Estimated Costs of the Ipilimumab–Nivolumab Therapy and Related Adverse Events in Metastatic Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Acquisition
2.2. Evaluation of Adverse Events Related to Immunotherapy and Their Costs
3. Results
3.1. Baseline Patient Characteristics
3.2. Clinical Safety
3.3. Efficacy
3.4. Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef] [Green Version]
- Ugurel, S.; Röhmel, J.; Ascierto, P.A.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Long, G.V.; Lorigan, P.; McArthur, G.A.; et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies-update 2017. Eur. J. Cancer Oxf. Engl. 2017, 83, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, E.; Wicky, A.; Bouchaab, H.; Imbimbo, M.; Delyon, J.; Gautron Moura, B.; Gérard, C.; Latifyan, S.; Özdemir, B.; Caikovski, M.; et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: An overlooked aspect in immunotherapy. Eur. J. Cancer Oxf. Engl. 2021, 149, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28 (Suppl. 4), iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Potluri, R.; Ranjan, S.; Bhandari, H.; Johnson, H.; Moshyk, A.; Kotapati, S. Healthcare cost comparison analysis of nivolumab in combination with ipilimumab versus nivolumab monotherapy and ipilimumab monotherapy in advanced melanoma. Exp. Hematol. Oncol. 2019, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- WMA-The World Medical Association-WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 11 December 2022).
- Human Research Act (HRA). Available online: https://www.admin.ch/opc/en/classified-compilation/20121176/201401010000/810.3 (accessed on 8 January 2020).
- Trials OoHRwtEoC. Updated January 2014. Available online: https://www.admin.ch/opc/en/classified-compilation/20121177/20 (accessed on 8 January 2020).
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer Oxf. Engl. 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Paly, V.F.; Hikichi, Y.; Baker, T.; Itakura, E.; Chandran, N.; Harrison, J. Economic evaluation of nivolumab combined with ipilimumab in the first-line treatment of advanced melanoma in Japan. J. Med. Econ. 2020, 23, 1542–1552. [Google Scholar] [CrossRef]
- Franken, M.G.; Leeneman, B.; Aarts, M.J.B.; van Akkooi, A.C.J.; van den Berkmortel, F.W.P.J.; Boers-Sonderen, M.J.; Eertwegh, A.V.D.; de Groot, J.; Hospers, G.; Kapiteijn, E. Trends in survival and costs in metastatic melanoma in the era of novel targeted and immunotherapeutic drugs. ESMO Open 2021, 6, 100320. [Google Scholar] [CrossRef]
- Leeneman, B.; Uyl-de Groot, C.A.; Aarts, M.J.B.; van Akkooi, A.C.J.; van den Berkmortel, F.W.P.J.; van den Eertwegh, A.J.M.; De Groot, J.W.B.; Herbschleb, K.H.; Van Der Hoeven, J.J.M.; Hospers, G.A.P.; et al. Healthcare Costs of Metastatic Cutaneous Melanoma in the Era of Immunotherapeutic and Targeted Drugs. Cancers 2020, 12, 1003. [Google Scholar] [CrossRef] [PubMed]
- Copley-Merriman, C.; Stevinson, K.; Liu, F.X.; Wang, J.; Mauskopf, J.; Zimovetz, E.A.; Chmielowski, B. Direct costs associated with adverse events of systemic therapies for advanced melanoma: Systematic literature review. Medicine 2018, 97, e11736. [Google Scholar] [CrossRef] [PubMed]
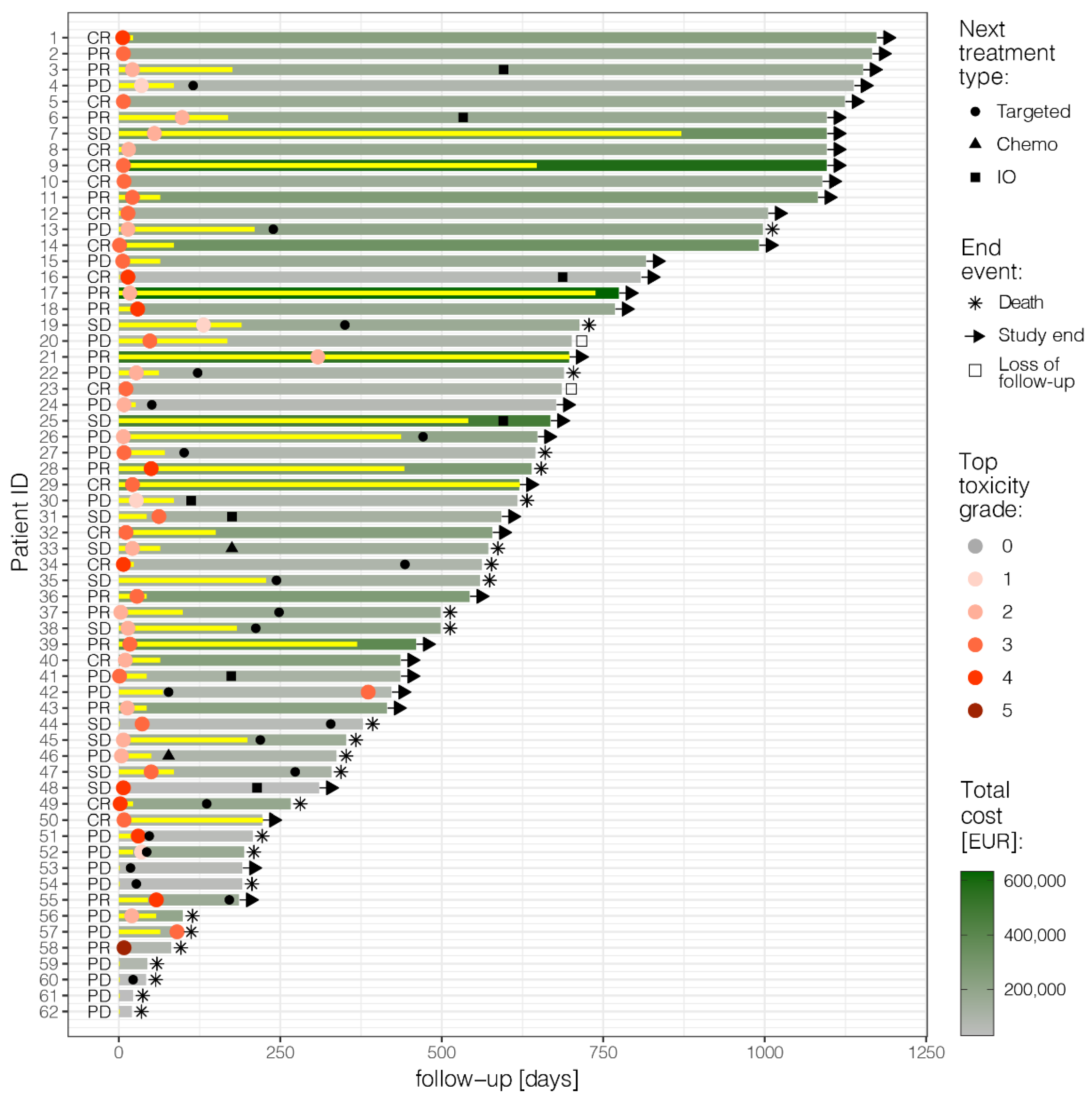




| Total cost | Total billing at the CHUV hospital during the follow-up period. Costs of subsequent treatments were not included. |
| Medication Costs | Cost of ipilimumab–nivolumab combination +/− nivolumab maintenance drugs for specific patients (doses administered depending on weight). |
| Infusion costs | Infusion, laboratory, medical and nurse visits related to the infusion day. |
| Surveillance costs | Medical and nurse visits and laboratory analysis occurring between the drug-infusion days during the concomitant phase. Additional procedures or hospitalizations in between the concomitant phase. |
| Toxicity-related costs | Global cost of toxicities (hospitalizations related to toxicities, cost of investigational and treatment procedures, expert evaluations). |
| Radiology cost | Cost of all radiology exams performed during follow-up, except those related to toxicities. |
| Radiotherapy cost | Cost of radiotherapy treatments. |
| Disease costs | Clinical and laboratory assessments during the follow-up period; hospitalizations due to disease, ambulatory costs from other experts due to disease, ambulatory biopsies, and were calculated as the difference between total costs and the sum of medication, infusion, surveillance, radiology and radiotherapy costs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautron Moura, B.; Gerard, C.L.; Testart, N.; Caikovski, M.; Wicky, A.; Aedo-Lopez, V.; Berthod, G.; Homicsko, K.; Prior, J.O.; Dromain, C.; et al. Estimated Costs of the Ipilimumab–Nivolumab Therapy and Related Adverse Events in Metastatic Melanoma. Cancers 2023, 15, 31. https://doi.org/10.3390/cancers15010031
Gautron Moura B, Gerard CL, Testart N, Caikovski M, Wicky A, Aedo-Lopez V, Berthod G, Homicsko K, Prior JO, Dromain C, et al. Estimated Costs of the Ipilimumab–Nivolumab Therapy and Related Adverse Events in Metastatic Melanoma. Cancers. 2023; 15(1):31. https://doi.org/10.3390/cancers15010031
Chicago/Turabian StyleGautron Moura, Bianca, Camille L. Gerard, Nathalie Testart, Marian Caikovski, Alexandre Wicky, Veronica Aedo-Lopez, Grégoire Berthod, Krisztian Homicsko, John O. Prior, Clarisse Dromain, and et al. 2023. "Estimated Costs of the Ipilimumab–Nivolumab Therapy and Related Adverse Events in Metastatic Melanoma" Cancers 15, no. 1: 31. https://doi.org/10.3390/cancers15010031
APA StyleGautron Moura, B., Gerard, C. L., Testart, N., Caikovski, M., Wicky, A., Aedo-Lopez, V., Berthod, G., Homicsko, K., Prior, J. O., Dromain, C., Kandalaft, L. E., Cuendet, M. A., & Michielin, O. (2023). Estimated Costs of the Ipilimumab–Nivolumab Therapy and Related Adverse Events in Metastatic Melanoma. Cancers, 15(1), 31. https://doi.org/10.3390/cancers15010031








