Long-Term Survival, Prognostic Factors, and Quality of Life of Patients Undergoing Pelvic Exenteration for Cervical Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Treatment Administration
2.3. Statistical Analysis and Data Assessment
2.4. Quality of Life Questionnaires
3. Results
The Results of the Quality of Life Study
4. Discussion
4.1. Survival and Prognostic Factors
4.2. Quality of Life
4.3. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunschwig, A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948, 1, 177–183. [Google Scholar] [CrossRef]
- Lewandowska, A.; Szubert, S.; Koper, K.; Koper, A.; Cwynar, G.; Wicherek, L. Analysis of long-term outcomes in 44 patients following pelvic exenteration due to cervical cancer. World J. Surg. Oncol. 2020, 18, 234. [Google Scholar] [CrossRef]
- Peiretti, M.; Zapardiel, I.; Zanagnolo, V.; Landoni, F.; Morrow, C.P.; Maggioni, A. Management of recurrent cervical cancer: A review of the literature. Surg. Oncol. 2012, 21, e59–e66. [Google Scholar] [CrossRef]
- Chiantera, V.; Rossi, M.; De Iaco, P.; Koehler, C.; Marnitz, S.; Ferrandina, G.; Legge, F.; Parazzini, F.; Scambia, G.; Schneider, A.; et al. Survival after curative pelvic exenteration for primary or recurrent cervical cancer: A retrospective multicentric study of 167 patients. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2014, 24, 916–922. [Google Scholar] [CrossRef]
- Jäger, L.; Nilsson, P.J.; Rådestad, A.F. Pelvic exenteration for recurrent gynecologic malignancy: A study of 28 consecutive patients at a single institution. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2013, 23, 755–762. [Google Scholar] [CrossRef]
- Petruzziello, A.; Kondo, W.; Hatschback, S.B.; Guerreiro, J.A.; Filho, F.P.; Vendrame, C.; Luz, M.; Ribeiro, R. Surgical results of pelvic exenteration in the treatment of gynecologic cancer. World J. Surg. Oncol. 2014, 12, 279. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Joniau, S.; D’Hoore, A.; Van Calster, B.; Van Limbergen, E.; Leunen, K.; Penninckx, F.; Van Poppel, H.; Amant, F.; Vergote, I. Pelvic exenterations for gynecological malignancies: A study of 36 cases. Int. J. Gynecol. Cancer 2012, 22, 889–896. [Google Scholar] [CrossRef]
- Marnitz, S.; Köhler, C.; Müller, M.; Behrens, K.; Hasenbein, K.; Schneider, A. Indications for primary and secondary exenterations in patients with cervical cancer. Gynecol. Oncol. 2006, 103, 1023–1030. [Google Scholar] [CrossRef]
- Höckel, M.; Dornhöfer, N. Pelvic exenteration for gynecological tumors: Achievements and unanswered questions. Lancet Oncol. 2006, 7, 837–847. [Google Scholar] [CrossRef]
- de Gregorio, N.; de Gregorio, A.; Ebner, F.; Friedl, T.W.P.; Huober, J.; Hefty, R.; Wittau, M.; Janni, W.; Widschwendter, P. Pelvic exenteration as ultimate ratio for gynecologic cancers: Single-center analyses of 37 cases. Arch. Gynecol. Obstet. 2019, 300, 161–168. [Google Scholar] [CrossRef]
- Bhangu, A.; Mohammed Ali, S.; Brown, G.; Nicholls, R.J.; Tekkis, P. Indications and outcome of pelvic exenteration for locally advanced primary and recurrent rectal cancer. Ann. Surg. 2014, 259, 315–322. [Google Scholar] [CrossRef]
- Li, L.; Ma, S.Q.; Tan, X.J.; Zhong, S.; Wu, M. Pelvic Exenteration for Recurrent and Persistent Cervical Cancer. Chin. Med. J. 2018, 131, 1541–1548. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.J.M.D.; et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Greimel, E.R.; Vlasic, K.K.; Waldenstrom, A.C.; Duric, V.M.; Jensen, P.T.; Singer, S.; Chie, W.; Nordin, A.; Radisic, V.B.; Wydra, D. The European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life questionnaire cervical cancer module: EORTC QLQ-CX24. Cancer 2006, 107, 1812–1822. [Google Scholar] [CrossRef]
- Căpîlna, M.E.; Moldovan, B.; Szabo, B. Pelvic exenteration—Our initial experience in 15 cases. Eur. J. Gynaecol. Oncol. 2015, 36, 142–145. [Google Scholar]
- Cǎpîlna, M.E.; Szabo, B.; Rusu, S.C.; Becsi, J.; Moldovan, B.; Neagoe, R.M.; Muhlfay, G. Anatomical variations of the obturator veins and their surgical implications. Eur. J. Gynaecol. Oncol. 2017, 38, 263–265. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Sonoda, Y.; Black, D.; Levine, D.A.; Chi, D.S.; Barakat, R.R. Fertility-sparing radical abdominal trachelectomy for cervical carcinoma: Technique and review of the literature. Gynecol. Oncol. 2006, 103, 807–813. [Google Scholar] [CrossRef]
- Jalloul, R.J.; Nick, A.M.; Munsell, M.F.; Westin, S.N.; Ramirez, P.T.; Frumovitz, M.; Soliman, P.T. The influence of surgeon volume on outcomes after pelvic exenteration for a gynecologic cancer. J. Gynecol. Oncol. 2018, 29, e68. [Google Scholar] [CrossRef]
- Baiocchi, G.; Guimaraes, G.C.; Faloppa, C.C.; Kumagai, L.Y.; Oliveira, R.A.R.; Begnami, M.D.; Soares, F.A.; Lopes, A. Does histologic type correlate to outcome after pelvic exenteration for cervical and vaginal cancer? Ann. Surg. Oncol. 2013, 20, 1694–1700. [Google Scholar] [CrossRef]
- Yoo, H.J.; Lim, M.C.; Seo, S.S.; Kang, S.; Yoo, C.W.; Kim, J.Y.; Park, S.Y. Pelvic exenteration for recurrent cervical cancer: Ten-year experience at national cancer center in Korea. J. Gynecol. Oncol. 2012, 23, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Moutardier, V.; Houvenaeghel, G.; Martino, M.; Lelong, B.; Bardou, V.J.; Resbeut, M.; Delpero, J.R. Surgical resection of locally recurrent cervical cancer: A single institutional 70 patient series. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2004, 14, 846–851. [Google Scholar] [CrossRef]
- Stanca, M.; Căpîlna, M.E. Prognostic Factors Associated with 5-Year Overall Survival in Cervical Cancer Patients Treated with Radical Hysterectomy Followed by Adjuvant Concurrent Chemoradiation Therapy at a Tertiary Care Center in Eastern Europe. Diagnostics 2021, 11, 570. [Google Scholar] [CrossRef]
- Stanca, M.; Căpîlna, D.M.; Trâmbițaș, C.; Căpîlna, M.E. The Overall Quality of Life and Oncological Outcomes Following Radical Hysterectomy in Cervical Cancer Survivors Results from a Large Long-Term Single-Institution Study. Cancers 2022, 14, 317. [Google Scholar] [CrossRef]
- Magrina, J.F.; Stanhope, C.R.; Weaver, A.L. Pelvic exenterations: Supralevator, infralevator, and with vulvectomy. Gynecol. Oncol. 1997, 64, 130–135. [Google Scholar] [CrossRef]
- Lago, V.; Poveda, I.; Padilla-Iserte, P.; Simón-Sanz, E.; García-Granero, Á.; Pontones, J.L.; Matute, L.; Domingo, S. Pelvic exenteration in gynecologic cancer: Complications and oncological outcome. Gynecol. Surg. 2019, 16, 317. [Google Scholar] [CrossRef]
- Căpîlna, M.E.; Palfalvi, L.; Ungar, L.; Cozlea, A.; Kiss, S.L.; Stanca, M. Orthotopic continent urinary diversion (the Budapest pouch) in 10 steps. Int. J. Gynecol. Cancer 2020, 30, 1842–1843. [Google Scholar] [CrossRef]
- Moreno-Palacios, E.; Diestro, M.D.; De Santiago, J.; Hernández, A.; Zapardiel, I. Pelvic exenteration in gynecologic cancer: La Paz university hospital experience. Int. J. Gynecol. Cancer 2015, 25, 1109–1114. [Google Scholar] [CrossRef]
- Urh, A.; Soliman, P.T.; Schmeler, K.M.; Westin, S.; Frumovitz, M.; Nick, A.M.; Fellman, B.; Urbauer, D.L.; Ramirez, P.T. Postoperative outcomes after continent versus incontinent urinary diversion at the time of pelvic exenteration for gynecologic malignancies. Gynecol. Oncol. 2013, 129, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Peters, W.A.; Liu, P.Y.; Barrett, R.J.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- Matsuo, K.; Mandelbaum, R.S.; Adams, C.L.; Roman, L.D.; Wright, J.D. Performance and outcome of pelvic exenteration for gynecologic malignancies: A population-based study. Gynecol. Oncol. 2019, 153, 368–375. [Google Scholar] [CrossRef]
- Kelly, M.E.; Ryan, E.J.; Aalbers, A.G.J.; Abdul, A.N.; Abraham-Nordling, M.; Alberda, W.; Antoniou, A.; Austin, K.K.; Baker, R.; Bali, M.; et al. Pelvic exenteration for advanced nonrectal pelvic malignancy. Ann. Surg. 2019, 270, 899–905. [Google Scholar] [CrossRef]
- Zoucas, E.; Frederiksen, S.; Lydrup, M.L.; Månsson, W.; Gustafson, P.; Alberius, P. Pelvic Exenteration for Advanced and Recurrent Malignancy. World J. Surg. 2010, 34, 2177–2184. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I.; Vilcu, M.; Neacsu, A.; Dima, S.; Croitoru, A.; Brezean, I. Pelvic Exenteration for Locally Advanced and Relapsed Pelvic Malignancies—An Analysis of 100 Cases. Vivo 2019, 33, 2205–2210. [Google Scholar] [CrossRef]
- Sardain, H.; Lavoue, V.; Redpath, M.; Bertheuil, N.; Foucher, F.; Levêque, J. Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review. Eur. J. Surg.Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2015, 41, 975–985. [Google Scholar] [CrossRef]
- Karmaniolou, I.; Arkadopoulos, N.; Vassiliou, P.; Nastos, C.; Dellaportas, D.; Siatelis, A.; Theodosopoulos, T.; Vezakis, A.; Parasyris, S.; Polydorou, A.; et al. Pelvic Exenteration Put into Therapeutical and Palliative Perspective: It Is Worth to Try. Indian J. Surg. Oncol. 2018, 9, 552–557. [Google Scholar] [CrossRef]
- Westin, S.N.; Rallapalli, V.; Fellman, B.; Urbauer, D.L.; Pal, N.; Frumovitz, M.M.; Ramondetta, L.M.; Bodurka, D.C.; Ramirez, P.T.; Soliman, P.T. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecol. Oncol. 2014, 134, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Benn, T.; Brooks, R.A.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Mutch, D.G.; Zighelboim, I. Pelvic exenteration in gynecologic oncology: A single institution study over 20 years. Gynecol. Oncol. 2011, 122, 14–18. [Google Scholar] [CrossRef] [Green Version]
- European Society of Gynaecological Oncology. Cervical Cancer Pocket Guidelines. Eur. Soc. Gynaecol. Oncol. 2018, 1–48. Available online: https://guidelines.esgo.org/cervical-cancer/guidelines/recommendations/ (accessed on 19 April 2022).
- Dessole, M.; Petrillo, M.; Lucidi, A.; Naldini, A.; Rossi, M.; De Iaco, P.; Marnitz, S.; Sehouli, J.; Scambia, G.; Chiantera, V. Quality of life in women after pelvic exenteration for gynecological malignancies: A multicentric study. Int. J. Gynecol. Cancer 2018, 28, 267–273. [Google Scholar] [CrossRef]
- Rezk, Y.A.; Hurley, K.E.; Carter, J.; Dao, F.; Bochner, B.H.; Aubey, J.J.; Caceres, A.; Einstein, M.H.; Abu-Rustum, N.R.; Barakat, R.R.; et al. A prospective study of quality of life in patients undergoing pelvic exenteration: Interim results. Gynecol. Oncol. 2013, 128, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Hawighorst-Knapstein, S.; Schönefuß, G.; Hoffmann, S.O.; Knapstein, P.G. Pelvic exenteration: Effects of surgery on quality of life and body image—A prospective longitudinal study. Gynecol. Oncol. 1997, 66, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Wilson, I.; Ira, B. Clinical understanding and clinical implications of response shift. Soc. Sci. Med. 1999, 48, 1577–1588. [Google Scholar] [CrossRef]
- Pilger, A.; Richter, R.; Fotopoulou, C.; Beteta, C.; Klapp, C.; Sehouli, J. Quality of Life and Sexuality of Patients after Treatment for Gynaecological Malignancies: Results of a Prospective Study in 55 Patients. Anticancer. Res. 2012, 32, 5045–5049. [Google Scholar]
- Wang, Z.; Huang, J.; Zeng, A.; Wu, M.; Wang, X. Vaginoplasty with Acellular Dermal Matrix after Radical Resection for Carcinoma of the Uterine Cervix. J. Investig. Surg. Off. J. Acad. Surg. Res. 2019, 32, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Crane, C.N.; Santucci, R.A. Vaginoplasty tips and tricks. Int. Braz J Urol Off. J. Braz. Soc. Urol. 2021, 47, 263–273. [Google Scholar] [CrossRef] [PubMed]
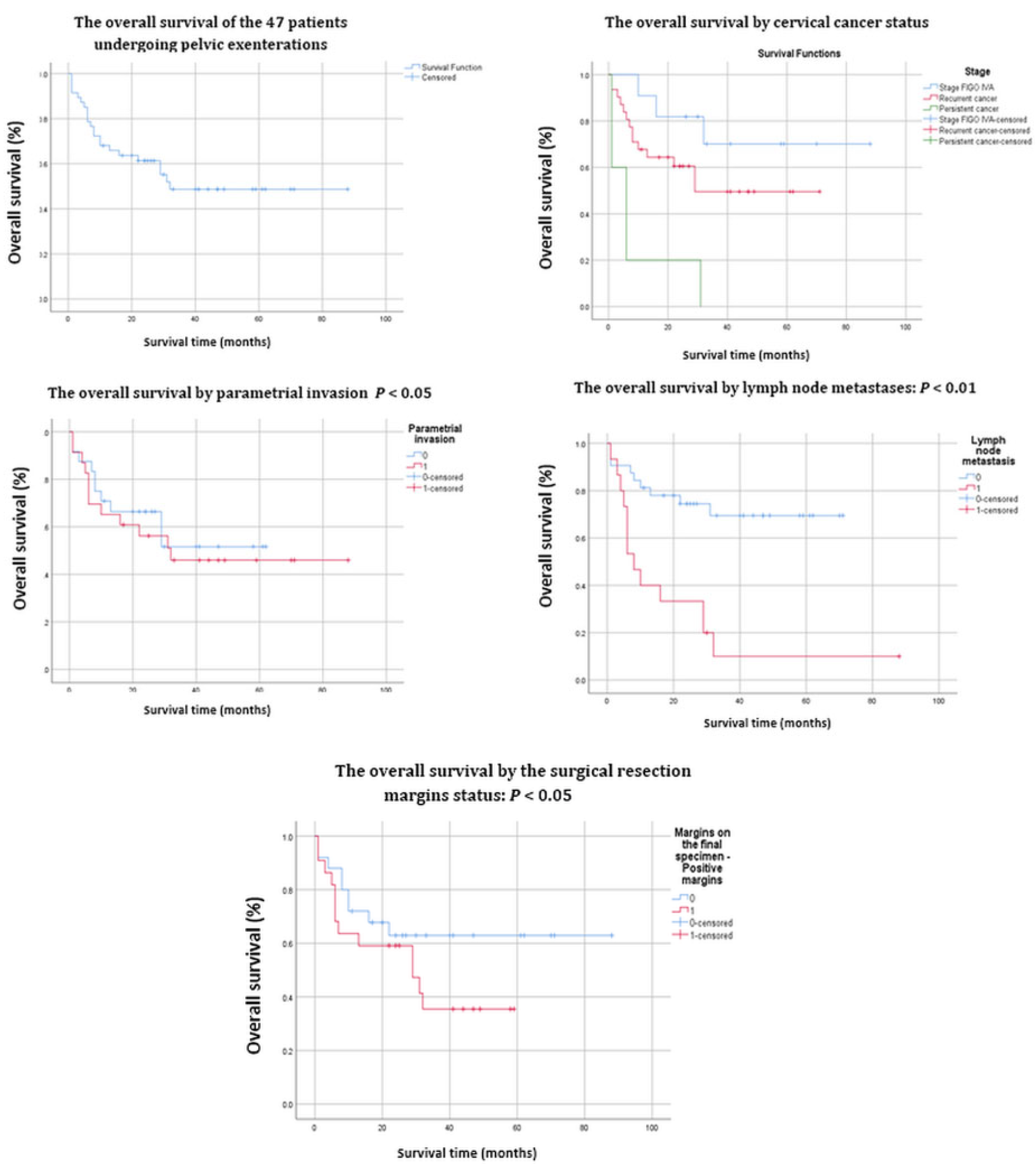

| Number (%) or Median (Range) | Overall Survival | ||||
|---|---|---|---|---|---|
| 5-Year Survival Rate | 95% CI | Mean Survival (Months) | p-Value | ||
| No. of Patients | 47 | ||||
| Age (Years) | 54 (36–67) | ||||
| 30–40 | 2 (4.3%) | 50.0% | 53.5–46.4 | 10.5 | 0.126 |
| 41–50 | 14 (29.8%) | 61.2% | 62.6–59.8 | 59.8 | 0.062 |
| 51–60 | 19 (40.4%) | 36.2% | 37.3–34.9 | 35.2 | 0.294 |
| 61–70 | 12 (25.5%) | 58.3% | 59.7–56.8 | 37.8 | 0.584 |
| Provenance | 0.940 | ||||
| Urban | 18 (38.3%) | 50.0% | 51.1–48.8 | 39.0 | |
| Rural | 29 (61.7%) | 49.6% | 50.6–48.5 | 51.6 | |
| Cancer status/Figo stage | |||||
| IVA | 11 (23.4%) | 70.1% | 71.5–68.9 | 67.8 | 0.220 |
| Recurrent cancer | 31 (66.0%) | 49.6% | 50.6–48.5 | 41.3 | 0.167 |
| Persistent cancer | 5 (10.6%) | 0% | 9.0 | 0.665 | |
| Tumor size | |||||
| <4 cm | 24 (51.1%) | 58.4% | 59.5–57.2 | 46.6 | 0.460 |
| ≥4 cm | 23 (48.9%) | 38.3% | 39.4–37.1 | 41.8 | 0.468 |
| Histology | |||||
| Squamous Cell Carcinoma | 40 (85.1%) | 49.2% | 50.1–48.3 | 49.7 | 0.356 |
| Adenocarcinoma | 7 (14.9%) | 42.9% | 44.7–41.0 | 35.2 | 0.356 |
| Tumor Differentiation Grade | |||||
| Grade 1 (Well Differentiated) | 8 (17.0%) | 21.9% | 23.8–19.9 | 34.3 | 0.795 |
| Grade 2 (Moderately Differentiated) | 14 (29.8%) | 56.3% | 57.6–54.9 | 43.8 | 0.996 |
| Grade 3 (Poorly Differentiated) | 25 (53.2%) | 50.8% | 51.8–49.7 | 48.6 | 0.848 |
| Depth of Cervical Stromal Invasion | |||||
| Inner 1/3 | 4 (8.5%) | 50.0% | 52.5–47.5 | 23.7 | 0.664 |
| Middle 1/3 | 14 (29.8%) | 46.8% | 48.3–45.2 | 38.5 | 0.222 |
| Outer 1/3 | 29 (61.7%) | 48.4% | 49.4–47.4 | 48.0 | 0.170 |
| Lymphovascular Space Invasion | |||||
| Positive | 26 (55.3%) | 26.6% | 27.5–25.6 | 32.3 | 0.249 |
| Negative | 21 (44.7%) | 77.% | 78–76 | 58.4 | |
| Parametrial Involvement | 0.059 | ||||
| Positive | 23 (48.9%) | 51.6% | 52.7–50.4 | 47 | |
| Negative | 24 (51.1%) | 46% | 47–45 | 38.4 | |
| Resection Margin Status | 0.052 | ||||
| Positive | 17 (36%) | 33.5% | 34.6–34.3 | 30.2 | |
| Negative | 30 (64%) | 62.9% | 63.8–61.9 | 58.7 | |
| Pelvic Lymph Nodes Metastases | 0.017 | ||||
| Positive | 15 (31.9%) | 10% | 10.8–9.12 | 20.2 | |
| Negative | 32 (68.1%) | 69.5% | 68.6–70.3 | 52.9 | |
| Overall Survival | |||||
|---|---|---|---|---|---|
| Number (%) or Median (Range) | 5-Year Survival Rate | 95% CI | Mean Survival (Months) | p-Value | |
| Treatment received prior to pelvic exenteration | |||||
| No prior treatment | 11 (24%) | 79% | 77.8–80.1 | 41.9 | 0.098 |
| Prior radical histerectomy + adjuvant radiotherapy | 2 (4.%) | 0% | 16.0 | 0.688 | |
| Prior concomitent definitive radiochemotherapy | 34 (72%) | 41.2% | 42.1–40.2 | 36.2 | 0.220 |
| Adjuvant treatment following pelvic exenteration | |||||
| Adjuvant chemotherapy | 13 (23%) | 70.1% | 71.5–68.6 | 67.8 | 0.220 |
| Type of pelvic exenteration (topographic) | |||||
| Total pelvic exenteration | 29 (61.7%) | 46.7 | 47.7–45.6 | 34.8 | 0.025 |
| Anterior pelvic exenteration | 17 (36.2%) | 50.3 | 51.5–49.0 | 51.3 | 0.011 |
| Posterior pelvic exenteration | 1 (2.1%) | 100% | 24 | 0.128 | |
| The type of pelvic exenterations concerning the levator ani muscle | |||||
| Supralevatorian | 28 (59.6%) | 52.1% | 53.1–51.0 | 53.3 | 0.021 |
| Infralevatorian | 19 (40.4) | 43.9% | 46.4–42.6 | 30.7 | 0.069 |
| Type of urinary tract reconstruction | |||||
| Bricker ileal incontinent conduit diversion | 46 (98%) | 52% | 51.2–52.9 | 27.3 | 0.718 |
| Type of intestinal tract reconstruction | |||||
| Colostomy | 27 (90%) | 53% | 52.1–54.1 | 22.9 | 0.817 |
| Colorectal anastomosis | 3 (10%) | 100% | 29.1 | 0.209 | |
| Complications | |||||
| Early complications | 18 (38.3%) | 20% | 20.9–19.01 | 15.1 | 0.007 |
| Late complications | 9 (19.1%) | 16.7% | 18.1–15.2 | 20.1 | 0.815 |
| Surgery time—minutes | 300–420 (360) | ||||
| Loss of blood—mL | 400–1800 (1.100) | ||||
| Transfused blood volume, mL | 0–1.350 (675) | ||||
| Days of hospitalization following pelvic exenteration | 19 (10–28) | ||||
| Mean follow-up time (months) | 44.5 (1–88) | ||||
| Status | |||||
| Dead | 22 (46.8%) | ||||
| Alive | 25 (53.2%) | 48.7% | 49.5–47.9 | 49.4 | |
| Patients who answered the quality of life questionnaires | 18 (28%) | ||||
| Number of Patients = 18 | Items ~ | Mean Score | SD * | Cronbach’s Alpha Coefficient # |
|---|---|---|---|---|
| QLQ-C30 | ||||
| Functioning scales α | ||||
| Physical α | 1–5 | 53.4 | 13.2 | 0.64 |
| Role α | 6, 7 | 28.5 | 20.6 | 0.69 |
| Cognitive α | 20, 25 | 46.4 | 21.0 | 0.84 |
| Emotional α | 21–24 | 37.0 | 23.1 | 0.93 |
| Social α | 26, 27 | 51.8 | 20.8 | 0.90 |
| Global quality of life α | 29, 30 | 32.6 | 16.1 | 0.66 |
| Symptom scales and/or items γ | ||||
| Fatigue γ | 10, 12, 18 | 60.0 | 16.8 | 0.58 |
| Nausea and vomiting γ | 14, 15 | 13.2 | 14.0 | 0.07 |
| Pain γ | 9, 19 | 73.2 | 20.0 | 0.60 |
| Dyspnea γ | 8 | 36.5 | 22.4 | NA |
| Sleep disturbance γ | 11 | 88.5 | 22.5 | NA |
| Appetite loss γ | 13 | 43.7 | 19.7 | NA |
| Constipation γ | 16 | 41.9 | 19.1 | NA |
| Diarrhea γ | 17 | 13.2 | 17.9 | NA |
| Financial impact γ | 28 | 90.2 | 21.9 | NA |
| QLQ-C24 | ||||
| Symptoms Experience γ | 31–37, 39, 41–43 | 21.8 | 12.1 | 0.71 |
| Body Image γ | 45–47 | 34.1 | 28.1 | 0.88 |
| Sexual/Vaginal Functioning γ | 50–53 | NA | NA | NA |
| Lymphoedema γ | 38 | 77.1 | 27.2 | NA |
| Peripheral Neuropathy γ | 40 | 29.7 | 31.2 | NA |
| Menopausal Symptoms γ | 44 | 77.7 | 27.9 | NA |
| Sexual Worry γ | 48 | 72.3 | 28.8 | NA |
| Sexual Activity α | 49 | NA | NA | NA |
| Sexual Enjoyment α | 54 | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanca, M.; Căpîlna, D.M.; Căpîlna, M.E. Long-Term Survival, Prognostic Factors, and Quality of Life of Patients Undergoing Pelvic Exenteration for Cervical Cancer. Cancers 2022, 14, 2346. https://doi.org/10.3390/cancers14092346
Stanca M, Căpîlna DM, Căpîlna ME. Long-Term Survival, Prognostic Factors, and Quality of Life of Patients Undergoing Pelvic Exenteration for Cervical Cancer. Cancers. 2022; 14(9):2346. https://doi.org/10.3390/cancers14092346
Chicago/Turabian StyleStanca, Mihai, Dan Mihai Căpîlna, and Mihai Emil Căpîlna. 2022. "Long-Term Survival, Prognostic Factors, and Quality of Life of Patients Undergoing Pelvic Exenteration for Cervical Cancer" Cancers 14, no. 9: 2346. https://doi.org/10.3390/cancers14092346
APA StyleStanca, M., Căpîlna, D. M., & Căpîlna, M. E. (2022). Long-Term Survival, Prognostic Factors, and Quality of Life of Patients Undergoing Pelvic Exenteration for Cervical Cancer. Cancers, 14(9), 2346. https://doi.org/10.3390/cancers14092346








