Current Biological, Pathological and Clinical Landscape of HER2-Low Breast Cancer
Abstract
:Simple Summary
Abstract
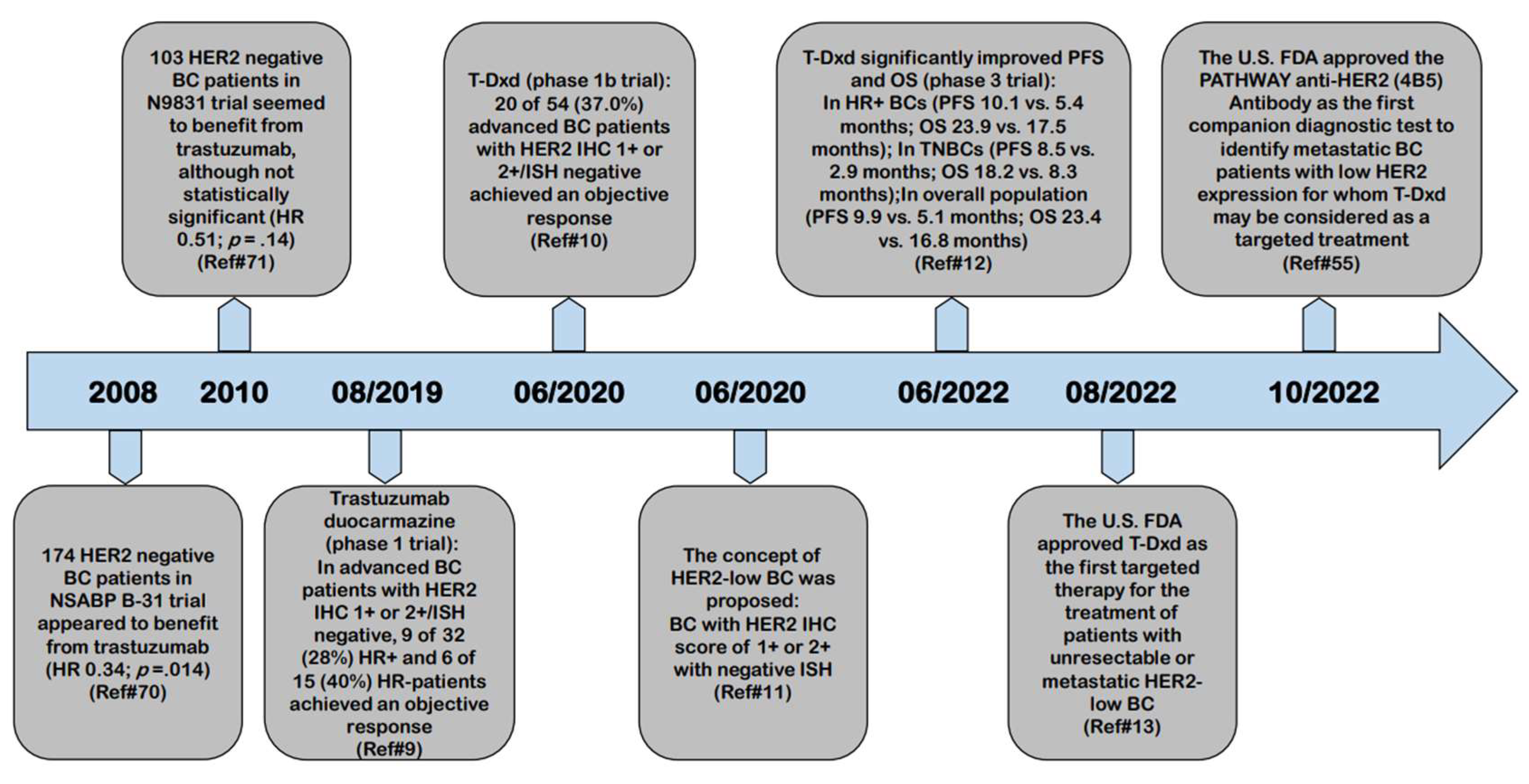
1. The Natural History of HER2-Low Breast Cancer
2. Current Biological Landscape of HER2-Low Breast Cancer
2.1. The Incidence of HER2-Low Breast Cancer
2.2. The Biology of HER2-Low Breast Cancer
2.3. The Molecular Basis of HER2-Low Breast Cancer
2.4. Factors may Contribute to the HER2-Low Expression in Breast Cancer
3. Current Pathological Landscape of HER2-Low Breast Cancer
3.1. The Fundamental Challenge in the Pathological Landscape of HER2-Low Breast Cancer: Accurate Definition
3.2. IHC Testing as the Primary Method for Identifying HER2-Low Breast Cancer
3.3. Current Developments in More Accurate and Reliable Methods for Identifying HER2-Low Expressing Tumors
3.4. HER2 Evaluation and Reporting in Breast Cancer in the New HER2-Low Era
4. Current Clinical Landscape of HER2-Low Breast Cancer: Role of HER2-Targeted Agents
4.1. Limited Activity of Anti-HER2 Monoclonal Antibodies in HER2-Low Breast Cancer
4.2. Limited Activity of First Generation of HER2-Targeted ADC in HER2-Low Breast Cancer
4.3. Significant Clinical Benefit of New HER2-Targeted ADC in HER2-Low BCs
4.4. Other Clinical Development of Agents in the Setting of HER2-Low Breast Cancer
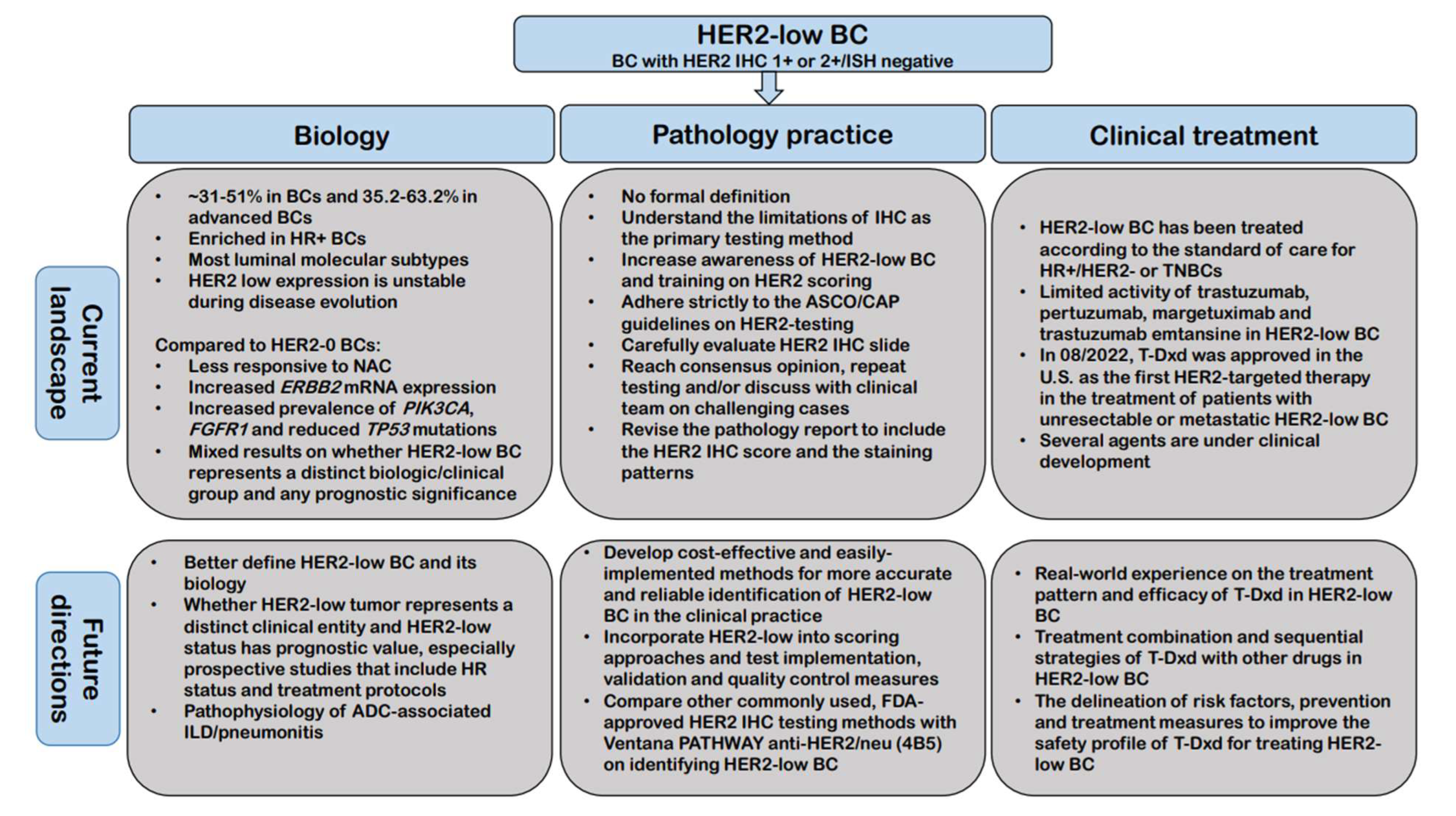
5. Future Directions
- What is the most appropriate/accurate assay to use for identifying HER2-low BC in the clinical practice;
- How to best incorporate this new classification of BC into scoring approaches and what changes are needed in terms of test implementation, validation and quality control measures;
- To establish a more accurate and reproducible definition of HER2-low BC;
- To further address whether HER2-low tumor represents a distinct clinical entity and whether HER2-low expression has prognostic value, especially prospective studies that include HR status and treatment protocols;
- Real-world experience with large multicenter case series on the treatment pattern and efficacy of T-Dxd in HER2-low BC;
- How should T-Dxd be sequenced with other treatment options for treating HER2-low BC and further evaluation of treatment combination strategies of T-Dxd with other drugs;
- To investigate the pathophysiology of ADC-associated adverse events especially ILD/pneumonitis along with the delineation of risk factors, prevention, and treatment measures, ultimately to improve the safety profile of these ADCs for treating HER2-low BC.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer:American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sáez, O.; Prat, A. Current and Future Management of HER2-Positive Metastatic Breast Cancer. JCO Oncol. Pract. 2021, 17, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Sakach, E.; O’Regan, R.; Meisel, J.; Li, X. Molecular Classification of Triple Negative Breast Cancer and the Emergence of Targeted Therapies. Clin. Breast Cancer 2021, 21, 509–520. [Google Scholar] [CrossRef]
- Schettini, F.; Venturini, S.; Giuliano, M.; Lambertini, M.; Pinato, D.J.; Onesti, C.E.; Vidal-Sicart, S.; Ganau, S.; Cebrecos, I.; Brasó-Maristany, F.; et al. Multiple Bayesian network meta-analyses to establish therapeutic algorithms for metastatic triple negative breast cancer. Cancer Treat. Rev. 2022, 111, 102468. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Karantza, V.; Aktan, G.; Lala, M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: A systematic literature review. Breast Cancer Res. 2019, 21, 143. [Google Scholar] [CrossRef] [Green Version]
- Başaran, G.A.; Twelves, C.; Diéras, V.; Cortés, J.; Awada, A. Ongoing unmet needs in treating estrogen receptor-positive/HER2-negative metastatic breast cancer. Cancer Treat. Rev. 2018, 63, 144–155. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: Results from a phase Ib study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- FDA approves fam-trastuzumab deruxtecan-nxki for HER2-low breast cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer (accessed on 21 October 2022).
- Marchiò, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Katerji, H.; Turner, B.M.; Hicks, D.G. HER2-Low Breast Cancers: New opportunities and challenges. Am. J. Clin. Pathol. 2022, 157, 328–336. [Google Scholar] [CrossRef]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. HER2-low breast cancer: Molecular characteristics and prognosis. Cancers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Zhang, H.; Katerji, H.; Turner, B.M.; Audeh, W.; Hicks, D.G. HER2-low breast cancers: Incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod. Pathol. 2022, 35, 1075–1082. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Rinnerthaler, G.; Tinchon, C.; Petzer, A.; Balic, M.; Heibl, S.; Schmitt, C.; Zabernigg, A.F.; Egle, D.; Sandholzer, M.; et al. Landscape of HER2-low metastatic breast cancer (MBC): Results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021, 23, 112. [Google Scholar] [CrossRef]
- Prat, A.; Bardia, A.; Curigliano, G.; Hammond, M.E.H.; Loibl, S.; Tolaney, S.M.; Viale, G. An Overview of Clinical Development of Agents for Metastatic or Advanced Breast Cancer Without ERBB2 Amplification (HER2-Low). JAMA Oncol. 2022, 8, 1676–1687. [Google Scholar] [CrossRef]
- Viale, G.; Niikura, N.; Tokunaga, E.; Aleynikova, O.; Hayashi, N.; Sohn, J.; O’Brien, C.; Higgins, G.; Varghese, D.; James, G.D.; et al. Retrospective study to estimate the prevalence of HER2-low breast cancer (BC) and describe its clinicopathological characteristics. J. Clin. Oncol. 2022, 40 (Suppl. 16), 1087. [Google Scholar] [CrossRef]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; De Bartolo, D.; Vernaci, G.; et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 2021, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Berrino, E.; Annaratone, L.; Bellomo, S.E.; Ferrero, G.; Gagliardi, A.; Bragoni, A.; Grassini, D.; Guarrera, S.; Parlato, C.; Casorzo, L.; et al. Integrative genomic and transcriptomic analyses illuminate the ontology of HER2-low breast carcinomas. Genome Med. 2022, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.R.; Gil, L.; Vasconcelos de Matos, L.; Baleiras, A.; Vasques, C.; Neves, M.T.; Ferreira, A.; Fontes-Sousa, M.; Miranda, H.; Martins, A. Impact of human epidermal growth factor receptor 2 (HER2) low status in response to neoadjuvant chemotherapy in early breast cancer. Cureus 2022, 14, e22330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ren, C.; Li, C.; Wang, Y.; Cheng, B.; Wen, L.; Jia, M.; Li, K.; Mok, H.; Cal, L.; et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Ahn, J.; Kim, Y.; Kim, J.S.; Song, J.Y.; Kim, H.K.; Lee, J.; Park, H.K.; Kim, Y.S. Clinical significance of HER2-low expression in early breast cancer: A nationwide study from the Korean breast cancer society. Breast Cancer Res. 2022, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Abudureheiyimu, N.; Mo, H.; Guan, X.; Lin, S.; Wang, Z.; Chen, Y.; Chen, S.; Li, Q.; Cai, R.; et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: A study of the national cancer center. China. Front. Oncol. 2022, 11, 774577. [Google Scholar] [CrossRef]
- Chen, M.; Chen, W.; Liu, D.; Chen, W.; Shen, K.; Wu, J.; Zhu, L. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: Features of HER2- low breast cancer. Breast Cancer 2022, 29, 844–853. [Google Scholar] [CrossRef]
- Rosso, C.; Voutsadakis, I.A. Characteristics, clinical differences and outcomes of breast cancer patients with negative or low HER2 expression. Clin. Breast Cancer 2022, 22, 391–397. [Google Scholar] [CrossRef]
- Tarantino, P.; Jin, Q.; Tayob, N.; Jeselsohn, R.M.; Schnitt, S.J.; Vincuilla, J.; Parker, T.; Tyekucheva, S.; Li, T.; Lin, N.U.; et al. Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol. 2022, 8, 1177–1183. [Google Scholar] [CrossRef]
- Di Cosimo, S.; La Rocca, E.; Ljevar, S.; De Santis, M.C.; Bini, M.; Cappelletti, V.; Valenti, M.; Baili, P.; de Braud, F.G.; Folli, S.; et al. Moving HER2-low breast cancer predictive and prognostic data from clinical trials into the real world. Front. Mol. Biosci. 2022, 9, 996434. [Google Scholar] [CrossRef] [PubMed]
- de Moura Leite, L.; Cesca, M.G.; Tavares, M.C.; Santana, D.M.; Saldanha, E.F.; Guimarães, P.T.; Sá, D.D.S.; Simões, M.F.E.; Viana, R.L.; Rocha, F.G.; et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res. Treat 2021, 190, 155–163. [Google Scholar] [CrossRef]
- Cherifi, F.; Da Silva, A.; Johnson, A.; Blanc-Fournier, C.; Abramovici, O.; Broyelle, A.; Levy, C.; Allouache, D.; Hrab, I.; Segura, C.; et al. HELENA: HER2-Low as a predictive factor of response to Neoadjuvant chemotherapy in early breast cancer. BMC Cancer 2022, 22, 1081. [Google Scholar] [CrossRef] [PubMed]
- de Nonneville, A.; Houvenaeghel, G.; Cohen, M.; Sabiani, L.; Bannier, M.; Viret, F.; Gonçalves, A.; Bertucci, F. Pathological complete response rate and disease-free survival after neoadjuvant chemotherapy in patients with HER2-low and HER2-0 breast cancers. Eur. J. Cancer 2022, 176, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, S.H.; Lee, H.J.; Jeong, H.; Jeong, J.H.; Kim, J.E.; Ahn, J.H.; Jung, K.H.; Gong, G.; Kim, H.H.; et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur. J. Cancer 2022, 176, 30–40. [Google Scholar] [CrossRef]
- Shao, Y.; Yu, Y.; Luo, Z.; Guan, H.; Zhu, F.; He, Y.; Chen, Q.; Liu, C.; Nie, B.; Liu, H. Clinical, Pathological Complete Response, and Prognosis Characteristics of HER2-Low Breast Cancer in the Neoadjuvant Chemotherapy Setting: A Retrospective Analysis. Ann. Surg. Oncol. 2022, 29, 8026–8034. [Google Scholar] [CrossRef]
- Domergue, C.; Martin, E.; Lemarié, C.; Jézéquel, P.; Frenel, J.S.; Augereau, P.; Campone, M.; Patsouris, A. Impact of HER2 Status on Pathological Response after Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer. Cancers 2022, 14, 2509. [Google Scholar] [CrossRef]
- Carlino, F.; Diana, A.; Ventriglia, A.; Piccolo, A.; Mocerino, C.; Riccardi, F.; Bilancia, D.; Giotta, F.; Antoniol, G.; Famiglietti, V.; et al. HER2-Low Status Does Not Affect Survival Outcomes of Patients with Metastatic Breast Cancer (MBC) Undergoing First-Line Treatment with Endocrine Therapy plus Palbociclib: Results of a Multicenter, Retrospective Cohort Study. Cancers 2022, 14, 4981. [Google Scholar] [CrossRef]
- Almstedt, K.; Heimes, A.S.; Kappenberg, F.; Battista, M.J.; Lehr, H.A.; Krajnak, S.; Lebrecht, A.; Gehrmann, M.; Stewen, K.; Brenner, W.; et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur. J. Cancer 2022, 173, 10–19. [Google Scholar] [CrossRef]
- Tan, R.S.Y.C.; Ong, W.S.; Lee, K.H.; Lim, A.H.; Park, S.; Park, Y.H.; Lin, C.H.; Lu, Y.S.; Ono, M.; Ueno, T.; et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: An international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022, 20, 105. [Google Scholar] [CrossRef]
- Douganiotis, G.; Kontovinis, L.; Markopoulou, E.; Ainali, A.; Zarampoukas, T.; Natsiopoulos, I.; Papazisis, K. Prognostic Significance of Low HER2 Expression in Patients With Early Hormone Receptor Positive Breast Cancer. Cancer Diagn. Progn. 2022, 2, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, N.; Adachi, Y.; Takatsuka, D.; Nozawa, K.; Endo, Y.; Ozaki, Y.; Sugino, K.; Kataoka, A.; Kotani, H.; Yoshimura, A.; et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 2022, 29, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Maran-Gonzalez, A.; Massol, O.; Sorbs, C.; Mollevi, C.; Guiu, S.; Boissière-Michot, F.; Ramos, J. Prognostic Value of HER2-Low Expression in Non-Metastatic Triple-Negative Breast Cancer and Correlation with Other Biomarkers. Cancers 2021, 13, 6059. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Gandini, S.; Nicolò, E.; Trillo, P.; Giugliano, F.; Zagami, P.; Vivanet, G.; Bellerba, F.; Trapani, D.; Marra, A.; et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur. J. Cancer 2022, 163, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; De Bartolo, D.; Vernaci, G.; et al. HER2-low-positive breast cancer: Evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer 2022, 8, 66. [Google Scholar] [CrossRef]
- van den Ende, N.S.; Smid, M.; Timmermans, A.; van Brakel, J.B.; Hansum, T.; Foekens, R.; Trapman, A.M.A.C.; Heemskerk-Gerritsen, B.A.M.; Jager, A.; Martens, J.W.M.; et al. HER2-low breast cancer shows a lower immune response compared to HER2-negative cases. Sci. Rep. 2022, 12, 12974. [Google Scholar] [CrossRef]
- Bayona, R.S.; Luna, A.M.; Tolosa, P.; De Torre, A.S.; Castelo, A.; Marín, M.; García, C.; Boni, V.; Hertfelder, E.B.; Vega, E.; et al. HER2-low vs HER2-zero metastatic breast carcinoma: A clinical and genomic descriptive analysis. Ann. Oncol. 2021, 32 (Suppl. 2), S29–S30. [Google Scholar] [CrossRef]
- Ross, J.S.; Fletcher, J.A.; Linette, G.P.; Stec, J.; Clark, E.; Ayers, M.; Symmans, W.F.; Pusztai, L.; Bloom, K.J. The Her-2/neu gene and protein in breast cancer 2003: Biomarker and target of therapy. Oncologist 2003, 8, 307–325. [Google Scholar] [CrossRef]
- Giuliano, M.; Trivedi, M.V.; Schiff, R. Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications. Breast Care 2013, 8, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Lousberg, L.; Collignon, J.; Jerusalem, G. Resistance to therapy in estrogen receptor positive and human epidermal growth factor 2 positive breast cancers: Progress with latest therapeutic strategies. Ther. Adv. Med. Oncol. 2016, 8, 429–449. [Google Scholar] [CrossRef]
- Diéras, V.; Deluche, E.; Lusque, A.; Pistilli, B.; Bachelot, T.; Pierga, J.Y.; Viret, F.; Levy, C.; Salabert, L.; Le Du, F.; et al. Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY) (meeting abstract). Cancer Res. 2022, 82 (Suppl. 4), PD8-02. [Google Scholar] [CrossRef]
- Zhang, H.; Karakas, C.; Tyburski, H.; Turner, B.M.; Peng, Y.; Wang, X.; Katerji, H.; Schiffhauer, L.; Hicks, D.G. HER2-low breast cancers: Current insights and future directions. Semin. Diagn. Pathol. 2022, 39, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Karakas, C.; Tyburski, H.; Turner, B.M.; Wang, X.; Schiffhauer, L.; Katerji, M.; Hicks, D.G.; Zhang, H. Inter-observer and Inter-antibody Reproducibility of HER2 Immunohistochemical Scoring in a HER2-low Expressing Breast Cancer Enriched Cohort. Am. J. Clin. Pathol. 2023; in press. [Google Scholar]
- Roche receives FDA approval for first companion diagnostic to identify patients with HER2 low metastatic breast cancer eligible for Enhertu. Available online: https://diagnostics.roche.com/global/en/news-listing/2022/roche-receives-fda-approval-for-first-companion-diagnostic-to-id.html. (accessed on 2 November 2022).
- Baez-Navarro, X.; Salgado, R.; Denkert, C.; Lennerz, J.K.; Penault-Llorca, F.; Viale, G.; Bartlett, J.M.S.; van Deurzen, C.H.M. Selecting patients with HER2-low breast cancer: Getting out of the tangle. Eur. J. Cancer 2022, 175, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Atallah, N.M.; Toss, M.S.; Green, A.R.; Mongan, N.P.; Ball, G.; Rakha, E.A. Refining the definition of HER2-low class in invasive breast cancer. Histopathology 2022, 81, 770–785. [Google Scholar] [CrossRef]
- Farahmand, S.; Fernandez, A.I.; Ahmed, F.S.; Rimm, D.L.; Chuang, J.H.; Reisenbichler, E.; Zarringhalam, K. Deep learning trained on hematoxylin and eosin tumor region of Interest predicts HER2 status and trastuzumab treatment response in HER2 + breast cancer. Mod. Pathol. 2022, 35, 44–51. [Google Scholar] [CrossRef]
- Qaiser, T.; Mukherjee, A.; Reddy, P.C.; Munugoti, S.D.; Tallam, V.; Pitkaaho, T.; Lehtimäki, T.; Naughton, T.; Berseth, M.; Pedraza, A.; et al. HER2 challenge contest: A detailed assessment of automated HER2 scoring algorithms in whole slide images of breast cancer tissues. Histopathology 2018, 72, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Helin, H.O.; Tuominen, V.J.; Ylinen, O.; Helin, H.J.; Isola, J. Free digital image analysis software helps to resolve equivocal scores in HER2 immunohistochemistry. Virchows Arch. 2016, 468, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Tuominen, V.J.; Tolonen, T.T.; Isola, J. ImmunoMembrane: A publicly available web application for digital image analysis of HER2 immunohistochemistry. Histopathology 2012, 60, 758–767. [Google Scholar] [CrossRef]
- Laurinaviciene, A.; Dasevicius, D.; Ostapenko, V.; Jarmalaite, S.; Lazutka, J.; Laurinavicius, A. Membrane connectivity estimated by digital image analysis of HER2 immunohistochemistry is concordant with visual scoring and fluorescence in situ hybridization results: Algorithm evaluation on breast cancer tissue microarrays. Diagn. Pathol. 2012, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Gustavson, M.; Haneder, S.; Spitzmueller, A.; Kapil, A.; Schneider, K.; Cecchi, F.; Sridhar, S.; Schmidt, G.; Lakis, S.; Teichert, R.; et al. Novel approach to HER2 quantification: Digital pathology coupled with AI-based image and data analysis delivers objective and quantitative HER2 expression analysis for enrichment of responders to trastuzumab deruxtecan (T-DXd; DS-8201), specifically in HER2-low patients (meeting abstract). Cancer Res. 2021, 81 (Suppl. 4), PD6-01. [Google Scholar]
- Wu, S.; Yue, M.; Zhang, J.; Li, X.; Li, Z.; Zhang, H.; Wang, X.; Han, X.; Cai, L.; Shang, J.; et al. The role of artificial intelligence in accurate interpretation of HER2 IHC 0 and 1+ in breast cancers. Mod. Pathol. 2023; in press. [Google Scholar]
- Paige Answers Call to Better Identify Breast Cancer Patients with Low Expression of HER2-Paige. Available online: https://www.businesswire.com/news/home/20220623005253/en/Paige-Answers-Call-to-Better-Identify-Breast-Cancer-Patients-with-Low-Expression-of-HER2 (accessed on 2 November 2022).
- Moutafi, M.; Robbins, C.J.; Yaghoobi, V.; Fernandez, A.I.; Martinez-Morilla, S.; Xirou, V.; Bai, Y.; Song, Y.; Gaule, P.; Krueger, J.; et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab. Invest 2022, 102, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.J.; Whiteaker, J.R.; Kennedy, L.C.; Bosch, D.E.; Lerch, M.L.; Schoenherr, R.M.; Zhao, L.; Lin, C.; Chowdhury, S.; Kilgore, M.R.; et al. Quantification of Human Epidermal Growth Factor Receptor 2 by Immunopeptide Enrichment and Targeted Mass Spectrometry in Formalin-Fixed Paraffin-Embedded and Frozen Breast Cancer Tissues. Clin. Chem. 2021, 67, 1008–1018. [Google Scholar] [CrossRef]
- Xu, K.; Bayani, J.; Mallon, E.; Pond, G.R.; Piper, T.; Hasenburg, A.; Markopoulos, C.J.; Dirix, L.; Seynaeve, C.M.; van de Velde, C.J.H.; et al. Discordance between Immunohistochemistry and ERBB2 mRNA to Determine HER2 Low Status for Breast Cancer. J. Mol. Diagn 2022, 24, 775–783. [Google Scholar] [CrossRef]
- Shu, L.; Tong, Y.; Li, Z.; Chen, X.; Shen, K. Can HER2 1+ Breast Cancer Be Considered as HER2-Low Tumor? A Comparison of Clinicopathological Features, Quantitative HER2 mRNA Levels, and Prognosis among HER2-Negative Breast Cancer. Cancers 2022, 14, 4250. [Google Scholar] [CrossRef]
- Paik, S.; Kim, C.; Wolmark, N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N. Engl. J. Med. 2008, 358, 1409–1411. [Google Scholar] [CrossRef]
- Perez, E.A.; Reinholz, M.M.; Hillman, D.W.; Tenner, K.S.; Schroeder, M.J.; Davidson, N.E.; Martino, S.; Sledge, G.W.; Harris, L.N.; Gralow, J.R.; et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J. Clin. Oncol. 2010, 28, 4307–4315. [Google Scholar] [CrossRef] [Green Version]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.F.; Provencher, L.; et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2+. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef]
- Gianni, L.; Lladó, A.; Bianchi, G.; Cortes, J.; Kellokumpu-Lehtinen, P.L.; Cameron, D.A.; Miles, D.; Salvagni, S.; Wardley, A.; Goeminne, J.C.; et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2010, 28, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Schneeweiss, A.; Park-Simon, T.W.; Albanell, J.; Lassen, U.; Cortés, J.; Dieras, V.; May, M.; Schindler, C.; Marmé, F.; Cejalvo, J.M.; et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with Schneethe anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest. New Drugs 2018, 36, 848–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordstrom, J.L.; Gorlatov, S.; Zhang, W.; Yang, Y.; Huang, L.; Burke, S.; Li, H.; Ciccarone, V.; Zhang, T.; Stavenhagen, J.; et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011, 13, R123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phase 2 Study of the Monoclonal Antibody MGAH22 (Margetuximab) in Patients with Relapsed or Refractory Advanced Breast Cancer—Study Results. Available online: https://clinicaltrials.gov/ (accessed on 2 November 2022).
- Burris, H.A., 3rd; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S.; et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 2011, 29, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; LoRusso, P.; Miller, K.D.; Modi, S.; Yardley, D.; Rodriguez, G.; Guardino, E.; Lu, M.; Zheng, M.; Girish, S.; et al. A Phase II Study of Trastuzumab Emtansine in Patients with Human Epidermal Growth Factor Receptor 2-positive Metastatic Breast Cancer Who Were Previously Treated with Trastuzumab, Lapatinib, an Anthracycline, a Taxane, and Capecitabine. J. Clin. Oncol. 2012, 30, 3234–3241. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Siddiqui, T.; Rani, P.; Ashraf, T.; Ellahi, A. Enhertu (Fam-trastuzumab-deruxtecan-nxki)—Revolutionizing treatment paradigm for HER2-Low breast cancer. Ann. Med. Surg 2022, 82, 104665. [Google Scholar] [CrossRef]
- Powell, C.A.; Modi, S.; Iwata, H.; Takahashi, S.; Smit, E.F.; Siena, S.; Chang, D.Y.; Macpherson, E.; Qin, A.; Singh, J.; et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 2022, 7, 100554. [Google Scholar] [CrossRef]
- Tarantino, P.; Modi, S.; Tolaney, S.M.; Cortés, J.; Hamilton, E.P.; Kim, S.B.; Toi, M.; Andrè, F.; Curigliano, G. Interstitial Lung Disease Induced by Anti-ERBB2 Antibody-Drug Conjugates: A Review. JAMA Oncol. 2021, 7, 1873–1881. [Google Scholar] [CrossRef]
- Kumagai, K.; Aida, T.; Tsuchiya, Y.; Kishino, Y.; Kai, K.; Mori, K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020, 111, 4636–4645. [Google Scholar] [CrossRef]
- Adams, E.; Wildiers, H.; Neven, P.; Punie, K. Sacituzumab govitecan and trastuzumab deruxtecan: Two new antibody-drug conjugates in the breast cancer treatment landscape. ESMO Open 2021, 6, 100204. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Peng, Y. Current Biological, Pathological and Clinical Landscape of HER2-Low Breast Cancer. Cancers 2023, 15, 126. https://doi.org/10.3390/cancers15010126
Zhang H, Peng Y. Current Biological, Pathological and Clinical Landscape of HER2-Low Breast Cancer. Cancers. 2023; 15(1):126. https://doi.org/10.3390/cancers15010126
Chicago/Turabian StyleZhang, Huina, and Yan Peng. 2023. "Current Biological, Pathological and Clinical Landscape of HER2-Low Breast Cancer" Cancers 15, no. 1: 126. https://doi.org/10.3390/cancers15010126
APA StyleZhang, H., & Peng, Y. (2023). Current Biological, Pathological and Clinical Landscape of HER2-Low Breast Cancer. Cancers, 15(1), 126. https://doi.org/10.3390/cancers15010126






