Controlling Hippo-YAP Pathway. Oncotarget 2015, 6, 34788–34799. [Google Scholar] [CrossRef]
- Song, S.; Wang, Z.; Li, Y.; Ma, L.; Jin, J.; Scott, A.W.; Xu, Y.; Estrella, J.S.; Song, Y.; Liu, B.; et al. PPARδ Interacts with the Hippo Coactivator YAP1 to Promote SOX9 Expression and Gastric Cancer Progression. Mol. Cancer Res. 2020, 18, 390–402. [Google Scholar] [CrossRef]
- Song, S.; Ajani, J.A.; Honjo, S.; Maru, D.M.; Chen, Q.; Scott, A.W.; Heallen, T.R.; Xiao, L.; Hofstetter, W.L.; Weston, B.; et al. Hippo Coactivator YAP1 Upregulates SOX9 and Endows Esophageal Cancer Cells with Stem-like Properties. Cancer Res. 2014, 74, 4170–4182. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.J.; Kook, M.-C.; Kim, Y.-I.; Cho, S.-J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.-H. Helicobacter Pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, G.; Chu, S.-J.; Zhu, J.-S.; Zhang, R.; Lu, W.-W.; Xia, L.-Q.; Lu, Y.-M.; Da, W.; Sun, Q. Loss of Large Tumor Suppressor 1 Promotes Growth and Metastasis of Gastric Cancer Cells through Upregulation of the YAP Signaling. Oncotarget 2016, 7, 16180–16193. [Google Scholar] [CrossRef] [Green Version]
- Yue, G.; Sun, X.; Gimenez-Capitan, A.; Shen, J.; Yu, L.; Teixido, C.; Guan, W.; Rosell, R.; Liu, B.; Wei, J. TAZ Is Highly Expressed in Gastric Signet Ring Cell Carcinoma. BioMed Res. Int. 2014, 2014, 393064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Zhou, K.; Wang, Q.; Huang, Y.; Ji, J.; Peng, Y.; Zhang, X.; Zheng, T.; Zhang, Z.; Chong, D.; et al. The Transcription Factor RUNX2 Fuels YAP1 Signaling and Gastric Cancer Tumorigenesis. Cancer Sci. 2021, 112, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-J.; Yang, L.; Qian, F.; Wang, Y.-X.; Yu, X.; Ji, C.-D.; Cui, W.; Xiang, D.-F.; Zhang, X.; Zhang, P.; et al. Transcription Factor RUNX2 Up-Regulates Chemokine Receptor CXCR4 to Promote Invasive and Metastatic Potentials of Human Gastric Cancer. Oncotarget 2016, 7, 20999–21012. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Chiwaki, F.; Takahashi, R.; Aoyagi, K.; Yanagihara, K.; Nishimura, T.; Tamaoki, M.; Komatsu, M.; Komatsuzaki, R.; Matsusaki, K.; et al. Identification and Characterization of CXCR4-Positive Gastric Cancer Stem Cells. PLoS ONE 2015, 10, e0130808. [Google Scholar] [CrossRef]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell 2007, 1, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Kucia, M.; Reca, R.; Miekus, K.; Wanzeck, J.; Wojakowski, W.; Janowska-Wieczorek, A.; Ratajczak, J.; Ratajczak, M.Z. Trafficking of Normal Stem Cells and Metastasis of Cancer Stem Cells Involve Similar Mechanisms: Pivotal Role of the SDF-1–CXCR4 Axis. Stem Cells 2005, 23, 879–894. [Google Scholar] [CrossRef]
- Kim, J.; Takeuchi, H.; Lam, S.T.; Turner, R.R.; Wang, H.-J.; Kuo, C.; Foshag, L.; Bilchik, A.J.; Hoon, D.S.B. Chemokine Receptor CXCR4 Expression in Colorectal Cancer Patients Increases the Risk for Recurrence and for Poor Survival. J. Clin. Oncol. 2005, 23, 2744–2753. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of Chemokine Receptors in Breast Cancer Metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Zhou, W.; Xian, Q.; Wang, Q.; Wu, C.; Yan, H.; Li, X.; Lu, L.; Wu, C.; Zhu, D.; Xu, X.; et al. M6A Methyltransferase 3 Promotes the Proliferation and Migration of Gastric Cancer Cells through the M6A Modification of YAP1. J. Oncol. 2021, 2021, 8875424. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Li, H.; Huang, T.; Wong, C.C.; Wu, F.; Wu, M.; Weng, N.; Liu, L.; Cheng, A.S.L.; et al. AMOTL1 Enhances YAP1 Stability and Promotes YAP1-Driven Gastric Oncogenesis. Oncogene 2020, 39, 4375–4389. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wong, C.C.; Leung, K.T.; Wu, F.; Zhou, Y.; Tong, J.H.M.; Chan, R.C.K.; Li, H.; Wang, Y.; Yan, H.; et al. FGF18-FGFR2 Signaling Triggers the Activation of c-Jun-YAP1 Axis to Promote Carcinogenesis in a Subgroup of Gastric Cancer Patients and Indicates Translational Potential. Oncogene 2020, 39, 6647–6663. [Google Scholar] [CrossRef]
- Tang, Y.; Fang, G.; Guo, F.; Zhang, H.; Chen, X.; An, L.; Chen, M.; Zhou, L.; Wang, W.; Ye, T.; et al. Selective Inhibition of STRN3-Containing PP2A Phosphatase Restores Hippo Tumor-Suppressor Activity in Gastric Cancer. Cancer Cell 2020, 38, 115–128.e9. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Huang, T.; Zhou, Y.; Zhang, J.; Lung, R.W.M.; Tong, J.H.M.; Chan, A.W.H.; Zhang, B.; Wong, C.C.; Wu, F.; et al. MiR-375 Is Involved in Hippo Pathway by Targeting YAP1/TEAD4-CTGF Axis in Gastric Carcinogenesis. Cell Death Dis. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Belair, C.; Baud, J.; Chabas, S.; Sharma, C.M.; Vogel, J.; Staedel, C.; Darfeuille, F. Helicobacter Pylori Interferes with an Embryonic Stem Cell Micro RNA Cluster to Block Cell Cycle Progression. Silence 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Huang, F.; Shi, Y.; Zhang, Q.; Xu, S.; Yao, Y.; Jiang, R. RP11-323N12.5 Promotes the Malignancy and Immunosuppression of Human Gastric Cancer by Increasing YAP1 Transcription. Gastric Cancer 2020, 24, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, B.-J.; Han, W.; Chi, C.-H.; Gu, C.; Wang, Y.; Fu, X.; Huang, W.; Liu, Z.; Song, X. CFIm25-Regulated LncRNA Acv3UTR Promotes Gastric Tumorigenesis via MiR-590-5p/YAP1 Axis. Oncogene 2020, 39, 3075–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Di, X.; Bi, Y.; Sun, S.; Wang, T. Long Non-Coding RNA LINC00649 Regulates YES-Associated Protein 1 (YAP1)/Hippo Pathway to Accelerate Gastric Cancer (GC) Progression via Sequestering MiR-16-5p. Bioengineered 2021, 12, 1791–1802. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Y.; Wang, H.; Xin, Y. The Novel Long Non-Coding RNA LATS2-AS1-001 Inhibits Gastric Cancer Progression by Regulating the LATS2/YAP1 Signaling Pathway via Binding to EZH2. Cancer Cell Int. 2020, 20, 204. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Wang, K.; Zhang, X.; Yu, Y.; Lv, Y.; Zhang, S.; Zhang, L.; Guo, Y.; Li, Y.; et al. A Novel YAP1/SLC35B4 Regulatory Axis Contributes to Proliferation and Progression of Gastric Carcinoma. Cell Death Dis. 2019, 10, 452. [Google Scholar] [CrossRef]
- Huang, S.; Cao, Y.; Guo, H.; Yao, Y.; Li, L.; Chen, J.; Li, J.; Xiang, X.; Deng, J.; Xiong, J. Up-Regulated Acylglycerol Kinase (AGK) Expression Associates with Gastric Cancer Progression through the Formation of a Novel YAP1-AGK-Positive Loop. J. Cell. Mol. Med. 2020, 24, 11133–11145. [Google Scholar] [CrossRef]
- Shi, J.; Li, F.; Yao, X.; Mou, T.; Xu, Z.; Han, Z.; Chen, S.; Li, W.; Yu, J.; Qi, X.; et al. The HER4-YAP1 Axis Promotes Trastuzumab Resistance in HER2-Positive Gastric Cancer by Inducing Epithelial and Mesenchymal Transition. Oncogene 2018, 37, 3022–3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, L.; Li, D.-S.; Chen, F.; Feng, J.-D.; Li, B.; Wang, T.-J. TAZ Overexpression Is Associated with Epithelial-Mesenchymal Transition in Cisplatin-Resistant Gastric Cancer Cells. Int. J. Oncol. 2017, 51, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T.; Sugihara, T.; Hoshino, Y.; Tarumoto, R.; Matsuki, Y.; Kanda, T.; Takata, T.; Nagahara, T.; Matono, T.; Isomoto, H. Photosensitizer Verteporfin Inhibits the Growth of YAP- and TAZ-Dominant Gastric Cancer Cells by Suppressing the Anti-Apoptotic Protein Survivin in a Light-Independent Manner. Oncol. Lett. 2021, 22, 703. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Rao, X.; Cui, Y.; Zhang, L.; Li, X.; Wang, B.; Zheng, Y.; Teng, L.; Zhou, T.; Zhuo, W. The Keratin 17/YAP/IL6 Axis Contributes to E-Cadherin Loss and Aggressiveness of Diffuse Gastric Cancer. Oncogene 2022, 41, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Liu, Q.; Salto-Tellez, M.; Yano, T.; Tada, K.; Ida, H.; Huang, C.; Shah, N.; Inoue, M.; Rajnakova, A.; et al. RUNX3, a Novel Tumor Suppressor, Is Frequently Inactivated in Gastric Cancer by Protein Mislocalization. Cancer Res. 2005, 65, 7743–7750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.-W.; Kim, M.-K.; Lee, Y.-S.; Lee, J.-W.; Kim, D.-M.; Song, S.-H.; Lee, J.-Y.; Choi, B.-Y.; Min, B.; Chi, X.-Z.; et al. RAC-LATS1/2 Signaling Regulates YAP Activity by Switching between the YAP-Binding Partners TEAD4 and RUNX3. Oncogene 2017, 36, 999–1011. [Google Scholar] [CrossRef]
- Shi, Z.; He, F.; Chen, M.; Hua, L.; Wang, W.; Jiao, S.; Zhou, Z. DNA-Binding Mechanism of the Hippo Pathway Transcription Factor TEAD4. Oncogene 2017, 36, 4362–4369. [Google Scholar] [CrossRef]
- Drug Approval Package: Visudyne (Verteporfin) Injection NDA 21-119. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-119_Visudyne.cfm (accessed on 21 January 2022).
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.-J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and Pharmacological Disruption of the TEAD–YAP Complex Suppresses the Oncogenic Activity of YAP. Genes Dev. 2012, 26, 1300. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-H.; Jeong, G.S.; Smoot, D.T.; Ashktorab, H.; Hwang, C.M.; Kim, B.S.; Kim, H.S.; Park, Y.-Y. Verteporfin Inhibits Gastric Cancer Cell Growth by Suppressing Adhesion Molecule FAT1. Oncotarget 2017, 8, 98887–98897. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zhao, L.; Chen, S.; Zheng, B.; Chen, H.; Zeng, T.; Sun, H.; Zhong, S.; Wu, W.; Lin, X.; et al. The Curcumin Analogue WZ35 Affects Glycolysis Inhibition of Gastric Cancer Cells through ROS-YAP-JNK Pathway. Food Chem. Toxicol. 2020, 137, 111131. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Tao, Z.; Zhao, L.; Zhu, Z.; Wu, W.; He, Y.; Chen, H.; Zheng, B.; Huang, X.; et al. Curcumin Derivative WZ35 Inhibits Tumor Cell Growth via ROS-YAP-JNK Signaling Pathway in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Wu, L.; Xiao, H.D.; Du, Q.; Liang, J. Inhibitory Effects of Dobutamine on Human Gastric Adenocarcinoma. World J. Gastroenterol. 2014, 20, 17092. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, Q.-L.; Wu, B.-W.; Zhu, T.; Cui, X.-X.; Jin, C.-J.; Wang, S.-Y.; Wang, S.-H.; Fu, D.-J.; Liu, H.-M.; et al. Discovery of Tertiary Amide Derivatives Incorporating Benzothiazole Moiety as Anti-Gastric Cancer Agents in Vitro via Inhibiting Tubulin Polymerization and Activating the Hippo Signaling Pathway. Eur. J. Med. Chem. 2020, 203, 112618. [Google Scholar] [CrossRef] [PubMed]
- Oku, Y.; Nishiya, N.; Shito, T.; Yamamoto, R.; Yamamoto, Y.; Oyama, C.; Uehara, Y. Small Molecules Inhibiting the Nuclear Localization of YAP/TAZ for Chemotherapeutics and Chemosensitizers against Breast Cancers. FEBS Open Bio 2015, 5, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xia, H.; Zhou, S.; Tang, Q.; Zhou, J.; Ren, M.; Bi, F. Simvastatin Inhibits the Malignant Behaviors of Gastric Cancer Cells by Simultaneously Suppressing YAP and β-Catenin Signaling. OncoTargets Ther. 2020, 13, 2057–2066. [Google Scholar] [CrossRef] [Green Version]
- Taccioli, C.; Sorrentino, G.; Zannini, A.; Caroli, J.; Beneventano, D.; Anderlucci, L.; Lolli, M.; Bicciato, S.; Del Sal, G. MDP, a Database Linking Drug Response Data to Genomic Information, Identifies Dasatinib and Statins as a Combinatorial Strategy to Inhibit YAP/TAZ in Cancer Cells. Oncotarget 2015, 6, 38854–38865. [Google Scholar] [CrossRef] [Green Version]
- Courtois, S.; Durán, R.V.; Giraud, J.; Sifré, E.; Izotte, J.; Mégraud, F.; Lehours, P.; Varon, C.; Bessède, E. Metformin Targets Gastric Cancer Stem Cells. Eur. J. Cancer Oxf. Engl. 2017, 84, 193–201. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Feng, W.; Yu, Y.; Jeong, K.; Guo, W.; Lu, Y.; Mills, G.B. Verteporfin Inhibits YAP Function through Up-Regulating 14-3-3σ Sequestering YAP in the Cytoplasm. Am. J. Cancer Res. 2015, 6, 27–37. [Google Scholar]
- Mae, Y.; Kanda, T.; Sugihara, T.; Takata, T.; Kinoshita, H.; Sakaguchi, T.; Hasegawa, T.; Tarumoto, R.; Edano, M.; Kurumi, H.; et al. Verteporfin-photodynamic Therapy Is Effective on Gastric Cancer Cells. Mol. Clin. Oncol. 2020, 13, 1. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, T.; Xu, Z.; Lin, Z.; Zhang, Z.; Feng, T.; Zhu, L.; Rong, Y.; Shen, H.; Luk, J.M.; et al. Targeting Hippo Pathway by Specific Interruption of YAP-TEAD Interaction Using Cyclic YAP-like Peptides. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 724–732. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Pan, Y.; Liao, D.; Tang, J.; Yao, D. Peptide 17, an Inhibitor of YAP/TEAD4 Pathway, Mitigates Lung Cancer Malignancy. Trop. J. Pharm. Res. 2018, 17, 1255. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, X.; Tan, J.; Tian, R.; Shen, P.; Cai, W.; Liao, H. Curcumin Suppresses the Stemness of Non-Small Cell Lung Cancer Cells via Promoting the Nuclear-Cytoplasm Translocation of TAZ. Environ. Toxicol. 2021, 36, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, W.; Pan, Y.; Gao, Y.; Deng, L.; Li, F.; Li, F.; Ma, X.; Hou, S.; Xu, J.; et al. YAP Suppresses Lung Squamous Cell Carcinoma Progression via Deregulation of the DNp63-GPX2 Axis and ROS Accumulation. Cancer Res. 2017, 77, 5769–5781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet, B.; Dupuis, B.; Chopin, C. Dobutamine: Mechanisms of action and use in acute cardiovascular pathology. Ann. Cardiol. Angeiol. 1991, 40, 397–402. [Google Scholar]
- Robbers-Visser, D.; Luijnenburg, S.E.; van den Berg, J.; Roos-Hesselink, J.W.; Strengers, J.L.; Kapusta, L.; Moelker, A.; Helbing, W.A. Safety and Observer Variability of Cardiac Magnetic Resonance Imaging Combined with Low-Dose Dobutamine Stress-Testing in Patients with Complex Congenital Heart Disease. Int. J. Cardiol. 2011, 147, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Drug Approval Package: Dobutrex Solution (Dobutamine) NDA #17-820/S-037. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/17-820_Dobutrex.cfm (accessed on 7 February 2022).
- Bao, Y.; Nakagawa, K.; Yang, Z.; Ikeda, M.; Withanage, K.; Ishigami-Yuasa, M.; Okuno, Y.; Hata, S.; Nishina, H.; Hata, Y. A Cell-Based Assay to Screen Stimulators of the Hippo Pathway Reveals the Inhibitory Effect of Dobutamine on the YAP-Dependent Gene Transcription. J. Biochem. 2011, 150, 199–208. [Google Scholar] [CrossRef]
- Drug Approval Package: Lescol XL (Fluvastatin Sodium) NDA #021192. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-192_Lescol.cfm (accessed on 23 January 2022).
- Drug Approval Package: Glucophage (Metformin Hydrochloride) NDA# 020357/S010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/020357s010.cfm (accessed on 7 February 2022).
- Votrient (Pazopanib) FDA Approval History—Drugs.Com. Available online: https://www.drugs.com/history/votrient.html (accessed on 7 February 2022).
- Bian, S.-B.; Yang, Y.; Liang, W.-Q.; Zhang, K.-C.; Chen, L.; Zhang, Z.-T. Leukemia Inhibitory Factor Promotes Gastric Cancer Cell Proliferation, Migration, and Invasion via the LIFR–Hippo–YAP Pathway. Ann. N. Y. Acad. Sci. 2021, 1484, 74–89. [Google Scholar] [CrossRef]
- Elisi, G.M.; Santucci, M.; D’Arca, D.; Lauriola, A.; Marverti, G.; Losi, L.; Scalvini, L.; Bolognesi, M.L.; Mor, M.; Costi, M.P. Repurposing of Drugs Targeting YAP-TEAD Functions. Cancers 2018, 10, 329. [Google Scholar] [CrossRef] [Green Version]
- Santucci, M.; Vignudelli, T.; Ferrari, S.; Mor, M.; Scalvini, L.; Bolognesi, M.L.; Uliassi, E.; Costi, M.P. The Hippo Pathway and YAP/TAZ-TEAD Protein-Protein Interaction as Targets for Regenerative Medicine and Cancer Treatment. J. Med. Chem. 2015, 58, 4857–4873. [Google Scholar] [CrossRef]
- Elbaz, H.A.; Stueckle, T.A.; Tse, W.; Rojanasakul, Y.; Dinu, C.Z. Digitoxin and Its Analogs as Novel Cancer Therapeutics. Exp. Hematol. Oncol. 2012, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Elbaz, H.A.; Stueckle, T.A.; Wang, H.-Y.L.; O’Doherty, G.A.; Lowry, D.T.; Sargent, L.M.; Wang, L.; Dinu, C.Z.; Rojanasakul, Y. Digitoxin and a Synthetic Monosaccharide Analog Inhibit Cell Viability in Lung Cancer Cells. Toxicol. Appl. Pharmacol. 2012, 258, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Lázaro, M.; Pastor, N.; Azrak, S.S.; Ayuso, M.J.; Austin, C.A.; Cortés, F. Digitoxin Inhibits the Growth of Cancer Cell Lines at Concentrations Commonly Found in Cardiac Patients. J. Nat. Prod. 2005, 68, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qian, D.Z.; Tan, Y.S.; Lee, K.; Gao, P.; Ren, Y.R.; Rey, S.; Hammers, H.; Chang, D.; Pili, R.; et al. Digoxin and Other Cardiac Glycosides Inhibit HIF-1alpha Synthesis and Block Tumor Growth. Proc. Natl. Acad. Sci. USA 2008, 105, 19579–19586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, K.-L.; Yu, F.; Ding, S. Inhibitors of Hippo-Yap Signaling Pathway. Application Number 14406749, 31 May 2013. [Google Scholar]
- Pobbati, A.V.; Han, X.; Hung, A.W.; Weiguang, S.; Huda, N.; Chen, G.-Y.; Kang, C.; Chia, C.S.B.; Luo, X.; Hong, W.; et al. Targeting the Central Pocket in Human Transcription Factor TEAD as a Potential Cancer Therapeutic Strategy. Struct. Lond. Engl. 1993 2015, 23, 2076–2086. [Google Scholar] [CrossRef] [Green Version]
- Bum-Erdene, K.; Zhou, D.; Gonzalez-Gutierrez, G.; Ghozayel, M.K.; Si, Y.; Xu, D.; Shannon, H.E.; Bailey, B.J.; Corson, T.W.; Pollok, K.E.; et al. Small-Molecule Covalent Modification of Conserved Cysteine Leads to Allosteric Inhibition of the TEAD⋅Yap Protein-Protein Interaction. Cell Chem. Biol. 2019, 26, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Kunig, V.B.K.; Potowski, M.; Akbarzadeh, M.; Klika Škopić, M.; Dos Santos Smith, D.; Arendt, L.; Dormuth, I.; Adihou, H.; Andlovic, B.; Karatas, H.; et al. TEAD-YAP Interaction Inhibitors and MDM2 Binders from DNA-Encoded Indole-Focused Ugi Peptidomimetics. Angew. Chem. Int. Ed. 2020, 59, 20338–20342. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Akbarzadeh, M.; Adihou, H.; Hahne, G.; Pobbati, A.V.; Yihui Ng, E.; Guéret, S.M.; Sievers, S.; Pahl, A.; Metz, M.; et al. Discovery of Covalent Inhibitors Targeting the Transcriptional Enhanced Associate Domain Central Pocket. J. Med. Chem. 2020, 63, 11972–11989. [Google Scholar] [CrossRef]
- Nouri, K.; Azad, T.; Ling, M.; Janse van Rensburg, H.J.; Pipchuk, A.; Shen, H.; Hao, Y.; Zhang, J.; Yang, X. Identification of Celastrol as a Novel YAP-TEAD Inhibitor for Cancer Therapy by High Throughput Screening with Ultrasensitive YAP/TAZ-TEAD Biosensors. Cancers 2019, 11, 1596. [Google Scholar] [CrossRef] [Green Version]
- Crook, Z.R.; Sevilla, G.P.; Friend, D.; Brusniak, M.-Y.; Bandaranayake, A.D.; Clarke, M.; Gewe, M.; Mhyre, A.J.; Baker, D.; Strong, R.K.; et al. Mammalian Display Screening of Diverse Cystine-Dense Peptides for Difficult to Drug Targets. Nat. Commun. 2017, 8, 2244. [Google Scholar] [CrossRef]
- Smith, S.A.; Sessions, R.B.; Shoemark, D.K.; Williams, C.; Ebrahimighaei, R.; McNeill, M.C.; Crump, M.P.; McKay, T.R.; Harris, G.; Newby, A.C.; et al. Antiproliferative and Antimigratory Effects of a Novel YAP–TEAD Interaction Inhibitor Identified Using in Silico Molecular Docking. J. Med. Chem. 2019, 62, 1291–1305. [Google Scholar] [CrossRef] [Green Version]
- Sturbaut, M.; Bailly, F.; Coevoet, M.; Sileo, P.; Pugniere, M.; Liberelle, M.; Magnez, R.; Thuru, X.; Chartier-Harlin, M.-C.; Melnyk, P.; et al. Discovery of a Cryptic Site at the Interface 2 of TEAD—Towards a New Family of YAP/TAZ-TEAD Inhibitors. Eur. J. Med. Chem. 2021, 226, 113835. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, J.; Li, Y.; Tao, H.; Xiong, H.; Lian, F.; Gao, J.; Ma, H.; Lu, T.; Zhang, D.; et al. Discovery and Biological Evaluation of Vinylsulfonamide Derivatives as Highly Potent, Covalent TEAD Autopalmitoylation Inhibitors. Eur. J. Med. Chem. 2019, 184, 111767. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, J.; Su, W.; Shan, H.; Zhang, B.; Wang, Y.; Shabanova, A.; Shan, H.; Liang, H. Melatonin Protects against Lung Fibrosis by Regulating the Hippo/YAP Pathway. Int. J. Mol. Sci. 2018, 19, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bristol-Myers Squibb Submits New Drug Application for Dasatinib. Available online: https://www.drugs.com/nda/sprycel_051228.html (accessed on 25 April 2022).
- Basu, D.; Lettan, R.; Damodaran, K.; Strellec, S.; Reyes-Mugica, M.; Rebbaa, A. Identification, Mechanism of Action and Anti-Tumor Activity of a Small Molecule Inhibitor of Hippo, TGF Beta, and WNT Signaling Pathways. Mol. Cancer Ther. 2014, 13, 1457–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, E.R.; Simov, V.; Valtingojer, I.; Venier, O. Recent Therapeutic Approaches to Modulate the Hippo Pathway in Oncology and Regenerative Medicine. Cells 2021, 10, 2715. [Google Scholar] [CrossRef]

| Expression Levels of YAP/TAZ | Regulation | Reference |
|---|---|---|
| Overexpression of YAP | Increase in pro-proliferation and pro-survival genes | [106] |
| Upregulated through RUNX3 inactivation in GC | [31] | |
| Induced by METTL3 found highly expressed in GC | [139] | |
| Regulation by fixation of lncRNA RP11-323N12.5 on its promoter | [145] | |
| Induced by HER4 and increases EMT, GC cells proliferation, and HER2-therapy resistance | [151] | |
| Overactivation of YAP | Activated by PAR1 through inhibition of LATS Upregulation of stem-like properties | [126] |
| Interacts with AMOTL1 to promote its nuclear translocation and activity | [140] | |
| Activation through MAPK-c-Jun pathway | [141] | |
| Inhibition is decreased through PP2A- inhibition of MST1/2 | [142] | |
| Overexpression of TAZ | Co-localisation with ZEB1 EMT transcription factor CSC tumorigenic properties | [107] |
| Upregulation by MiR-125a-5p, leading to stimulation of genes involved in cell survival, EMT, invasion, and tumour growth | [87] | |
| Highly expressed in SRCC poorly undifferentiated GC | [153] | |
| YAP/TAZ overexpression in CSCs and residual cells after chemotherapy-treatment | Overexpression of associated target genes | [125] |
| Strategies | Molecules | Mechanism | Reference |
|---|---|---|---|
| Targeting oncogenic YAP/TAZ-TEAD signalling | RUNX3 | YAP-TEAD interaction competitor | [31,151,152] |
| VGLL4 | YAP-TEAD interaction competitor | [30,113,153] | |
| Super-TDU | YAP-TEAD interaction competitor | [113] | |
| Verteporfin | YAP-TEAD interaction competitor Targets FAT1 and Survivin Induces 14-3-3 proteins PDT and induces cell death through singlet oxygen production | [121,149,154,155,156,157,158] | |
| Peptide 17 | YAP-TEAD interaction inhibitor, Targets N6-methyladenosine (m6A)’s methyltransferase 3 | [135,159,160] | |
| WZ35, a Curcumin analogue | Cell death through increase in cellular ROS level | [161,162] | |
| Hippo kinases and/or side-pathways stimulation | Dobutamine | Recruits YAP to the cytoplasm through PKA signalling | [163] |
| Compound F10 | MST1/2 activation through cytoskeletal alteration | [164] | |
| Statins (Lovastatin, Fluvastatin, Simvastatin) | Modulates actin dynamics and activate Hippo kinases Inhibit β-catenin expression and YAP activity | [165,166,167] | |
| Metformin | Induces AMPK, which stabilizes AMOTL1 and induces Hippo kinases | [67,168] | |
| Pazopanib | Promotes effectors degradation by the ubiquitin-proteasome system | [165] | |
| SHAP | Alters STRN3-PP2Aa interaction and restores MST1/2 activity | [142] | |
| Leukaemia Inhibitory Factor | Induces LATS1/2 phosphorylation by MST1/2 and through Scribble activation in some cases | [42,43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seeneevassen, L.; Dubus, P.; Gronnier, C.; Varon, C. Hippo in Gastric Cancer: From Signalling to Therapy. Cancers 2022, 14, 2282. https://doi.org/10.3390/cancers14092282
Seeneevassen L, Dubus P, Gronnier C, Varon C. Hippo in Gastric Cancer: From Signalling to Therapy. Cancers. 2022; 14(9):2282. https://doi.org/10.3390/cancers14092282
Chicago/Turabian StyleSeeneevassen, Lornella, Pierre Dubus, Caroline Gronnier, and Christine Varon. 2022. "Hippo in Gastric Cancer: From Signalling to Therapy" Cancers 14, no. 9: 2282. https://doi.org/10.3390/cancers14092282
APA StyleSeeneevassen, L., Dubus, P., Gronnier, C., & Varon, C. (2022). Hippo in Gastric Cancer: From Signalling to Therapy. Cancers, 14(9), 2282. https://doi.org/10.3390/cancers14092282






